Ventilator-associated pneumonia (VAP) is a major health care associated infection which usually emanates from aspiration, immigration of pathogens from aerodigestive tract, adulterated appliance uses or medications. The mortality rate due to VAP is approximately 13% and the causative organisms are bacteria, viruses, and fungi. Many studies have investigated the causative organisms as Pseudomonas spp., Acinetobacter spp., Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus with varying prevalence. Intensive Care Unit (ICU) admitted patients who are ventilated, are more prone to the infections where the pathogens adhere to the mucosa of lower respiratory tract of mechanically ventilated patients and start infections. Clinical diagnosis based on Clinical Pulmonary Infection Score (CPIS) has poor specificity and microbiological findings takes 48-72 hrs, that can delay the treatment of patients. Lymphopenia on complete blood count is a predictor of mortality in VAP patients, but decreased lymphocyte count occurs in various other infections too. Multiplex PCR is a better diagnostic technique for VAP which can even diagnose atypical bacteria along with other etiological agents. Effectively employing sampling techniques is a vital step in the diagnosis of VAP, enabling the identification of pathogens responsible for lung infections. Furthermore, the emergence of novel therapeutic options approved by regulatory bodies, adds significant advancements in VAP treatment. In this review article, we have performed an in-depth study on the pathogenesis, diagnosis and therapeutic strategies involved in VAP. This study will help the researchers working in this area to design their work appropriately with the updated knowledge on VAP.
Ventilator Associated Pneumonia, Bacteria, Multiplex PCR, Pathogenesis, Therapy
Ventilator-associated pneumonia (VAP) is a significant infection related to healthcare that occurs when a patient is on mechanical ventilation for two or more consecutive days, where the ventilator tube passes through the patient’s mouth and windpipe. The machine that breathes for patients sometimes carries harmful pathogens which infect the lungs, causing pneumonia.1 VAP is defined as a pneumonia where the patient is on mechanical ventilation for more than 2 days on the date of event, with the day of ventilator placement being day 1, and the ventilator was in place on the date of event or the day before.2 VAP typically arises from various sources, including aspiration, the movement of pathogens from the aerodigestive tract, the use of contaminated equipment or medications.3 The mortality rate associated with VAP is around 13%, and diagnosing it early remains a challenge because there is no standard diagnostic method available.4,5 The clinical findings and the Clinical Pulmonary Infection Score (CPIS) have limited diagnostic accuracy.6 A recent study revealed that lung ultrasonography is a useful tool for assessing lung conditions in the ICU, and the Lung Ultrasound Score (LUS) can be used with confidence to diagnose patients with VAP.7
If VAP is not treated promptly and appropriately, it leads to a high mortality rate.8-12 Microbial culture technology may take around 48-72 hr to diagnose the VAP, which causes delay in starting the antibiotic therapy, and can increase mortality rate.13-16 Besides, many other diagnostic techniques are not sensitive or specific enough to detect the causative agent at an early stage.17 In the present context, early diagnosis of VAP with suitable technique is very important to initiate the antibiotic therapy targeting the particular microorganisms and saving the life along with the avoidance of antibiotic resistance development.
In this review article, we have discussed thoroughly about the pathogenesis, diagnosis and therapeutic strategies involved in VAP which will help the researchers working in this area to design their future research work effectively with updated informations.
Different microorganisms associated with VAP
Different microorganisms associated with VAP are bacteria (typical and atypical bacteria), viruses and fungi (Table 1, 2, and 3). Many studies have investigated the causative organisms as Pseudomonas spp., Acinetobacter spp., Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus with varying prevalence.18-20 One study showed that Pseudomonas aeruginosa was an etiological agent in 33.3% cases and Klebsiella pneumoniae in 20.8% cases of VAP infection, followed by Escherichia coli, Candida albicans and Staphylococcus aureus each causing 8.3% of VAP infections.21
Table (1):
List of different microorganisms (typical bacteria) causing ventilator associated pneumonia (VAP)
Pathogens |
Specimens collected |
Identification techniques |
References |
|---|---|---|---|
E. coli |
NB-BAL (Nonbronchoscopic bronchoalveolar lavage), Tracheal aspirates, Blood and Sputum, sinus lavage |
Using Microbiological techniques like NB-BAL Culture, Tracheal aspirates culture, Blood Culture, Sputum culture, Gram staining, standard biochemical test, and pleural fluid culture, Kirby-Bauer disc diffusion technique, VITEK® 2 automated system, MALDI-TOF (Matrix-Assisted Laser Desorption Ionization-Time of Flight) mass spectrometry, and PCR. |
(21, 24-40) |
Klebsiella spp. |
NB-BAL, Tracheal aspirates, Blood, Sputum, and Pleural Fluid. |
Using Microbiological techniques like NB-BAL Culture, Tracheal aspirates culture, Blood Culture, pleural fluid culture, Sputum culture, Gram staining, standard biochemical test, Kirby-Bauer disc diffusion technique, VITEK® 2 automated system, MALDI-TOF mass spectrometry, and Molecular typing by ERIC-PCR ((Enterobacterial Repetitive Intergenic Consensus- Polymerase chain reaction)) amplification. |
(21, 25-34, 38, 39, 41, 42) |
Pseudomonas aeruginosa |
NB-BAL, Tracheal aspirates, Blood, pleural fluid, and sputum, sinus lavage |
Using Microbiological techniques like NB-BAL Culture, Tracheal aspirates culture, Blood Culture, Sputum culture, Gram staining, standard biochemical test, Kirby-Bauer disc diffusion technique, VITEK® 2 automated system, and pleural fluid cultures, and MALDI-TOF mass spectrometry |
(19, 21, 24-32, 34, 35, 37-44) |
Acinetobacter spp. |
NB-BAL, Tracheal aspirates, Blood, pleural fluid, Sputum |
Using Microbiological techniques like NB-BAL Culture, Tracheal aspirates culture, Blood Culture by the Bactec method, Sputum culture, Gram staining, standard biochemical test, Kirby-Bauer disc diffusion technique, VITEK® 2 automated system, and pleural fluid cultures, |
(21, 24-29, 32-35, 37-40, 42) |
Citrobacter spp. |
NB-BAL, Tracheal aspirates, Blood |
Using Microbiological techniques like NB-BAL Culture, Tracheal aspirates culture, Blood Culture by the Bactec method, abdominal radiography, |
(21, 25, 32, 34, 37-40) |
Staphylococcus spp. |
NB-BAL, Tracheal aspirates, Blood, Sputum, sinus lavage |
Using Microbiological techniques like NB-BAL Culture, Tracheal aspirates culture, Blood Culture by the Bactec method, Gram staining, standard biochemical test, Kirby-Bauer disc diffusion technique, VITEK 2 system, MALDI-TOF mass spectrometry |
(19, 21, 25-40, 42) |
Enterococci spp. |
Sputum, ETA (Endotracheal aspirates), Blood |
Sputum culture, endotracheal aspirates culture, Gram staining, standard biochemical test, Kirby-Bauer disc diffusion technique, VITEK® 2 automated system, Blood culture |
(26-28, 32, 33, 35-38) |
Enterobacter spp. |
Sputum, ETA, Blood, BAL, sinus lavage |
Sputum culture, endotracheal aspirates culture, Gram staining, standard biochemical test, Kirby-Bauer disc diffusion technique, VITEK 2 system, MALDI-TOF mass spectrometry, Blood culture |
(26, 27, 30, 31, 34, 35, 37, 38-40, 42, 43) |
Streptococcus spp. |
Sputum, ETA, BAL, sinus
Lavage |
Sputum culture, endotracheal aspirates culture, Gram staining, standard biochemical test, Kirby-Bauer disc diffusion technique, VITEK 2 system |
(19, 24, 26, 27, 31, 37-40) |
MRSA (Methicillin-resistant Staphylococcus aureus) |
Sputum, ETA, BAL |
Sputum culture, endotracheal aspirates culture, Gram staining, standard biochemical test |
(24, 26, 32, 40, 43) |
MSSA (Methicillin-susceptible Staphylococcus aureus) |
Sputum, ETA, BAL |
Sputum culture, endotracheal aspirates culture, Gram staining, standard biochemical test |
(24, 26, 32, 40, 43) |
Morganella morganii |
Respiratory samples |
Bacteria were cultured using standard microbiological methods, VITEK 2 system |
(27) |
Proteus spp. |
ETA, BAL |
Bacteria were cultured using standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system, Endotracheal aspirates culture |
(21, 24, 27, 32, 36-40) |
Pseudomonas fluorescens |
Respiratory samples |
Bacteria were cultured using standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system, Direct fluorescent antibody, Soluble antigen testing, PCR, |
(27, 44, 40) |
Serratia marcescens |
Respiratory samples, Blood sample |
Bacteria were cultured using standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system, Blood culture |
(27, 33, 35-40, 43) |
Listeria monocytogenes |
Respiratory samples |
Bacteria were cultured using standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system |
(27) |
Stenotrophomonas maltophilia |
Respiratory samples, sinus
lavage |
Bacteria were cultured using standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system |
(21, 27, 28, 31, 36-38, 40) |
Burkholderia pseudomallei |
Respiratory samples |
VAP was diagnosed based on both the clinical and microbiological criteria |
(21) |
Providencia spp. |
ETA |
Endotracheal aspirates culture |
(24) |
Miscellaneous pathogens |
Respiratory samples, sinus
lavage |
Microbiological culture, Kirby-Bauer disc diffusion technique, VITEK 2 system, sinus lavage culture |
(21, 27, 28, 31-34, 36, 39, 40, 43, 45) |
Table (2):
List of different microorganisms (atypical bacteria) causing ventilator associated pneumonia (VAP)
Pathogens |
Specimens collected |
Identification techniques |
References |
|---|---|---|---|
Haemophilus sp. |
ETA, BAL, Blood, Pleural Fluid |
Bacteria were cultured using standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system, Tracheal secretion culture, Bronchoscopy, Blood culture, and pleural fluid cultures |
(19, 24, 27, 36-41) |
Mycoplasma pneumoniae |
ETA, BAL, Blood, Pleural Fluid |
Tracheal secretion culture, Bronchoscopy, Blood culture, and pleural fluid cultures |
(36) |
Moraxella catarrhalis |
ETA, BAL, Blood, Pleural Fluid |
