ISSN: 0973-7510
E-ISSN: 2581-690X
Endophytic bacteria reside within the plant cell and are beneficial to it in a number of ways like growth, protection to environmental conditions and sustainability in favor of the hosts. Throughout the ages, Garlic (Allium sativum L.) has legendary therapeutic importance. The important step in endophyte isolation is the removal of plant tissue surface microflora, called as surface sterilization. Endophytes from medicinal plants may mimic the compound produced by the host plant and also plays an important role in production of bioactive compound, so it is necessary to isolate endophytes, not the epiphytic microbes. The present investigation was undertaken with an aim to optimize the sterilization parameters viz: time and concentration of ethanol and sodium hypochlorite for isolation of endophytic bacteria as well as exploring their antibacterial activity from Allium sativum. The results revealed that concentration and exposure time of sterilizing agent caused prominent surface sterilization but have negative effect on isolation of endophytes. Experimental results revealed that 70% ethanol (6 min), 2% sodium hypochlorite (1 min) followed by 70% ethanol (30 sec) is effective for surface sterilization of leaf and 70% ethanol (6 min), 3% sodium hypochlorite (1 min) followed by ethanol (30 sec) for bulb of Allium sativum. A total of 86 bacterial endophytes were recovered and screened for antibacterial activity against Enterococcus faecalis, Bacillus cereus ATCC 11778, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Enterobacter, Klebsiella pneumoniae, Salmonella typhi and Proteus. Out of 86 bacterial endophytes, only (9%) endophytes were found to be inhibiting one or all test human pathogen. The findings of the present study suggest that use of optimized sterilization conditions are effective for removing surface bacterial strains without affecting endophytes as well as the Allium sativum plant of the Jaunpur district represents an excellent reservoir of endophytic bacteria and novel source of bioactive compounds. On the other hand different concentration and time of their exposure to different parts of plant is required for proper surface sterilization of that part and isolation of endophytes.
Endophytic Bacteria, Allium sativum, Surface Sterilization, Antibacterial Activity
Endophytes are microorganisms that reside within the tissues of plants without causing damage to host cell.1,2 Recently, endophytic microorganisms gained attention as they are potential producer of variety of secondary metabolite with enormous applications in agriculture, medical and pharmaceutical industries.3-6 Endophytes are ubiquitous and present in all parts of the plant like root, leaf, seed, fruit and flower.7-10 Microorganism associated with medicinal plant act as reservoir of bioactive metabolites.11 Endophytes have been isolated from various plants.12,13 Three types of beneficial microbial groups (eg. Phyllospheric, endophytic and rhizospheric) are associated with the plants. Both the rhizospheric and endophytic microorganisms are associated with the plants. However, isolation and cultivation of endophytic bacteria is very difficult in comparison to rhizospheric bacteria. Probably because they are more closely associated and dependent on plant, an effective procedure should be adopted for proper surface sterilization.14 The time required for each sterilizing agent can be determined in a pilot study which usually varies with host plant tissue type, age, sensitivity and thickness in such a way to avoid damage of host tissue.15-18 Isolate obtained after surface sterilization from the plant samples are assumed to be endophytic, and it is crucial to be confident in surface sterilization process. Optimization of concentration and time of exposure for these sterilizing agents is tedious but essential part of isolation techniques, mainly because chemical agents applied for surface sterilization are generally toxic to plant tissues. Different processess used for surface sterilization mainly depend on three parameters (i) the type of sterilizing agent used, (ii) the concentration of sterilizing agent (iii) the exposure time of sterilizing agent. In endophyte isolation research commonly used surface sterilizing agent are sodium hypochlorite (2% – 10%), ethanol (70% – 90%), mercuric chloride (0.1%) and formaldehyde (40%). To increase the efficacy of surface sterilization combination of these sterilizing agent are used. Only a few reports are available on optimization of surface sterilization process for isolation of bacterial endophytes.19-22 Therefore, proper exposure time and concentration of sterilizing agent and the sequences of using them play pivotal role in imparting minimum explants damage to achieve better harness of endophytes diversity.