Bacteria were cultured using standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system, Tracheal secretion culture, Bronchoscopy, Blood culture, and pleural fluid cultures |
(27, 41) |
Burkholderia cepacian |
Respiratory samples, ETA |
The microorganisms were identified by standard microbiological methods using MALDI-TOF mass spectrometry, Endotracheal aspirates culture, standard biochemical test, VITEK® 2 automated system |
(24, 28, 30) |
Aeromonas salmonicida |
Respiratory samples |
Microbiological culture techniques, VITEK® 2 automated system, standard biochemical test |
(28) |
Cupriavidus pauculus |
Respiratory samples |
Microbiological culture techniques, VITEK® 2 automated system, standard biochemical test |
(28) |
Enterococcus faecalis |
Respiratory samples |
Microbiological culture techniques, VITEK® 2 automated system, standard biochemical test |
(28) |
Nocardia spp. |
BAL, Sputum, blood, serum, |
Gram stain and culture, Mycological culture, Soluble antigen testing, PCR, Ziehl Neelsen stain, Modified Ziehl Neelsen or Kinyoun acid fast stain |
(44) |
Mycobacterium tuberculosis |
BAL, Sputum, blood, serum, |
Gram stain and culture, Mycological culture, Soluble antigen testing, PCR, Ziehl Neelsen stain |
(44) |
Miliary tuberculosis |
BAL, Sputum, blood, serum, |
Gram stain and culture, Mycological culture, Soluble antigen testing, PCR, Ziehl Neelsen stain, Modified Ziehl Neelsen or Kinyoun acid fast stain |
(44) |
Legionella spp. |
BAL, Sputum, blood, serum, |
PCR, culture method, Gram stain, Mycological culture, soluble antigen testing |
(44, 45) |
non-tuberculous mycobacteria |
BAL, Sputum, blood, serum, |
Gram stain and culture, Mycological culture, soluble antigen testing, PCR, Ziehl Neelsen stain, Modified Ziehl Neelsen or Kinyoun acid fast stain |
(44) |
Bosea massiliensis |
Respiratory samples |
PCR, Culture method. |
(45) |
Mycoplasma hominis |
Respiratory samples |
PCR |
(36) |
Neisseria meningitidis, |
BAL |
Quantitative cultures performed |
(40) |
Haemophilus parahaemolyticus |
BAL |
Quantitative cultures performed |
(40) |
Table (3):
List of different microorganisms (fungi) causing ventilator associated pneumonia (VAP)
Pathogens |
Specimens collected |
Identification techniques |
References |
|---|---|---|---|
Pneumocystis jirovecii |
BAL, Sputum, blood, serum, |
Gram stain and culture, Mycological culture, soluble antigen testing, PCR, |
(44) |
Aspergillus spp. |
BAL, Sputum, blood, serum, |
Gram stain and culture, Mycological culture, soluble antigen testing, PCR, |
(44) |
Cryptococcus spp. |
BAL, Sputum, blood, serum, |
India Ink Preparation, Gram stain and culture, Mycological culture, soluble antigen testing, PCR, Cryptococcal antigen. |
(44) |
Candida spp. |
ETA, Blood sample, sinus lavage |
Endotracheal aspirates culture, Blood culture, standard microbiological methods, Kirby-Bauer disc diffusion technique, VITEK 2 system, sinus lavage culture on Sabouraud’s dextrose agar |
(21, 24, 27, 31-33, 36, 42) |
VAP usually develops by micro-aspiration of the microorganisms which colonize in the upper respiratory tract in ICU patients. The colonization by pathogens results in the replacement of normal mixed microbial flora by the virulent microorganisms in the upper respiratory tract, which may be monomicrobial or polymicrobial in nature. Studies have revealed that approximately 58% of the total infections are caused by Gram negative bacilli and 20% due to Staphylococcus aureus infection.21-23 The most common multi-drug resistant (MDR) pathogens included in the VAP infection are Pseudomonas spp, Acinetobacter spp. and some Enterobacteriaceae strains which are extended spectrum β-lactamase (ESBL), AmpC β-lactamase or metallo-β-lactamase (MBL) producers. The aetiological agents vary according to the patient population and the hospital settings. 24 Most of the MDR pathogens have been isolated from late onset VAP compared to early onset VAP and the reason could be a prolonged stay in the hospital and associated prior antibiotic therapy.21
Pathogenesis
The ICU admitted patients who are ventilated, have several reasons like critical illness, comorbidities, and malnutrition that affect their immune system.46,47 Endotracheal intubations supress the cough impulse, hamper muco-ciliary clearance, damage the tracheal epithelial surface and make a path for pathogenic bacteria which migrate from upper respiratory tract to lower respiratory tract.48-50
These pathogens then adhere to the mucosa of lower respiratory tract of mechanically ventilated patients and start infections. Generally, four routes are responsible for providing access to microorganisms in order to produce VAP infections a) Aspiration from oropharynx or from stomach into oropharynx then into the lower respiratory tract,51-53 b) Extension of contiguous infection,54 c) Contaminated air or ICU aerosol inhalation,55 and d) Vascular or urinary catheter-related blood-stream infection seeding the lungs.56-58
Few studies have revealed that use of contaminated respiratory therapy equipment in bronchoscopy and endoscopy can also be the reason for VAP infection.55,59 Critically ill patients in the ICU get exposed with these contaminated equipments and the ICU aerosol inhalation makes changes in their oral flora dramatically to aerobic gram-negative bacilli and Staphylococcus aureus microorganisms.60 These pathogenic bacteria adhere to the orotracheal mucosa of ventilated patient, which is eased by reduced IgA and increased protease production, exposed and denuded mucous membranes, elevated airway pH, and increased number of airway receptors for bacteria.61
Some studies showed that gastric pathogens can also be the source for VAP infections.51 The number of pathogens enhances when the pH level increases inside the gastric tract.62-65 It is revealed that the gastric pathogens or gastric contents can be aspirated to the lower respiratory tract and cause direct or indirect VAP infections.66-70
Pathogens can also make biofilms near the endotracheal tube and become highly resistant to antibiotics and host defense mechanisms. Many antibiotic-resistant nosocomial pathogens colonize in the biofilms.71,72 The presence of an endotracheal tube is one of the major culprits for VAP development: air flow moves pathogens toward the distal airways, while clearance of the trachea is blunted due to reduced tracheal ciliary movement and impaired cough.73
It is found that unsterilized contaminated hospital water and air can also infect critically ill patients.61 Nosocomial infections can spread through water which is contaminated with bacteria, mycobacteria, fungi, and parasites.74
Sampling methods for the diagnosis of VAP
Several sampling methods are available for the diagnosis of VAP, but the choice of sampling method may affect the accuracy and reliability of the results. In this context, we have discussed here the various sampling methods available for the diagnosis of VAP, their advantages and disadvantages, and the evidences supporting their use.
Endotracheal aspirate (ETA)
ETA is the most commonly used sampling method for the diagnosis of VAP. This method involves suctioning secretions from the endotracheal tube and is relatively easy to perform. ETA has high sensitivity and specificity, and it is less invasive than other sampling methods. However, ETA may not accurately reflect the lower respiratory tract microbiome, and contamination with oropharyngeal flora may lead to false-positive results.75 The use of quantitative cultures can improve the accuracy of ETA-based diagnosis of VAP. A threshold of 106 CFU/mL is often used to differentiate between contamination and true infection.76 A meta-analysis of 25 studies comparing the accuracy of ETA and BAL for the diagnosis of VAP showed that ETA had a sensitivity of 75.7% and a specificity of 67.9%, while BAL had a sensitivity of 71.1% and a specificity of 79.6%.77 ETA remains a useful and practical method for the diagnosis of VAP in resource-limited settings.
Bronchoalveolar Lavage (BAL)
BAL involves the instillation and aspiration of sterile saline into the lower respiratory tract. BAL provides a more accurate representation of the lower respiratory tract microbiome and is more specific for the diagnosis of VAP than ETA.78 However, BAL is more invasive and requires more expertise and resources to perform. A threshold of 104 CFU/mL is often used to diagnose VAP based on BAL samples.76 A meta-analysis of 25 studies comparing the accuracy of BAL and PSB for the diagnosis of VAP showed that BAL had a sensitivity of 71.1% and a specificity of 79.6%, while PSB had a sensitivity of 61.4% and a specificity of 76.5%.77 BAL is considered the gold standard for the diagnosis of VAP, but its use is limited by its invasiveness and the need for specialized equipment and expertise.
Protected Specimen Brush (PSB)
PSB involves the insertion of a sterile brush through the endotracheal tube into the lower respiratory tract. The brush is then rotated to collect a sample from the bronchial mucosa. PSB has similar sensitivity and specificity to BAL, and it is less invasive than BAL. However, PSB may cause bleeding or trauma to the respiratory tract. A threshold of 104 CFU/mL is often used to diagnose VAP based on PSB samples. A meta-analysis of 25 studies comparing the accuracy of BAL and PSB for the diagnosis of VAP showed that BAL had a higher sensitivity than PSB but nearly similar specificity.77
Mini-BAL
Mini-BAL is a modification of the BAL technique that uses a smaller volume of sterile saline to lavage the lower respiratory tract. Mini-BAL has similar sensitivity and specificity to BAL and is less invasive than BAL. However, it may be less accurate than BAL in patients with high lung compliance. A threshold of 104 CFU/mL is often used to diagnose VAP based on mini-BAL samples. A randomized controlled trial comparing the accuracy of mini-BAL and BAL for the diagnosis of VAP showed that mini-BAL had a lower sensitivity and similar specificity than BAL.79
Diagnostic methods for VAP
The diagnosis of VAP is typically made based on a combination of clinical and radiographic findings, as well as laboratory testing (Figure 1). It is crucial to diagnose the VAP as early as possible and adopt effective strategies to curb the infections and manage the condition (Figure 2). Some of the common diagnostic criteria used to diagnose VAP are discussed here.