Allium sativum (L.) is well known from ancient time as important medicinal plant having antidiabetic, hypocholesterolemic, antilipidemic, fibrinolytic and anticancer activity.23 The medicinal properties are assigned due to the presence of Allicin (allyl 2-propenethiosulfinate or diallyl thiosulfinate), the principal bioactive compound present in the garlic. Other important compounds present in garlic homogenate are 1-propenyl allyl thiosulfonate, allyl methyl thiosulfonate, (E,Z)-4,5,9-trithiadodecal, 6,11-triene 9-oxide (ajoene), and y-L-glutamyl- S-alkyl- L-cysteine. The endophytes of A. sativum might be of interest to characterize. Therefore, an attempt was undertaken to isolate the bacterial endophytes from Allium sativum by using ethanol and sodium hypochlorite solution for proper surface sterilization. The effectiveness of both ethanol (70%) and sodium hypochlorite (2 and 3%) for surface sterilization has not yet been reported and perhaps it will be first comprehensive report to establish a standard protocol for optimization of surface sterilization for isolation of bacterial endophytes from Allium sativum (leaf and bulb) from district Jaunpur. The present study was undertaken with an aim to optimize effectiveness of surface sterilizing agent for isolation of endophytic bacteria from leaf and bulb of Allium sativum and their antibacterial activity.
Collection of samples
For isolation of bacterial endophytes, Allium sativum healthy plants were collected from different Allium sativum grown field of district Jaunpur (25.5906°N, 83.0062°E) Uttar Pradesh during November 2019-Feburary 2020. The plants were uprooted, placed in clean sterile polyethylene bags (Himedia) and brought to laboratory for experimental purpose. Healthy intact bulb and fresh leaves of Allium sativum were selected for isolation of endophytes. Plants that showed any symptoms of pathogenic infection and damage were not included in the study. During entire investigation all the material used viz: sterilizing agent and media were used of AR grade and procured from Merk and Himedia, respectively.
Isolation of Endophytic bacteria
Pretreatment
In the laboratory selected healthy plants were thoroughly washed under running tap water followed by several washes with sterile distilled water to remove adhering soil and majority of rhizospheric and phyllospheric bacteria. The plants were then cut in to two pieces i.e. leaf and bulb by using sterile knife in laminar air flow. Cut end of plant were sealed using liquid wax to avoid the entry of surface sterilizing agent inside through cut end.
Experimental design
Surface sterilization processes representing 16 sterilization conditions for leaf and 40 sterilization conditions for bulb of Allium sativum with variable concentration and times of ethanol and sodium hypochlorite solution. Concentration and time of sterilizing agent were varied as: 70% ethanol for 4 min, 6 min, 8 min, 10 min followed by, 2% and 3% sodium hypochlorite for 1 min, 2 min, 3 min, 4 min and 5 min respectively. For optimization of surface sterilization ethanol and sodium hypochlorite concentration was kept constant and times of sterilization were varied.
Surface sterilization
Surface sterilization is initial and critical step for isolation of endophytic bacteria. Sterilizing agent generally kills microorganism either by oxidizing or reducing properties of the compounds being used. Concentration of individual sterilizing agent for sterilization of explants has already been optimized using ethanol and sodium hypochlorite solution.24 After optimization of concentration of individual agent in present investigation combination of 70% ethanol with 2% and 3% of sodium hypochlorite solution were used to optimize the time duration for treatment with these sterilizing agent. After pretreatment outer most layers of the bulbs were peeled off and then treated with ethanol and sodium hypochlorite respectively and plants materials dried on sterile filter paper under sterile condition finally inoculated on nutrient agar plates.
Test for effectiveness of surface sterilization
To assess the effectiveness of surface sterilization procedure, explants after surface sterilization were washed three times in 50 ml of sterile distilled water followed by spreading of 0.1 ml of final wash water on nutrient agar plate (g/l; Peptone 5 gm, Beef extract 3 gm and NaCl 5 gm, agar 20 gm, pH 7.0 ± 0.1) as control and incubated at 30°C for 48-72 hrs and observed for possible microbial growth.25,26 In each step of surface sterilization sample were washed in sterilized distilled water. Samples were used for isolation of endophytes when there is no appearance of colonies on control plate of final wash. All the experiments were carried out in triplicate.