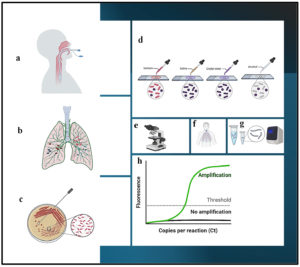
Figure 1. Diagrammatic representation of various diagnostic techniques for VAP infection: a) Intubated patient, b) Infected lung, c) Microbiological culture, d) Gram’s staining, e) Microscopic examination, f) X-ray diagnosis, g) Polymerase Chain Reaction, h) Real-Time PCR
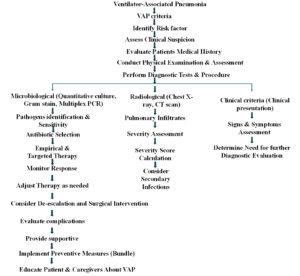
Figure 2. Illustrates VAP criteria, various diagnostic techniques, their associated risk factors, and patient management strategies
Clinical, radiological, and microbiological criteria
Clinical criteria: The patient should have a new or progressive infiltrate on chest X-ray, along with two or more of the following: fever (>38°C), leukocytosis (>10,000 cells/mm³), purulent sputum, or a decrease in oxygen saturation. Radiological criteria: The patient should have a new or progressive infiltrate on chest X-ray, with consolidation or cavitation, and/or a new or persistent infiltrate on computed tomography (CT) scan of the chest. Microbiological criteria: The patient should have a positive culture from a lower respiratory tract sample, such as bronchoalveolar lavage (BAL) or endotracheal aspirate (ETA), with a significant growth of pathogenic bacteria (>104 CFU/mL).76
It is important to note that the diagnosis of VAP can be challenging, as many of the clinical and radiographic findings can be non-specific and may be present in other respiratory conditions. Therefore, it is essential to rule out other potential causes of respiratory symptoms, such as acute lung injury, acute respiratory distress syndrome, and pulmonary embolism. In addition to the above criteria, physicians should consider the patient’s clinical history, risk factors, and response to treatment when making a diagnosis. Early diagnosis and prompt treatment with appropriate antibiotics are critical for improving outcomes in patients with VAP. Etiological diagnosis of VAP is very challenging and limited diagnostic tests are available so far (Figure 1). Broad differential diagnosis is performed for the confirmation of VAP infections in patients suffering from increasing oxygen requirements, leukocytosis, fever, and productive cough. Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) suggests that clinical pulmonary infection score (CPIS) can help determine the presence or absence of VAP and its management to some extent.80,81 The CPIS score of >6 (between 0-12) shows good amount of VAP infection.17,82
VAP diagnosis through the clinical indicators like fever, purulent secretions, leukocytosis, chest radiography, routine aerobic cultures from ETA, PSB (protected specimen brush) and BAL samples, and CPIS had poor diagnostic specificity. Presence of any of these indicators might misguide the antibiotic therapy to the patients. Clinicians need to have better tools along with these clinical findings which will help in proper antibiotic therapy.78 Limited studies explain the usefulness of CPIS score for VAP detection. Based on CPIS score irrational antibiotic prescription may occur.83-86
Microbial culture
The microbiological diagnosis of VAP is typically made through the collection and analysis of respiratory secretions from the lower respiratory tract. The goal of microbiological diagnosis is to identify the causative organism(s) responsible for the infection, so that appropriate antibiotic therapy can be initiated. There are several methods used for collecting respiratory secretions in VAP, including bronchoalveolar lavage (BAL), protected specimen brush (PSB), and endotracheal aspirate (ETA).76 Each method has its own advantages and disadvantages, and the choice of method may depend on factors such as the severity of illness, the patient’s clinical status, and the availability of resources. Once respiratory secretions have been collected, Gram staining is performed and further the samples are cultured for antibiotic sensitivity testing. Gram staining involves staining a sample of respiratory secretions with crystal violet and iodine, then decolorizing with alcohol and counterstaining with safranin. The resulting staining pattern can help identify the type of bacteria present in the sample. Gram staining is quick and easy to perform, but it has a low sensitivity and specificity for diagnosing VAP, and it cannot identify specific bacterial species. The results of microbiological testing can take several days to become available. In the meantime, broad-spectrum antibiotic therapy is usually initiated based on the patient’s clinical presentation and risk factors for infection. Once the results of microbiological testing are available, antibiotic therapy may be adjusted based on the specific pathogens identified and their susceptibility to antibiotics.87,88 Microbiological culture techniques are performed using different types of nutrient support such as nutrient agar, blood agar, chocolate agar, MacConkey agar, and sabouraud dextrose agar. Selective media is used for bacterial isolates and CHROM agar (Chromogenic culture media) is used for fungi (Candida species is commonly isolated fungi). Microbial culture is the gold standard for diagnosis of VAP, but it can take several days to obtain results, and it may be subject to contamination with other microorganisms.89
It is important to note that microbiological diagnosis of VAP can be challenging, as respiratory secretions may be contaminated with normal flora or other opportunistic pathogens. In addition, antibiotic therapy prior to sample collection may interfere with the ability to isolate and identify pathogens. Many organisms implicated in VAP are fastidious in nature and difficult to culture using routine culture methods. Viral agents require special cell lines, technical expertise and infrastructure for their detection. Hence, traditional culture methods are less sensitive and detect small range of etiological agents. Therefore, careful interpretation of microbiological results is important, and the diagnosis of VAP should be made based on a combination of clinical, radiographic, and microbiological findings.
Antigen testing
This technique involves detecting specific bacterial antigens in a sample of respiratory secretions using immunoassay methods. Antigen testing is quick and easy to perform, and it can provide results within hours. However, it has a lower sensitivity and specificity than culture or PCR, and it may be subject to cross-reactivity with non-pathogenic bacteria.
Multiplex PCR
Early Diagnosis of VAP with the help of multiplex PCR can resolve the time barrier for the treatment of VAP patients. Multiplex PCR can detect very low levels of pathogenic nucleic acid with high specificity and independent of viability of the target microbe. Moreover, supplementary information like presence of antibiotic resistance genes can also be gathered by this technique.90 Previous antibiotic treatments, poor infection control practices and irrational antibiotic use may cause pathogens to become antibiotic resistant. Multidrug resistance due to production of ESBL, AmpC or MBL by these bacteria add to the morbidity, making the infection even more difficult to treat.28,91 About 50% of VAP is polymicrobial; making it difficult to define the bacteria having particular role in VAP. Hence, appropriate and rapid diagnosis is important to adequately manage this condition.
Molecular techniques may overcome this problem. Over the past two decades PCR technology has become an important tool of molecular diagnosis of clinical pathogenic samples. Molecular methods are more sensitive and rapid so that the treatment can be initiated at an early. Apart from being expensive and highly sophisticated, one of the important limitations of molecular techniques is that they can detect both dead and as well as live organisms which demands clinical correlation of the test results. However, multiplex PCR technique has shown to be a valuable method for simultaneous identification of viruses, bacteria, fungi, and parasites and has the potential to save time and effort without compromising the accuracy.
According to Buchan et al., standard culture methods are mostly unable to detect respiratory infectious pathogens because of the polymicrobial nature and simultaneous detection of viral agents is also necessary for urgent identification of hospital-acquired and ventilator-associated pneumonia.92 To get rid of all these problems scientists have implemented semiquantitative BioFire FilmArray Pneumonia panel (PN panel) test (multiplex PCR). All bacterial targets reported as >105 CFU/mL in culture were reported as ≥105 genomic copies/ml by the PN panel.92 The BIOFIRE® FILMARRAY® Pneumonia plus Panel can be used for rapid and accurate automated testing of pneumonia by detection of LRTI (lower respiratory tract infections) causing bacteria, viruses along with seven genetic markers for antibiotic resistance. In this technique hands-on time is only 2 minutes and no pipetting is required. The turnaround time is 1 hr and it can detect 34 targets simultaneously. The sputum/BAL/ET samples could be analysed by PNplus Panel (BioFire Diagnostics, USA) system which is a multiplex PCR system used to determine 9 viruses (coronavirus, human metapneumovirus, enterovirus, influenza A and B virus, parainfluenza virus, respiratory syncytial virus, MERS virus, and adenovirus), 3 atypical pathogens (C. pneumoniae, L. pneumophila, and M. pneumoniae), 15 bacteria (Acinetobacter calcoaceticus-baumannii complex, Enterobacter cloacae, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Klebsiella aerogenes, Moraxella catarrhalis, Proteus spp., Serratia marcescens, Haemophilus influenzae, Pseudomonas aeruginosa, Staphylococcus aureus, S. pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae), and 7 resistance genes i.e., CTX-M, KPC (Klebsiella pneumoniae carbapenemase), OXA-48-like (oxacillinase), NDM (New Delhi metallo-β-lactamase), VIM (Verona Integron-encoded MBL), IMP (Imipenemase), mecA/C/MREJ (methicillin resistance geneA/ methicillin resistance geneC/mec right-extremity junction). The sensitivity and specificity reported for this method by using sputum samples are 96.3% and 97.2%, respectively.93
Study suggested that the Unyvero platform (Curetis AG, Holzgerlingen, Germany) is a multiplex PCR method used to diagnose severe infectious pathogens within 5 hrs. The Unyvero Hospitalized Pneumonia Multiplex PCR panel detects 21 bacteria (including Enterobacterales, P. aeruginosa, S. maltophilia, S. aureus etc.) with 21 antimicrobial resistant genes including ESBL (Extended-spectrum beta-lactamases), carbapenemases, mecA gene, mecC, blaOXA-23, blaOXA-24, blaOXA-48, blaOXA-58, blaVIM, blaIMP, blaKPC, blaNDM, and blaCTX-M. It is observed that multiplex PCR is suitable for the diagnosis of VAP/HAP (Hospital acquired pneumonia) by detecting the pathogens very fast and thus it can aid to early antimicrobial therapy.94 The Unyvero pneumonia cartridges correctly detected the VAP causing pathogens for 73% of the episodes and identified the resistance mechanism in 67% of them with P. aeruginosa having the highest discordance rate.95 Recently VAPs were diagnosed with various types of multiplex PCR platforms with different sensitivity and specificity. One of the studies performed to evaluate the performance of rapid screening test, GeneXpert MRSA/SA ETA (Methicillin-resistant Staphylococcus aureus/Staphylococcus aureus Endotracheal aspirates) assay test (in patients assuming at risk of VAP) compared with 3 cultures based and nuc gene-based qRT-PCR test (thermostable nuclease gene based quantitative Realtime PCR) for the detection of S. aureus in ETA samples of mechanically ventilated patients. GeneXpert MRSA/SA ETA assay rapidly and accurately diagnosed S. aureus directly from ETA samples. S. aureus is a common pathogen that causes VAP infections with increased mortality and morbidity rate. S. aureus (prior colonization with potential pathogens) plays a significant role for the development of nosocomial infections. The GeneXpert MRSA/SA ETA was a rapid and sensitive technology for the identification of S. aureus in ETA samples. GeneXpert assay machine (RMRSA/SA-ETA-10, Cepheid, USA) detects sequences within the staphylococcal protein A (spa) gene, the methicillin resistance gene (mecA), and staphylococcal cassette chromosome (SCCmec) gene inserted in attB insertion site (S. aureus chromosome). Extraction of DNA, amplification and the detection of the targets (by GeneXpert machine) was completed within 66 min. The nuc gene-based qRT-PCR was performed to target nuc gene, with SAnucF2 (TAAAGCGATTGATGGTGATACG), and SAnucR2 (TTCTTTGACCTTTGTCAAACTCG) primers (200 nM) and TaqMan probe (cy5-TGGTCCTGAAGCAAGTGCATTTACg-BBQ) to identify the S. aureus.96 The Xpert Carba-R detection is also performed with GeneXpert machine (Cepheid) to identify the resistance gene carbapenemase (from carbapenemase-producing organisms). The Xpert Carba-R (Carbapenem Resistance Molecular Test) detects 5 targets for carbapenemase-producing organisms (blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP-1).97 One of the previous studies was aimed to determine the OXA resistance gene and ISAba-1 gene by molecular typing (the repetitive extragenic palindromic sequence-based PCR in short called ‘rec-PCR’). In this study, the nosocomial MDR A. baumannii isolates were highly resistant to antibiotics and carry the blaOXA-23 resistance gene. The analysis of these isolates by rep‑PCR revealed that they were very closely related strains of the same origin, recently separated from each other.98 Study of Morris et al. has assessed the diagnostic accuracy of a novel application for 16S PCR. This study revealed that 16S PCR in BAL could be used as a rapid test in suspected VAP and may allow for judicious use of antibiotics.99
Hematological assay
Complete blood count also aids to the diagnosis of VAP and many studies suggest that VAP patients have decreased lymphocyte count (lymphocytopenia) which predicts bad outcome. When a patient acquires pneumonia from hospital, the marked changes observed in the patient are immunological changes, along with lymphocytopenia.100-103 Patients with sepsis also exhibit decreased lymphocyte count. This lymphopenia can be attributed to the recruitment of lymphocytes in the site of infection or their destruction at the site of inflammation or infection due to apoptosis.104,105 At the time of diagnosis, < 595 cells/mm3 of lymphocyte count is considered as a risk factor for mortality (approx. 90 days) in VAP patients.106
Flow cytometric analysis
Flow cytometric analysis in the pneumonia infected mouse revealed that CD4+ T-helper 17 (Th17) cells and interleukin (IL)-17A play an important role in clearing pathogens. Based on these observations, it has been hypothesized that number of Th17 cells and level of IL-17A implicate the risk of nosocomial pneumonia in humans. The Th17 cells could be a protective agent from development of nosocomial pneumonia in patients having mechanical ventilation.107 Study by Ma et al. showed that hyper-IgE syndromic patients are deficient with healthy Th17 cells.108 Similarly, Milner et al. observed depleted Th17 cell number in HIV patients.109 A study by Paats et al. revealed that in community acquired pneumonia (CAP) patients, BAL and peripheral blood have significantly increased IL-17A+IL-22+ cells compared to healthy controls.110 In the early stage of sepsis, patients may suffer from systemic inflammatory response syndrome (SIRS) with a “cytokine storm” also called as CARS (Compensatory anti-inflammatory response syndrome), which may lead to immune paralysis. CARS may lead to increased susceptibility to secondary infections by immune cell apoptosis, impaired lymphocyte and phagocyte function, and the phenotypic shift from Th1 to Th2.107,111-113
Mass spectrometry analysis
Mass spectrometry (MS) is an essential analytical technology for the modern health care laboratories because of its high sensitivity, selectivity and suitability for quantitative evaluations.114 Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) drastically shortens the time for identification of the pathogens. thereby initiating early antibiotic therapy. Management of VAP patients by early identification of causative pathogens is very crucial and mass spectrometry plays essential role in this aspect.115 MALDI-TOF MS identification can be failed if we proceed with complex, faintly multi-contaminated biological matrix. Moreover, this technique is not compatible with ETA samples for the identification of bacteria.115 ESI-MS (Electrospray Ionisation-Mass Spectrometry) has the potential to diagnose VAP samples within 6 hr and identify the species of bacteria that usually cause VAP, prior to clinical diagnosis. Periodical monitoring of pulmonary specimen through PCR/ESI-MS has clinical benefits for ventilated subjects by guiding appropriate and adequate initial level of antibiotic therapy to attain better outcomes and lower the use of broad-spectrum antibiotics.116
Gene and protein expression studies
Studies have shown that VAP is associated with up and down regulation of certain genes. It is found that overexpression of tumor necrosis factor-α (TNF-α) gene is associated with a 2.1 to 13-fold increase in the development of severe sepsis from various causes including pneumonia. PIK3R3 (Phosphoinositide-3-Kinase Regulatory Subunit 3) encodes for phosphoinositide 3-kinase regulatory subunit gamma (PI3Kγ) and it is predominantly expressed in immune cells and plays a role in chemoattractant-induced cell migration. Furthermore, genome wide screening approach and microarray analysis would be useful for identification of new genetic factors that are associated with the development of infection in VAP. To identify differentially expressed genes in patients who develop VAP compared to similar patients who do not develop VAP, a logistic regression model was developed with five genes like PIK3R3, ATP2A1 (ATPase Sarcoplasmic/Endoplasmic Reticulum Ca2+ Transporting 1), PI3, ADAM8 (ADAM Metallopeptidase Domain 8) and HCN4 (hyperpolarization activated cyclic nucleotide-gated potassium channel 4) and was able to accurately categorize 95% of patients that developed VAP.117 ADAM8 gene encodes a disintegrin and metalloproteinase domain-containing protein 8 which helps in cell adhesion. HCN4 encodes for the hyperpolarization activated cyclic nucleotide-gated potassium channel for the cardiac rhythm maintenance. The over-expression of HCN4 gene is significantly associated to the infectious complications in VAP patients. The gene ATP2A1 encodes for the sarcoplasmic reticulum calcium transporting ATPase (SERCA1) in the skeletal muscle. Lipopolysaccharides (LPS) can cause myocardial dysfunction in ICU patients and that could be the reason SERCA comes into the picture related to cardiac relaxation. In VAP positive patients ATP2A1 was found to be down regulated after LPS stimulation.117,118
Therapeutic strategies
Antibiotic therapy
The use of antibiotics is the mainstay of treatment for VAP, with the aim of eradicating the causative organisms and reducing morbidity and mortality. However, the selection of appropriate antibiotics and the duration of treatment are critical to optimize outcomes while minimizing the emergence of antibiotic resistance. Appropriate antibiotic therapy in VAP patients depends on duration of mechanical ventilation, late onset VAP (> 4 days) or early onset VAP (< 4 days) and the type of pathogens detected in culture and molecular techniques. Selection of best possible antibiotics for the treatment of VAP patients is also assisted by clinical information, such as patient risk factors/comorbidities and previous antibiotic exposure.119
Proper antibiotic treatment is very challenging for VAP patients, because of the traditionally followed techniques for the diagnosis which takes 24-48 hrs for the confirmation of pathogenic agents. Treatment of patients empirically or without the knowledge of pathogens, can help pathogens to become multidrug resistant. Knowing all this, the American Thoracic Society (ATS) currently recommends antibiotic therapy based on the patients’ risk of colonization by an organism with multidrug resistance. Inadequate initial antimicrobial treatment is directly associated with increased mortality and longer ICU stay. Study showed that the pathogens associated with initial inappropriate empiric antimicrobial therapy are Pseudomonas aeruginosa, Acinetobacter species, Klebsiella pneumonia, Enterobacter species, and MRSA. Delayed/inappropriate initial antibiotic therapy can lead to fatality in critically ill patients with infections, including VAP.1
Empirical treatment of VAP patients starts very soon, after the detection of pathogens through microscopic findings. However, the treatment should be readjusted according to culture findings as soon as the results are available. The criteria for the selection of antibiotic treatment to the patients is determined by the type of pathogens detected, patients’ history of exposure to antibiotics and comorbidities, and compatibility with particular antibiotics type. The type of pathogens treated according to their role in different type of patients, like MSSA (Methicillin sensitive Staphylococcus aureus) is frequently seen in coma patients. The bacteria MRSA (Methicillin resistant Staphylococcus aureus) is not seen in patients who haven’t exposed with antibiotics. Combination of antibiotic therapy should be prescribed in Pseudomonas aeruginosa infection. Antifungal therapy is given in fungal infections but not recommended in non-neutropenic patients, if intubated. Gram-positive bacteria should be treated with vancomycin in VAP infection.119 Combined treatment with meropenem and imipenem, ciprofloxacin and vancomycin seem to be appropriate and could cover all possible infective agents. To reduce mortality rate, reasonable prescription of antibiotics and corticosteroids could be effective.26 Knowledge on local distribution of pathogens and their resistance pattern can also determine the empirical treatment of VAP patients. According to Grief and Loza, the antibiotics that can be prescribed with early onset VAP without associated MDR risk factors, are ceftriaxone, fluoroquinolone, ampicillin-sulbactam, and ertapenem.120 They further advocated that for late onset/MDR patients, prescription should include individual or combination of antibiotics such as antipseudomonal cephalosporins (Cefepime, ceftazidime), antipseudomonal carbapenems (imipenem or meropenem), beta-lactam/beta-lactamase inhibitors (piperacillin-tazobactam) with an antipseudomonal fluoroquinolone (ciprofloxacin) or aminoglycoside plus linezolid or vancomycin (For MRSA), Telavancin is indicated for VAP for susceptible isolates of S. aureus (when other therapies are not suitable).120
Antibiotics treatment duration
The optimal duration of antibiotic treatment for VAP remains a topic of debate among clinicians. Traditionally, a 10–14 day course of antibiotics has been recommended for VAP, but recent evidence suggests that shorter courses may be equally effective and associated with fewer adverse effects and less chance of emergence of antibiotic resistance. The Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) issued guidelines in 2016 that recommend a duration of 7 days for most patients with VAP, with the possibility of shorter courses (i.e., 5-7 days) in selected patients based on clinical and microbiological response.5 The guidelines suggest that the decision to shorten the duration of treatment should be based on the following factors:
- The patient’s clinical stability, as evidenced by resolution of fever, improvement in oxygenation, and stable vital signs.
- The patient’s microbiological response, as evidenced by clearance of the causative organism(s) from respiratory specimens.
- The absence of comorbidities or immunosuppression that could increase the risk of treatment failure or relapse.
- The presence of biomarkers (such as procalcitonin) that indicate a low risk of ongoing infection.
Several studies have investigated the efficacy and safety of shorter courses of antibiotics for VAP. A randomized controlled trial by Chastre et al. compared a 15-day course of antibiotics to a 8-day course in patients with VAP caused by Gram-negative bacilli. The study found no significant difference in clinical cure rates or mortality between the two groups, but the 8-day group had a lower incidence of superinfections and fewer days of antibiotic exposure.121
Another randomized controlled trial by Uranga et al. compared a 5-day course of antibiotics to a 10-day course in patients with VAP caused by Gram-negative bacilli. The study found no significant difference in clinical cure rates or mortality between the two groups, but the 5-day group had a shorter duration of mechanical ventilation and a lower incidence of antibiotic-related adverse effects.122
However, it is important to note that shorter courses of antibiotics may not be appropriate for all patients with VAP, particularly those with comorbidities, immunosuppression, or polymicrobial infections. In addition, the decision to shorten the duration of treatment should be based on clinical judgment and individualized for each patient.