Test for effectiveness of sterilizing agent on bacterial endophytes
To assess the effectiveness of sterilizing agent on bacterial endophytes, 1 gm of sterilized plant tissue macerated by using sterile mortar and pestle in 5 ml of autoclaved distilled water to form a homogenous suspension. 0.1 ml of aliquot was spread on nutrient agar plate followed by incubation at 30°C for 48-72 hrs and observed for possible microbial growth.27-29 Validation of surface sterilization process also verified by imprinting surface sterilized plant tissue on nutrient agar plate.30,31
Media used for isolation of endophytic bacteria
The choice of growth media is imperative as it directly affect the number and the type of endophytic bacteria isolated from plant tissue. The isolation of enophytic bacteria was done by using three different media, Nutrient agar (NA), Tryptic soya agar (TSA) and water agar (WA). The surface sterilized plant tissues were cut into 0.5×0.5cm pieces aseptically by using sterile surgical blade and transferred on to petri plate by using sterile forceps containing NA, TSA and WA. The plates were sealed with parafilm incubated for 28±2°C for 3-4 days and regular observation were done after every 12 hrs. The colony were sub cultured on their respective medium and culture were stored at 4°C.
Determination of Antibacterial Activity
Agar plug diffusion method was used for primary screening of bacterial isolates having potential to produce antimicrobial compounds.32 Agar culture plate of the bacterial isolates was made on Nutrient agar medium by dense streaks on the agar surface. After suitable (24-48 hrs) incubation, an agar plug was cut aseptically with the help of sterilized cork borer (8mm). The plug was then placed on already prepared lawn of test bacteria (from log phase culture of 0.5 McFarland standard in nutrient broth) on Muller Hinton agar. The plates were incubated at 30°C for 24 hours and the antimicrobial activity were determined by measuring the diameter of zone of inhibition.
Test microorganism and their multiple antibiotic resistance (MAR) index
Antibacterial potential of bacterial endophytes were tested against three gram-positive (Enterococcus faecalis, Bacillus cereus ATCC 11778, Staphylococcus aureus) and six gram-negative (Pseudomonas aeruginosa, Escherichia coli, Enterobacter, Klebsiella pneumoniae, Salmonella typhi and Proteus) multi-drug resistant bacterial isolates. The test organism were bacterial isolates obtained from the Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University Varanasi, India. The culture were maintained in the department laboratory. The antibiotics susceptibility pattern of test organism were performed as per standard procedure and their MAR index was calculated by the ratio of number of antibiotics ineffective over the organism to the number of antibiotic exposed.33
Multiple antibiotic resistance (MAR) index= number of ineffective antibiotics/number of antibiotics exposed.
Molecular identification
Genomic DNA extraction
Bacterial genomic DNA was extracted by using Nucleopore DNA extraction kit. The quality of genomic DNA extracted from the bacterial isolates was determined on 0.8% Agarose gel. 16S rRNA gene was amplified by using pA (8-27f) and pH (1541-1522r) primer.34 After completion of PCR, aliquot of mixture was mixed with loading dye and loaded into the 1.2% agarose gel. The amplicon was separated by running the gel at 60V/cm for 1 hour and visualized under UV light. The 16S rRNA fragments were precisely cut under UV light and removed from gel. They were eluted and purified by using Nucleopore sure extract PCR clean-up kit and sequencing were done by using an automated capillary sequencer.