Antibiotics commonly used to treat VAP patients
Cefoperazone–Sulbactam
Cefoperazone-sulbactam is a combination of a third generation cephamycin and a traditional β-lactamase inhibitor. It exhibits effectiveness against Enterobacterales and Pseudomonas spp. The inclusion of sulbactam in the combination extends its activity to include Acinetobacter and anaerobic bacteria. Additionally, sulbactam enhances the stability of cefoperazone against certain β-lactamases and reduces the impact of the high inoculum effect. The recommended dosage for treatment is 2g administered every 12 hours for a duration of 7 days.123
Tedizolid
Tedizolid operates by attaching itself to a specific region called the 23S rRNA within the 50 S subunit of the bacterial ribosome. This binding action has the effect of preventing the formation of the 70 S initiation complex, which in turn disrupts the normal process of protein synthesis within the bacteria. As a result, the growth and proliferation of the bacteria are inhibited.124
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have both granted approval for the use of tedizolid in treating acute bacterial skin and skin structure infections (ABSSSIs). It can be administered either orally or intravenously, with a recommended daily dose of 200 mg, for a duration of six days.125
Tedizolid demonstrates activity against a wide range of gram-positive pathogens, including vancomycin-resistant Enterococcus species, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant S. aureus. When compared to another drug called linezolid, tedizolid has been found to be significantly more potent against various gram-positive pathogens, ranging from four to eight times more effective.126-130 It has shown promising results as a therapeutic agent to treat infections caused by MRSA or methicillin-susceptible S. aureus in individuals with cystic fibrosis.131 At a minimum inhibitory concentration (MIC) of less than 0.5 mg/L, tedizolid has the ability to inhibit the growth of 99.7% of bacterial isolates tested.132 Furthermore, it is effective against S. aureus and Streptococcus pneumoniae, two commonly encountered bacteria in clinical infections.133
Ceftaroline-Avibactam
Ceftaroline, a fifth-generation cephalosporin, exhibits strong efficacy against common respiratory pathogens such as MRSA, PRP, and non-ESBL producing Enterobacterial strains. However, it demonstrates limited effectiveness against anaerobes, ESBL and AmpC producing strains such as A. baumannii, and P. aeruginosa.134 When ceftaroline is combined with avibactam, it effectively counteracts the breakdown caused by class A (including ESBLs and KPC), class C (AmpC), and certain class D beta-lactamases, while simultaneously preserving its activity against gram-positive bacteria. This medication is utilized for the treatment of HAP/VAP.123
Plazomicin
This is a newly developed semisynthetic aminoglycoside that exhibits resistance to inactivation by aminoglycoside-modifying enzymes. It demonstrates effectiveness against a significant portion of carbapenem-resistant Enterobacterales (CRE),135 specifically over 95% of Enterobacterales strains with a susceptibility breakpoint below 2 mg/L.136 However, it is susceptible to the impact of 16S rRNA methyltransferase.137 No efficacy has been observed against anaerobes, Enterococcus, Streptococcus, and Stenotrophomonas.138 The drug has received FDA approval for intravenous administration at a dosage of 15mg/kg, for the treatment of complicated urinary tract infections (cUTI) caused by aerobic gram-negative bacteria, including AP (acute pyelonephritis).123
Aztreonam-Avibactam
Azetreonam is the sole beta-lactam compound that exhibits activity against metallo-beta-lactamases (MBL). However, it is vulnerable to hydrolysis by the majority of ESBLs or AmpC enzymes, which are frequently co-produced in carbapenem-resistant strains.139 When combined with avibactam, aztreonam gains stability against most multidrug-resistant (MDR) pathogens, including those carrying class A, C, and D beta-lactamases.140,141 Azetreonam-avibactam effectively inhibits 99.9% of Enterobacterales, even in the presence of NDM, KPC, or OXA-48 producers.142-144 Over 75% of tested isolates of P. aeruginosa exhibited in vitro susceptibility to aztreonam-avibactam, with a MIC value below 8 mg/L.142 Although the drug has not yet received approval from the FDA or EMA, clinical trials are underway for the treatment of severe gram-negative infections. However, until more robust data is available, aztreonam has been utilized in combination with ceftazidime-avibactam to treat serious infections caused by MBL-producing strains.123
Eravacycline
Eravacycline is a newly developed fluorocycline compound that shares a structural similarity with tigecycline. It is accessible in both oral and intravenous forms. Eravacycline demonstrates a broad spectrum of activity, encompassing both gram-positive and gram-negative bacteria, as well as anaerobic bacteria, except for P. aeruginosa. Notably, eravacycline exhibits effectiveness against A. baumannii isolates that are resistant to sulbactam. During treatment with eravacycline, there have been reports of a low incidence of C. difficile infection.123
Some examples of newly developed drug combinations that are used for treatment of VAP with promising results, are ceftazidime-avibactam, ceftolozane-tazobactam, meropenem-vaborbactam, imipenem-relebactam cilastatin, and cefiderocol.123
Phyto-therapeutic approach
Phyto-therapeutic treatment can be the best alternative treatment for antibiotics against VAP infection as they have no side effects, and no chance of development of antibiotic resistant microorganisms. Since the ancient times people are using plant products as medicines for eradication of lung infection, chronic bronchitis, sinusitis, and respiratory tract mycoses.145-147 The essential oil and phytochemicals derived from medicinal plants, have found to play significant role against many diseases. Previous studies showed that essential oil has anti-bacterial, anti-viral and antifungal properties.148,168 Antibiotics target only one type of pathogen, but phytochemicals target many pathogens and are either water/buffer soluble, show lower leftover impact, and there is no molecular catalysis and cross reactivity. Essential oils easily bind to the lipids of bacterial cell membrane and mitochondrial cell membrane.149 The stability of bacteria is affected when some important change occurs in its membrane structure through the interaction of essential oils.150 Some acids present in medicinal plants like anacardic, gallic acid and flavonoids play very important role for the eradication of pathogenic microorganisms. Various studies are going on for the development of phyto-therapeutics that can reduce the use of antibiotics.151 Tea oil and thyme oil can stop spreading of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA).148 Essential oils being volatile at room temperature used as aroma therapy that were effective against Haemophillus influenzae, Streptococcus pneumoniae and Staphylococcus aureus.147 It was seen that in liquid and solid media essential oil has antimicrobial activity and its diffusion capacity in agar shows inhibition of bacterial growth.152-155 Barley and wheat extracted thionin peptide contains 47 amino acid which is very toxic to yeast and Gram-positive bacteria.156,157 Febatin from faba beans, is effective against E. coli, P. aeruginosa and Enterococcus hirae. The ethanol soluble fractions of purple clove yield terpenoids, which show excellent activity against Bacillus subtilis, Staphylococcus aureus and Candida albicans.158,159 Oil from the plant Melaleuca alternifolia showed activity against E. coli, S. aureus and skin pathogens.160,161 Few essential oils and their major constituents were found effective in gaseous state against Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus pyogenes and Staphylococcus aureus.162 The plant Sida cordifolia contains many volatile components which have antimicrobial effect against many bacteria like S. aureus, Staphylococcus epidermidis, Candida guilliermondii and Trichosporon inkin.163 The phytotherapeutic agents derived from Sida cordifolia, are effective against many diseases like rheumatism, inflammation, asthma and nasal congestion.164 Broad spectrum pharmacological activities of Jupiner berry oil target against many bacteria and fungi.165,166 Currently antifungal medicines are also in use from some plants extracts like Inula viscosa, Artemisia velotorum, Lavandula augustifolia, and Ocimum gratissimum.167 Some essential oils inhibit microbial DNA/RNA which ultimately affect the synthesis of proteins and stop their growth.168 Essential oils extracted from various seed spices (Anethum graveolens, Carum capticum, Coriandrum sativum, Cuminum cyminum, Foeniculum vulgare, Pimpinella anisum and Seseli indicum) have shown antibacterial activity against many bacterial pathogens (Corynebacterium diphtheriae, Staphylococcus aureus, Streptococcus haemolyticus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Klebsiella species and Proteus vulgaris). The oils extracted from Carum capticum, Cuminum cyminum, Coriandrum sativum, Anethum graveolens, and Pimpinella anisum can control hospital acquired infections/VAP/urogenital tract infections/diarrhoea (the associated pathogens being P. aeruginosa, E. coli, Klebsella species and Proteus vulgaris).151
Alternative therapeutic approach
Other than antibiotics therapy, some other approaches have been evaluated to control bacterial infections, like cytokines which can modulate phagocyte functions to control the infections. Cytokines, namely, interferon-g, granulocyte colony stimulating factor, granulocyte-macrophage colony stimulating factor and macrophage colony stimulating factor, have been used as adjunctive agents for treatment of infections. Archeocins (myxopyronin) are also an alternative antibiotic agent that inhibit bacterial enzyme like RNA polymerase, which lead to bacterial death. Preventive strategies can also be used to check the spread of infection through disinfectant.148 Also, oral hygiene with Chlorhexidine (CHX), CHX body washing, selective oral decontamination (SOD) and/or digestive decontamination (SDD), multiple decontamination regimens, probiotics, subglottic secretions drainage (SSD), special cuff material and shape, silver-coated endotracheal tubes (ETTs), universal use of gloves and contact isolation, alcohol-based hand gel, vaporized hydrogen peroxide, and bundles of care have been addressed as effective preventive approaches.169
Bacteriophage therapy could be a good therapeutic strategy for the treatment of VAP patients because it was seen that bacteriophage treatment against E. coli, Pseudomonas spp., Acinetobacter spp., and S. aureus showed good efficacy.148
BAL is considered a suitable sample for studying the cellular and biological changes induced by drugs. This means that it can be used in proof-of-concept studies during the clinical development of new drugs, allowing researchers to evaluate the effects of drugs on the respiratory system.170
VAP associated with COVID-19
During the COVID-19 pandemic a lot of patients admitted in ICU, needed mechanical ventilation and nearly 1 out of 2 of these COVID-19 infected patients developed VAP with 42.7% mortality.171 ICU admitted COVID-positive patients were severely hypoxemic with microvascular and parenchymal lung damage.172 These patients were under high-risk zone to develop VAP, especially if compared with cohorts with ARDS (acute respiratory distress syndrome) patients and large mixed COVID+ve ICU admitted patients.173,174 COVID Patients with severe lung damage had increased infection rate.171
Viruses have ability to damage immune cells such as lymphocytes, B cells, natural killer cells and T cells which lead to immune cell dysfunction, which is one of the reasons for bacterial and fungal coinfections. SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) damages the lung epithelial cells and also slows down ciliary clearance, thereby inviting bacteria as well as fungi infection (Aspergillus and Mucor).175 This is the reason why, at the time of 2nd wave of COVID-19, there were multiple cases of mucormycosis reported in immunocompromised patients (uncontrolled diabetes mellitus and leukemia patients) or patients under treatment with corticosteroids and tocilizumab.176,177 COVID-19 infected patients showed cytokine storm due to increased level of IL-6. Tocilizumab (recombinant humanized monoclonal antibody) reduced the level of IL-6 in a patient’s body to protect lung damage and multiorgan failure.178,179 But at the same time blockage of IL-6 also had adverse consequences due to its central role in innate immunity and microbial clearance.180 Tocilizumab had a higher risk of serious secondary bacterial and fungal infections with subsequent higher mortality.181
One of the studies reported that approximately 50% of COVID-19 positive patients were infected with both bacteria as well as fungal agents which were highly resistant to multiple antibiotics.182 This study was performed during the 2nd wave of COVID-19 when an unexpected high rate of VAP in mechanically ventilated COVID-19 cases were seen in the ICUs.177 One of the studies conducted in Iran reported that every Covid 19 patient who were mechanically ventilated had also bacterial pneumonia infections, and all died in ICU except one.183 China and UK also reported that 13.9% and 6.1% patients had COVID as well as secondary bacterial infections respectively.184,185
Invasive and prolonged mechanical ventilation, use of immunomodulant therapies i.e., corticosteroids and anti-IL-6 drugs, overcrowded ICU during COVID-19 pandemic wave (inadequate staffing with more episode of cross-contamination) combinedly increased the risk for VAP development.186-187
Pathogens can adhere to the mucosa of the lower respiratory tract of VAP patients and start infections by aspiration from the oropharynx to the lower respiratory tract, contiguous infection extension, ICU aerosols inhalation and also by vascular or urinary catheter-related blood stream infection moving to the lung. Diagnosis of VAP is very challenging and there are limited diagnostic tests available. Clinical diagnosis has poor specificity and microbiological findings take 48-72 hrs which delays treatment of patients. Moreover, a lot of organisms implicated in VAP are fastidious in nature and are difficult to culture using routine aerobic culture methods. Molecular methods are more sensitive, rapid and specific in identification of the etiological agents, and help in initiating the early treatment. Multiplex PCR, flow cytometry, and mass spectrometry analysis are some of the sensitive techniques used, although culture method is the gold standard for microbial identification. VAP treatment has witnessed notable advancements with the emergence of novel therapeutic options that have obtained approval from regulatory bodies like the Food and Drug Administration (FDA) or the European Medicines Agency (EMA). The availability of diverse treatment options presents new opportunities for improved patient outcomes in the management of VAP. Development of antibiotic resistance is the biggest challenge the world is facing now. Phyto-therapeutic approach, bacteriophage therapy, cytokine treatment could be some of the alternative therapeutic strategies to overcome this issue, although it needs in-depth research.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
BT, AB, and MKJ Conceptualized the study. HKT designed the figures and wrote the original draft. BT, AB, PS, PKR, BPM, and MKJ wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Arthur LE, Kizor RS, Selim AG, van Driel ML, Seoane L. Antibiotics for ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;10(10):CD004267.
Crossref - Center for Disease Control and Prevention. Pneumonia (ventilator-associated [VAP] and Non- ventilator- associated pneumonia [PNEU]) event. National Healthcare safety network, 2023:1-19.
- Daneman N, Sarwar S, Fowler RA, Cuthbertson BH, SuDDICU Canadian Study Group. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta- analysis. Lancet Infect Dis. 2013;13(4):328-341.
Crossref - Ferrer M, Torres A. Epidemiology of ICU-acquired pneumonia. Curr Opin Crit Care. 2018;24(5):325-331.
Crossref - Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator- associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63(5):e61-e111.
Crossref - Fabregas N, Ewig S, Torres A, et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax. 1999;54(10):867-873.
Crossref - Mongodi S, Via G, Girard M, et al. Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest. 2016;149(4):969-980.
Crossref - Siempos II, Vardakas KZ, Kyriakopoulos CE, Ntaidou TK, Falagas ME. Predictors of mortality in adult patients with ventilator-associated pneumonia: a meta- analysis. Shock. 2010;33(6):590-601.
Crossref - Kett DH, Cano E, Quartin AA, et al. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11(3):181-189.
Crossref - Muscedere JG, Shorr AF, Jiang X, Day A, Heyland DK, Canadian Critical Care Trials Group. The adequacy of timely empiric antibiotic therapy for ventilator- associated pneumonia: an important determinant of outcome. J Crit Care. 2012;27(3):322.e7-322.14.
Crossref - Piskin N, Aydemir H, Oztoprak N, et al. Inadequate treatment of ventilator-associated and hospital- acquired pneumonia: risk factors and impact on outcomes. BMC Infect Dis. 2012;12:268.
Crossref - Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36(11):1999- 2006.
Crossref - Burillo A, de Egea V, Onori R, et al. Gradient diffusion antibiogram used directly on bronchial aspirates for a rapid diagnosis of ventilator-associated pneumonia. Antimicrob Resist Infect Control. 2019;8:176.
Crossref - Celis R, Torres A, Gatell JM, Almela M, Rodriguez- Roisin R, Agusti-Vidal A. Nosocomial pneumonia. A multivariate analysis of risk and prognosis. Chest. 1988;93(2):318-324.
Crossref - Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-acquired pneumonia study group. Intensive Care Med. 1996;22(5):387-394.
Crossref - Kuti EL, Patel AA, Coleman CI. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care. 2008;23(1):91- 100.
Crossref - Kalanuria AA, Ziai W, Mirski M. Ventilator-associated monia in the ICU. Crit Care. 2014;18(2):208.
Crossref - Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: a nine months’ prospective study. Ann Thorac Med. 2007;2(2):52-57.
Crossref - Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163(6):1371-1375.
Crossref - Edis EC, Hatipoglu ON, Tansel O, Sut N. Acinetobacter pneumonia: is the outcome different from the pneumonias caused by other agents. Ann Thorac Med. 2010;5(2):92-96.
Crossref - Charles MP, Easow JM, Joseph NM, Ravishankar M, Kumar S, Sivaraman U. Aetiological agents of ventilator-associated pneumonia and its resistance pattern – a threat for treatment. Australas Med J. 2013;6(9):430-434.
Crossref - Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903.
Crossref - Park DR. The microbiology of ventilator-associated pneumonia. Respir Care. 2005;50(6):742-763.
- Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: role of multi-drug resistant pathogens. J Infect Dev Ctries. 2010;4(4):218-225.
Crossref - Afjeh SA, Sabzehei MK, Karimi A, Shiva F, Shamshiri AR. Surveillance of ventilator-associated pneumonia in a neonatal intensive care unit: characteristics, risk factors, and outcome. Arch Iran Med. 2012;15(9):567-571.
- Japoni A, Vazin A, Davarpanah MA, et al. Ventilator- associated pneumonia in Iranian intensive care units. J Infect Dev Ctries. 2011;5(4):286-293.
Crossref - Aly NY, Al-Mousa HH, Al Asar el SM. Nosocomial infections in a medical-surgical intensive care unit. Med Princ Pract. 2008;17(5):373-377.
Crossref - Farag AM, Tawfick MM, Abozeed MY, Shaban EA, Abo-Shadi MA. Microbiological profile of ventilator- associated pneumonia among intensive care unit patients in tertiary Egyptian hospitals. J Infect Dev Ctries. 2020;14(2):153-161.
Crossref - Galhardo LF, Ruivo GF, Santos FO, et al. Impact of oral care and antisepsis on the prevalence of ventilator- associated pneumonia. Oral Health Prev Dent. 2020;18(1):331-336.
Crossref - Herkel T, Uvizl R, Doubravska L, et al. Epidemiology of hospital-acquired pneumonia: results of a Central European multicenter, prospective, observational study compared with data from the European region. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(3):448-455.
Crossref - Souweine B, Mom T, Traore O, et al. Ventilator-associated sinusitis: microbiological results of sinus aspirates in patients on antibiotics. Anesthesiology. 2000;93(5):1255-1260.
Crossref - Patro S, Sarangi G, Das P, et al. Bacteriological profile of ventilator-associated pneumonia in a tertiary care hospital. Indian J Pathol Microbiol. 2018;61(3):375- 379.
Crossref - Samudio GC, Monzon R, Ortiz LM, Godoy GM. Late onset neonatal sepsis in an intensive care neonatal unit: etiological agents and most frequent location. Rev Chilena Infectol. 2018;35(5):547-552.
Crossref - Siniscalchi A, Aurini L, Benini B, et al. Ventilator associated pneumonia following liver transplantation: etiology, risk factors and outcome. World J Transplant. 2016;6(2):389-395.
Crossref - Urzedo JE, Levenhagen MM, Pedroso RS, Abdallah VO, Sabino SS, Brito DV. Nosocomial infections in a neonatal intensive care unit during 16 years: 1997- 2012. Rev Soc Bras Med Trop. 2014;47(3):321-326.
Crossref - Kushnareva MV, Keshishyan ES, Balashova ED. The etiology of neonatal pneumonia, complicated by bronchopulmonary dysplasia. J Neonatal Perinatal Med. 2019;12(4):429-436.
Crossref - Walaszek M, Rozanska A, Walaszek MZ, Wojkowska- Mach J, Polish Society of Hospital Infections Team. Epidemiology of Ventilator-Associated Pneumonia, microbiological diagnostics and the length of antimicrobial treatment in the Polish Intensive Care Units in the years 2013-2015. BMC Infect Dis. 2018;18(1):308.
Crossref - Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS. Microbiology of ventilator- associated pneumonia compared with that of hospital- acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28(7):825-831.
Crossref - Woske HJ, Roding T, Schulz I, Lode H. Ventilator- associated pneumonia in a surgical intensive care unit: epidemiology, etiology and comparison of three bronchoscopic methods for microbiological specimen sampling. Crit Care. 2001;5(3):167-173.
Crossref - Zaccard CR, Schell RF, Spiegel CA. Efficacy of bilateral bronchoalveolar lavage for diagnosis of ventilator-associated pneumonia. J Clin Microbiol. 2009;47(9):2918-2924.
Crossref - Boots RJ, Lipman J, Bellomo R, Stephens D, Heller RE. The spectrum of practice in the diagnosis and management of pneumonia in patients requiring mechanical ventilation. Australian and New Zealand practice in intensive care (ANZPIC II). Anaesth Intensive Care. 2005;33(1):87-100.
Crossref - Santucci SG, Gobara S, Santos CR, Fontana C, Levin AS. Infections in a burn intensive care unit: experience of seven years. J Hosp Infect. 2003;53(1):6-13.
Crossref - Palacio F, Reyes LF, Levine DJ, et al. Understanding the concept of health care-associated pneumonia in lung transplant recipients. Chest. 2015;148(2):516-522.
Crossref - Gudiol C, Sabe N, Carratala J. Is hospital-acquired pneumonia different in transplant recipients? Clin Microbiol Infect. 2019;25(10):1186-1194.
Crossref - La Scola B, Boyadjiev I, Greub G, Khamis A, Martin C, Raoult D. Amoeba-resisting bacteria and ventilator-associated pneumonia. Emerg Infect Dis. 2003;9(7):815-821.
Crossref - Johanson WG, Pierce AK, Sanford JP. Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N Engl J Med. 1969;281(21):1137-1140.
Crossref - Sigalet DL, Mackenzie SL, Hameed SM. Enteral nutrition and mucosal immunity: implications for feeding strategies in surgery and trauma. Can J Surg. 2004;47(2):109-116.
- Klainer AS, Turndorf H, Wu WH, Maewal H, Allender P. Surface alterations due to endotracheal intubation. Am J Med. 1975;58(5):674-683.
Crossref - Levine SA, Niederman MS. The impact of tracheal intubation on host defenses and risks for nosocomial pneumonia. Clin Chest Med. 1991;12(3):523-543.
Crossref - Bone DK, Davis JL, Zuidema GD, Cameron JL. Aspiration pneumonia. Prevention of aspiration in patients with tracheostomies. Ann Thorac Surg. 1974;18(1):30-37.
Crossref - de Latorre FJ, Pont T, Ferrer A, Rossello J, Palomar M, Planas M. Pattern of tracheal colonization during mechanical ventilation. Am J Respir Crit Care Med. 1995;152(3):1028-1033.
Crossref - Ewig S, Torres A, El-Ebiary M, et al. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999;159(1):188-198.
Crossref - George DL, Falk PS, Wunderink RG, et al. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158(6):1839-1847.
Crossref - Estes RJ, Meduri GU. The pathogenesis of ventilator- associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med. 1995;21(4):365-383.
Crossref - Hamill RJ, Houston ED, Georghiou PR, et al. An outbreak of Burkholderia (formerly Pseudomonas) cepacia respiratory tract colonization and infection associated with nebulized albuterol therapy. Ann Intern Med. 1995;122(10):762-766.
Crossref - Alcon A, Fabregas N, Torres A. Hospital-acquired pneumonia: etiologic considerations. Infect Dis Clin North Am. 2003;17(4):679-695.
Crossref - Maki DG. Preventing infection in intravenous therapy. Anesth Analg. 1977;56(1):141-153.
Crossref - Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. II. Long-term devices. Clin Infect Dis. 2002;34(10):1362-1368.
Crossref - Edmondson EB, Reinarz JA, Pierce AK, Sanford JP. Nebulization equipment. A potential source of infection in gram-negative pneumonias. Am J Dis Child. 1966;111(4):357-360.
Crossref - Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20(6):740- 745.
Crossref - Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care. 2005;50(6):725-39.
- du Moulin GC, Paterson DG, Hedley-Whyte J, Lisbon A. Aspiration of gastric bacteria in antacid- treated patients: a frequent cause of postoperative colonisation of the airway. Lancet. 1982;1(8266):242- 245.
Crossref - Daschner F, Kappstein I, Engels I, et al. Stress ulcer prophylaxis and ventilation pneumonia: prevention by antibacterial cytoprotective agents? Infect Control Hosp Epidemiol. 1988;9(2):59-65.