Identification using EzTaxon server
To determine the closest taxa, the isolates’ whole 16S rDNA sequences are compared to sequences found in the EzTaxon-Nezbiocloud database.35
Statistical Analysis
All the data pertaining to assess the effectiveness of sterilizing agent (70% ethanol, 2 and 3% Sodium hypochlorite solution) for surface sterilization and growth of endophytes from leaf and bulb of Allium sativum were subjected to linear regression analysis. Mean as well as standard deviation was calculated by using Microsoft Excel 2007 and the experiments were carried out in triplicates. All the data were exposed as mean ±SE. The experimental data of antibacterial activity were analyzed using one way analysis of variance (ANOVA) followed by Duncan’s multi-range test (DMRT). Values with P=0.05 were considered statistically significant. ANOVA was conducted using SPSS software, ver. 16
Surface sterilization is most important aspect of endophytes isolation, as the surface of plant is in contact with external environment all the time. In the present investigation for optimizing the time duration of surface sterilization process combination of 70% ethanol and sodium hypochlorite solution (2% and 3%) were used for different set time intervals. Sixteen combination of sterilization conditions for leaf and forty combination of sterilization conditions for bulb were tested but only two sterilization conditions for leaves and nine sterilization conditions for bulb were found to effective with less harms to the growth of endophytes (Table 1). The result obtained suggested that the combination of 70% ethanol with 2% sodium hypochlorite was found to be effective for surface sterilization of leaf and combination of 70% ethanol with 3% sodium hypochlorite was most efficient for surface sterilization of bulb of Allium sativum. In present investigation total 86 bacterial endophytes from Allium sativum in which seventy seven (ESS01-ESS077) bacterial endophyte were isolated from bulb and nine (ESS101-ESS109) were isolated from leaf of Allium sativum.
Table (1):
Number of endophytic bacteria isolated following surface sterilization and the efficiency of surface sterilization methods
| Plant parts | Method used | Number of isolatesa | Survived validityb test | Imprintc |
|---|---|---|---|---|
| Leaf | 70% ethanol(6 min)+2% sodium hypochlorite solution (1min) | 4 | + | + |
| 70%ethanol(10min)+2% sodium hypochlorite solution (1min) | 2 | + | + | |
| Bulb | 70%ethanol(6min)+2% sodium hypochlorite solution (4min) | 4 | + | + |
| 70%ethanol(8min)+2% sodium hypochlorite solution (4min) | 3 | + | + | |
| 70% ethanol(6 min)+3% sodium hypochlorite solution (1min) | 7 | + | + | |
| 70% ethanol(8 min)+3% sodium hypochlorite solution (1min) | 5 | + | + | |
| 70%ethanol(10min)+3% sodium hypochlorite solution (1min) | 2 | + | + | |
| 70%ethanol(10min)+3% sodium hypochlorite solution (2min) | 2 | + | – | |
| 70%ethanol(10min)+3% sodium hypochlorite solution (3min) | 1 | + | – | |
| 70% ethanol(4 min)+3% sodium hypochlorite solution (4min) | 1 | + | – | |
| 70% ethanol(6 min)+3% sodium hypochlorite solution (4min) | 1 | + | – |
aResult of validity test= +, survival i.e. endophytic bacteria survived after surface sterilization method; -, dead i.e. isolates was killed after surface sterilization.
bNumber of isolates= on the basis of colony morphology.