Crossref - Giannella RA, Broitman SA, Zamcheck N. Influence of gastric acidity on bacterial and parasitic enteric infections. A perspective. Ann Intern Med. 1973;78(2):271-276.
Crossref - Donowitz LG, Page MC, Mileur BL, Guenthner SH. Alteration of normal gastric flora in critical care patients receiving antacid and cimetidine therapy. Infect Control. 1986;7(1):23-26.
Crossref - Heyland D, Mandell LA. Gastric colonization by gram-negative bacilli and nosocomial pneumonia in the intensive care unit patient. Evidence for causation. Chest. 1992;101(1):187-193.
Crossref - Torres A, El-Ebiary M, González J, et al. Gastric and pharyngeal flora in nosocomial pneumonia acquired during mechanical ventilation. Am Rev Respir Dis. 1993;148(2):352-357.
Crossref - Inglis TJ, Sherratt MJ, Sproat LJ, Gibson JS, Hawkey PM. Gastroduodenal dysfunction and bacterial colonisation of the ventilated lung. Lancet. 1993;341(8850):911-913.
Crossref - Torres A, Serra-Batlles J, Ros E, et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med. 1992;116(7):540-543.
Crossref - Ibanez J, Penafiel A, Marse P, Jorda R, Raurich JM, Mata F. Incidence of gastroesophageal reflux and aspiration in mechanically ventilated patients using small-bore nasogastric tubes. JPEN J Parenter Enter Nutr. 2000;24(2):103-106.
Crossref - Koerner RJ. Contribution of endotracheal tubes to the pathogenesis of ventilator-associated pneumonia. J Hosp Infect. 1997;35(2):83-89.
Crossref - Adair CG, Gorman SP, Feron BM, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25(10):1072- 1076.
Crossref - Coppadoro A, Bellani G, Foti G. Non-pharmacological interventions to prevent ventilator-associated pneumonia: A literature review. Respir Care. 2019;64(12):1586-1595.
Crossref - Anaissie EJ, Penzak SR, Dignani MC. The hospital water supply as a source of nosocomial infections: a plea for action. Arch Intern Med. 2002;162(13):1483-1492.
Crossref - Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888-906.
Crossref - Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637-657.
Crossref - Fernando SM, Tran A, Cheng W, et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients-a systematic review and meta-analysis. Intensive Care Med. 2020;46(6):1170-1179.
Crossref - Mayhall CG. Ventilator-associated pneumonia or not? Contemporary diagnosis. Emerg Infect Dis. 2001;7(2):200-204.
Crossref - Fujitani S, Yu VL. Diagnosis of ventilator-associated pneumonia: focus on nonbronchoscopic techniques (nonbronchoscopic bronchoalveolar lavage, including mini-BAL, blinded protected specimen brush, and blinded bronchial sampling) and endotracheal aspirates. J Intensive Care Med. 2006;21(1):17-21.
Crossref - Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.
Crossref - Modi AR, Kovacs CS. Hospital-acquired and ventilator- associated pneumonia: diagnosis, management, and prevention. Cleve Clin J Med. 2020;87(10):633-639.
Crossref - Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143(5 Pt 1):1121-1129.
Crossref - Papazian L, Thomas P, Garbe L, et al. Bronchoscopic or blind sampling techniques for the diagnosis of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1982-1991.
Crossref - Schurink CAM, Nieuwenhoven CAV, Jacobs JA, et al. Clinical pulmonary infection score for ventilator- associated pneumonia: accuracy and inter-observer variability. Intensive Care Med. 2004;30(2):217-224.
Crossref - Fartoukh M, Maitre B, Honore S, Cerf C, Zahar JR, Brun- Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168(2):173- 179.
Crossref - Luyt CE, Chastre J, Fagon JY. Value of the clinical pulmonary infection score for the identification and management of ventilator-associated pneumonia. Intensive Care Med. 2004;30(5):844-852.
Crossref - Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50(3):1700582.
Crossref - O’Horo JC, Thompson D, Safdar N. Is the Gram stain useful in the microbiologic diagnosis of VAP? A meta- analysis. Clin Infect Dis. 2012;55(4):551-561.
Crossref - Calderaro A, Buttrini M, Farina B, Montecchini S, De Conto F, Chezzi C. Respiratory tract infections and laboratory diagnostic methods: a review with A focus on syndromic panel-based assays. Microorganisms. 2022;10(9):1856.
Crossref - Murdoch DR, O‘Brien KL, Driscoll AJ, et al. Laboratory methods for determining pneumonia etiology in children. Clin Infect Dis. 2012;54(Suppl 2):S146-S152.
Crossref - Garland JS. Strategies to prevent ventilator-associated pneumonia in neonates. Clin Perinatol. 2010;37(3):629- 643.
Crossref - Buchan BW, Windham S, Balada-Llasat JM, et al. Practical comparison of the BioFire FilmArray pneumonia panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J Clin Microbiol. 2020;58(7):e00135-20.
Crossref - BioFire® FilmArray® pneumonia plus panel. 2021. https://www.biomerieux-diagnostics.com/biofire- filmarray-pneumonia-panel. Accessed July 07 2021.
- Peiffer-Smadja N, Bouadma L, Mathy V, et al. Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Crit Care. 2020;24(1):366.
Crossref - Luyt CE, Hékimian G, Bonnet I, et al. Usefulness of point-of-care multiplex PCR to rapidly identify pathogens responsible for ventilator-associated pneumonia and their resistance to antibiotics: an observational study. Crit Care. 2020;24(1):378.
Crossref - Coppens J, Van Heirstraeten L, Ruzin A, et al. Comparison of GeneXpert MRSA/SA ETA assay with semi-quantitative and quantitative cultures and nuc gene-based qPCR for detection of Staphylococcus aureus in endotracheal aspirate samples. Antimicrob Resist Infect Control. 2019;8:4.
Crossref - Burillo A, Marin M, Cercenado E, et al. Evaluation of the Xpert carba-R (Cepheid) assay using contrived bronchial specimens from patients with suspicion of ventilator- associated pneumonia for the detection of prevalent carbapenemases. PLOS ONE. 2016;11(12):e0168473.
Crossref - Ozyurt S, Kostakoglu U, Yildiz IE, et al. Investigation of the clonal associations in Acinetobacter baumannii strains isolated from the respiratory samples of patients in a tertiary research hospital. Niger J Clin Pract. 2020;23(8):1155-1162.
Crossref - Conway Morris A, Gadsby N, McKenna JP, et al. 16S pan-bacterial PCR can accurately identify patients with ventilator-associated pneumonia. Thorax. 2017;72(11):1046-1048.
Crossref - Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862- 874.
Crossref - Bermejo-Martin JF, Almansa R, Martin-Fernandez M, Menendez R, Torres A. Immunological profiling to assess disease severity and prognosis in community-acquired pneumonia. Lancet Respir Med. 2017;5(12):e35-e36.
Crossref - Lopez-Mestanza C, Andaluz-Ojeda D, Gomez-Lopez JR, Bermejo-Martín JF. Lymphopenic hospital acquired sepsis (L-HAS): an immunological phenotype conferring higher risk of mortality. Med Intensiva. 2019;43(8):510- 512.
Crossref - Warny M, Helby J, Nordestgaard BG, Birgens H, Bojesen SE. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLOS Med. 2018;15(11):e1002685.
Crossref - Chung KP, Chang HT, Lo SC, et al. Severe lymphopenia is associated with elevated plasma interleukin-15 levels and increased mortality during severe sepsis. Shock. 2015;43(6):569-575.
Crossref - Girardot T, Rimmelé T, Venet F, Monneret G. Apoptosis- induced lymphopenia in sepsis and other severe injuries. Apoptosis. 2017;22(2):295-305.
Crossref - Ceccato A, Panagiotarakou M, Ranzani OT, et al. Lymphocytopenia as a predictor of mortality in patients with ICU-acquired pneumonia. J Clin Med. 2019;8(6):843.
Crossref - Orlov M, Dmyterko V, Wurfel MM, Mikacenic C. Th17 cells are associated with protection from ventilator associated pneumonia. PLOS ONE. 2017;12(8):e0182966.
Crossref - Ma CS, Chew GY, Simpson N, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205(7):1551-1557.
Crossref - Milner JD, Sandler NG, Douek DC. Th17 cells, Job’s syndrome and HIV: opportunities for bacterial and fungal infections. Curr Opin HIV AIDS. 2010;5(2):179- 183.
Crossref - Paats MS, Bergen IM, Hanselaar WE, et al. T helper 17 cells are involved in the local and systemic inflammatory response in community-acquired pneumonia. Thorax. 2013;68(5):468-474.
Crossref - Arens C, Bajwa SA, Koch C, et al. Sepsis-induced long-term immune paralysis-results of a descriptive, explorative study. Crit Care. 2016;20:93.
Crossref - Muenzer JT, Davis CG, Chang K, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78(4):1582-1592.
Crossref - Merlino JI, Yowler CJ, Malangoni MA. Nosocomial infections adversely affect the outcomes of patients with serious intraabdominal infections. Surg Infect. 2004;5(1):21-27.
Crossref - Pu F, Chiang S, Zhang W, Ouyang Z. Direct sampling mass spectrometry for clinical analysis. Analyst. 2019;144(4):1034-1051.
Crossref - Bardet C, Barraud O, Clavel M, et al. Early and specific targeted mass spectrometry-based identification of bacteria in endotracheal aspirates of patients suspected with ventilator-associated pneumonia. Eur J Clin Microbiol Infect Dis. 2021;40(6):1291-1301.
Crossref - Hou D, Ju M, Wang Y, et al. PCR coupled to electrospray ionization mass spectrometry for microbiological diagnosis and surveillance of ventilator-associated pneumonia. Exp Ther Med. 2020;20(4):3587-3594.
Crossref - Swanson JM, Wood GC, Xu L, et al. Developing a gene expression model for predicting ventilator-associated pneumonia in trauma patients: a pilot study. PLOS ONE. 2012;7(8):e42065.
Crossref - Xu X, Yuan B, Liang Q, et al. Gene expression profile analysis of ventilator-associated pneumonia. Mol Med Rep. 2015;12(5):7455-7462.
Crossref - Sandiumenge A, Diaz E, Bodí M, Rello J. Therapy of ventilator-associated pneumonia. A patient-based approach based on the ten rules of “The Tarragona Strategy”. Intensive Care Med. 2003;29(6):876-883.
Crossref - Grief SN, Loza JK. Guidelines for the evaluation and treatment of pneumonia. Prim Care. 2018;45(3):485- 503.
Crossref - Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator- associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588-2598.
Crossref - Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: A multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-1265.
Crossref - Bassetti M, Mularoni A, Giacobbe DR, Castaldo N, Vena A. New antibiotics for hospital-acquired pneumonia and ventilator-associated pneumonia. Semin Respir Crit Care Med. 2022;43(2):280-294.
Crossref - Locke JB, Finn J, Hilgers M, et al. Structure-activity relationships of diverse oxazolidinones for linezolid- resistant Staphylococcus aureus strains possessing the cfr methyltransferase gene or ribosomal mutations. Antimicrob Agents Chemother. 2010;54(12):5337- 5343.
Crossref - SIVEXTRO tedizolid phosphate: prescribing information. Accessed October 30, 2021. https://www.accessdata. fda.gov/drugsatfda_docs/label/2019/205436s005lbl. pdf; 2019. Merck Sharp & Dohme Corp.
- Schaadt R, Sweeney D, Shinabarger D, Zurenko G. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent. Antimicrob Agents Chemother. 2009;53(8):3236-3239.
Crossref - Prokocimer P, Bien P, Deanda C, Pillar CM, Bartizal K. In vitro activity and microbiological efficacy of tedizolid (TR-700) against Gram-positive clinical isolates from a phase 2 study of oral tedizolid phosphate (TR-701) in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother. 2012;56(9):4608-4613.
Crossref - Barber KE, Smith JR, Raut A, Rybak MJ. Evaluation of tedizolid against Staphylococcus aureus and enterococci with reduced susceptibility to vancomycin, daptomycin or linezolid. J Antimicrob Chemother. 2016;71(1):152-155.
Crossref - Pfaller MA, Sader HS, Shortridge D, Castanheira M, Flamm RK, Mendes RE. Activity of tedizolid against gram-positive clinical isolates causing infections in Europe and surrounding areas (2014- 2015). J Chemother. 2019;31(4):188-194.
Crossref - Carvalhaes CG, Sader HS, Flamm RK, Streit JM, Mendes RE. Assessment of tedizolid in vitro Activity and Resistance Mechanisms against a Collection of Enterococcus spp. causing Invasive Infections, Including Isolates Requiring an Optimized Dosing Strategy for Daptomycin from U.S. and European Medical Centers, 2016 to 2018. Antimicrob Agents Chemother. 2020;64(4):e00175-20.
Crossref - Roch M, Varela MC, Taglialegna A, Rosato AE. Tedizolid is a promising antimicrobial option for the treatment of Staphylococcus aureus infections in cystic fibrosis patients. J Antimicrob Chemother. 2020;75(1):126-134.
Crossref - Bensaci M, Sahm D. Surveillance of tedizolid activity and resistance: in vitro susceptibility of Gram- positive pathogens collected over 5 years from the United States and Europe. Diagn Microbiol Infect Dis. 2017;87(2):133-138.
Crossref - Carvalhaes CG, Sader HS, Rhomberg PR, Mendes RE. Tedizolid activity against a multicentre worldwide collection of Staphylococcus aureus and Streptococcus pneumoniae recovered from patients with pneumonia (2017-2019). Int J Infect Dis. 2021;107:92-100.
Crossref - Russo A. Spotlight on new antibiotics for the treatment of pneumonia. Clin Med Insights Circ Respir Pulm Med. 2020;14.
Crossref - Lopez-Diaz MD, Culebras E, Rodriguez-Avial I, et al. Plazomicin activity against 346 extended- spectrum-β-lactamase/AmpC-producing Escherichia coli urinary isolates in relation to aminoglycoside- modifying enzymes. Antimicrob Agents Chemother. 2017;61(2):e02454-16.
Crossref - Castanheira M, Sader HS, Mendes RE, Jones RN. Activity of Plazomicin tested against Enterobacterales isolates collected from U.S. Hospitals in 2016-2017: effect of different breakpoint criteria on susceptibility rates among Aminoglycosides. Antimicrob Agents Chemother. 2020;64(5):e02418-19.
Crossref - Livermore DM, Mushtaq S, Warner M, et al. Activity of Aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother. 2011;66(1):48-53.
Crossref - Clark JA, Burgess DS. Plazomicin: a new aminoglycoside in the fight against antimicrobial resistance. Ther Adv Infect Dis. 2020;7.
Crossref - Brogden RN, Heel RC. Aztreonam. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1986;31(2):96-130.
Crossref - Shields RK, Doi Y. Aztreonam combination therapy: an answer to metallo-β-lactamase-producing Gram- negative bacteria? Clin Infect Dis. 2020;71(4):1099- 1101.
Crossref - Sader HS, Mendes RE, Pfaller MA, Shortridge D, Flamm RK, Castanheira M. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) clinical Enterobacteriaceae i so l ates. Antimicrob Agents Chemother. 2018;62(1):e01856-17.
Crossref - Sader HS, Duncan LR, Arends SJR, Carvalhaes CG, Castanheira M. Antimicrobial activity of aztreonam- avibactam and comparator agents when tested against a large collection of contemporary Stenotrophomonas maltophilia isolates from medical centers worldwide. Antimicrob Agents Chemother. 2020;64(11):e01433-20.
Crossref - Dupont H, Gaillot O, Goetgheluck AS, et al. Molecular characterization of carbapenem-nonsusceptible enterobacterial isolates collected during a prospective interregional survey in France and susceptibility to the novel ceftazidime-avibactam and aztreonam- avibactam combinations. Antimicrob Agents Chemother. 2016;60(1):215-221.
Crossref - Karlowsky JA, Kazmierczak KM, de Jonge BLM, Hackel MA, Sahm DF, Bradford PA. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother. 2017;61(9):e00472-17.
Crossref - Federspil P, Wulkow R, Zimmermann T. Effects of standardized Myrtol in therapy of acute sinusitis- results of a double-blind, randomized multicenter study compared with placebo. Laryngorhinootologie. 1997;76(1):23-27.
Crossref - Boyd EM, Sheppard P. Nutmegoiland camphene as inhaled expectorants. Arch Otolaryngol. 1970;92(4):372-378.
Crossref - Singh HB, Srivastava M, Singh AB, Srivastava AK. Cinnamon bark oil, a potent fungitoxicant against fungi causing respiratory tract mycoses. Allergy. 1995;50(12):995-999.
Crossref - Upadhyay RK. Emergence of drug resistance in microbes, its dissemination and target modification of antibiotics: a life threatening problem to human society. Int J Pharm Biol Res. 2011;2(5):119-126.
- Knobloch K, Weigand H, Weis N, Schwarm HM, Vigenschow H. Action of terpenoids on energy metabolism. In: Progress in Essential Oil Research. De Gruyter; 2019:429-446.
- Griffin SG, Wyllie SG, Markham JL, Leach DN. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flaour Fragr J. 1999;14(5):322-332.
Crossref - Singh G, Kapoor IPS, Pandey SK, Singh UK, Singh RK. Studies on essential oils: part 10; Antibacterial Activity of Volatile Oils of Some Spices. Phytother Res. 2002;16(7):680-682.
Crossref - Pandey DK, Tripathi NN, Tripathi RD, Dixit SN. Fungitoxic and phytotoxic properties of the essential oil of Hyptis suaveolens. Z Pflanzenkr Pflanzenschutz J Plant Dis Prot. 1982:344-349.
- Gocho S. Antibacterial action of aroma compounds in vapor state. Int J Antimicrob Agents. 1991;19:329-334.
- Inoue S. Inhibitory effect of volatile constituents of plants on the proliferation of bacteria. J Antibact Antifung Agents. 1983;11(11):609-615.
- Goi H. Antifungal activity of powdery black mustard, powdery wasabi (Japanese horseradish) and allyl isothiocyanate by gaseous contact. J Antibact Antifung Agents. 1985;13(5):199-204.
- Mendez E, Moreno A, Colilla F, et al. Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, γ-hordothionin, from barley endosperm. Eur J Biochem. 1990;194(2):533-539.
Crossref - Fernandez MA, Garcia MD, Saenz MT. Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J Ethnopharmacol. 1996;53(1):11-14.
Crossref - Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564-582.
Crossref - Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6(1):39.
Crossref - Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother. 2002;46(6):1914-20.
Crossref - Hammer KA, Carson CF, Riley TV. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil). Am J Infect Control. 1996;24(3):186-189.
Crossref - Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J Antimicrob Chemother. 2001;47(5):565-573.
Crossref - Nunes XP, Maia GLDA, Almeida JRGDS, Pereira FDO, Lima EDO. Antimicrobial activity of the essential oil of Sida cordifolia L. Rev Bras Farmacognosia. 2006;16:642-644.
Crossref - Cleeland R, Squires E. Evaluation of new antimicrobials “in vitro” and in experimental animal infections. In: Lorian V, ed. Antibiotics in Laboratory Medicine. 1991.
- Filipowicz N, Kaminski M, Kurlenda J, Asztemborska M, Ochocka JR. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytother Res. 2003;17(3):227-231.
Crossref - Pepeljnjak S, Kosalec I, Kalodera Z, Blazevic N.Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005;55(4):417-422.
- Cafarchia C, De Laurentis N, Milillo MA, Losacco V, Puccini V. Antifungal activity of essential oils from leaves and flowers of Inula viscosa (Asteraceae) by Apulian region. Parassitologia. 2002;44(3-4):153-156.
- Upadhyay RK. Essential oils: antimicrobial, antihelminthic, antiviral, anticancer and antiinsect properties. J Appl Biol Sci. 2010;36(1):1-22.
- Cotoia A, Spadaro S, Gambetti G, Koulenti D, Cinnella G. Pathogenesis-targeted preventive strategies for multidrug resistant ventilator-associated pneumonia: A narrative review. Microorganisms. 2020;8(6):821.
Crossref - Stanzel F. Bronchoalveolar lavage. Princ Pract Interv Pulmonol. 2012:165-176.
Crossref - Ippolito M, Misseri G, Catalisano G, et al. Ventilator-associated pneumonia in patients with COVID-19: A systematic review and meta-analysis. Antibiotics (Basel). 2021;10(5):545.
Crossref - Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir Med. 2020;8(12):1201-1208.
Crossref - Maes M, Higginson E, Pereira-Dias J, et al. Ventilator- associated pneumonia in critically ill patients with COVID-19. Crit Care. 2021;25(1):25.
Crossref - Bekaert M, Timsit JF, Vansteelandt S, et al. Attributable mortality of ventilator-associated pneumonia: A reappraisal using causal analysis. Am J Respir Crit Care Med. 2011;184(10):1133-1139.
Crossref - Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4+ T-cell count. Int J Infect Dis. 2020;96:148-150.
Crossref - Eucker J, Sezer O, Graf B, Possinger K. Mucormycoses. Mycoses. 2001;44(7-8):253-260.
Crossref - Meawed TE, Ahmed SM, Mowafy SMS, Samir GM, Anis RH. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J Infect Public Health. 2021;14(10):1375- 1380.
Crossref - Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13( 9):1972-1988.
Crossref - Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474-e484.
Crossref - Narazaki M, Kishimoto T. The two-faced cytokine IL-6 in host defense and diseases. Int J Mol Sci. 2018;19(11):3528.
Crossref - Pawar A, Desai RJ, Solomon DH, et al. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study. Ann Rheum Dis. 2019;78(4):456-464.
Crossref - Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057-1098.
Crossref - Sharifipour E, Shams S, Esmkhani M, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20(1):646.
Crossref - Fu Y, Yang Q, Xu M, et al. Secondary bacterial infections in critical ill patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(6):ofaa220.
Crossref - Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395-1399.
Crossref - Chang R, Elhusseiny KM, Yeh YC, Sun WZ. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis. PLOS ONE. 2021;16(2):e0246318.
Crossref - Giacobbe DR, Battaglini D, Enrile EM, et al. Incidence and prognosis of ventilator-associated pneumonia in critically ill patients with COVID-19: A multicenter study. J Clin Med. 2021;10(4):555.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.