cImprint= + growth occur; – No growth (4 layer of Allium sativum)
Effect of 70% ethanol and 2% sodium hypochlorite solution on surface sterilization and growth of endophytes of leaf of Allium sativum
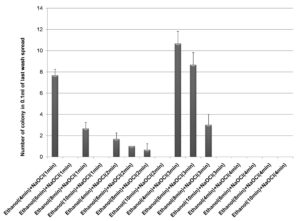
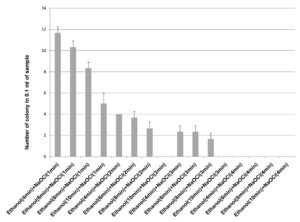
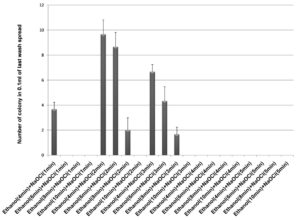
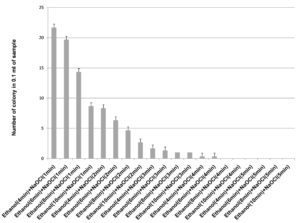
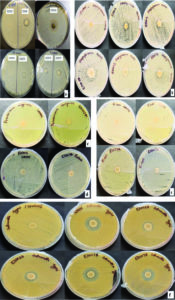
70% ethanol and 2% sodium hypochlorite solution were used for sterilization of leaf and the results obtained suggested that 70% ethanol (6 min) and 2% sodium hypochlorite solution (1 min) found to be effective for surface sterilization. There are so many concentrations such as 70% ethanol for 10 min and 2% sodium hypochlorite solution for 1, 2, 3 and 4 minutes were effective for surface sterilization of leaf (Figure 1) but when these concentrations were used for surface sterilization of leaf, reduced the appearance of endophytic bacteria drastically on isolation plate (Figure 2). Treatment of 2% sodium hypochlorite solution for 3 minutes was responsible for rapid increase in number of colonies in control plate. This concentration of sodium hypochlorite for 3 minutes treatment was probably responsible for damaging the outer layer of leaf of plant due to which some of the endophytes might leak in solution. Thus, resulting in sudden rise in number of colonies on control plate. When 2% sodium hypochlorite solution were used for 4 minutes treatment than there is no colony observed in control plate and treated plate which indicates that this treatment of 2% Sodium hypochlorite completely inhibitory for growth of endophytes (Figure 2).
Figure 1 . Effect of 70% ethanol and 2% sodium hypochlorite on surface sterilization of Leaf of Allium sativum
Figure 2. Effect of 70% ethanol and 2% sodium hypochlorite on growth of endophytes after surface sterilization of leaf of Allium sativum
Effect of 70% ethanol and 2% sodium hypochlorite solution on surface sterilization and growth of endophytes of bulb of Allium sativum
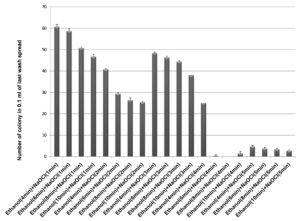
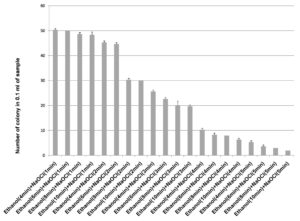
70% ethanol and sodium hypochlorite solution (2% and 3%) were used for surface sterilization of bulb and the result obtained suggested that 70% ethanol with 2% sodium hypochlorite solution were not found to be effective for removing surface bacteria (Figure 3), in comparison to 3% sodium hypochlorite. When 70% ethanol were used with 2% sodium hypochlorite solution for surface sterilization of bulb, 70% ethanol for 6 and 8 minutes with 2% sodium hypochlorite solution for 4 minutes found to be highly effective for surface sterilization. Although when these concentrations were used for surface sterilization appearance of endophytic bacteria and variability also decreased (Figure 4 & Table 1). When 2% sodium hypochlorite solution were used for 5 minutes treatment then there were increase in number of colony in control plate as compared to 4 minutes treatment because 5 minutes treatment of Sodium hypochlorite solution caused damaged of outer three to four layer of Allium sativum that might be resulting to leakage of bacteria in solution and thus visible on control plate. Damage caused by 2% sodium hypochlorite solution were assessed by imprinting the layer of Allium sativum on nutrient agar plate and were incubated at 30°C for 48-72 hrs. Therefore, 2% sodium hypochlorite solution was not used for surface sterilization of bulb of Allium sativum.
Figure 3. Effect of ethanol (70%) and sodium hypochlorite solution (2%) on surface sterilization of bulb of Allium sativum
Figure 4. Effect of ethanol (70%) and sodium hypochlorite solution (2%) on growth of endophytes after surface sterilization of bulb of Allium sativum
Effect of 70% ethanol and 3% sodium hypochlorite solution on surface sterilization and growth of endophytes of bulb of Allium sativum
Ethanol (70%) with sodium hypochlorite solution (2%) was not found to be effective for surface sterilization, so sodium hypochlorite 3% solutions with 70% ethanol were used for surface sterilization of bulb (Table 1). The results obtained suggested that the 3% sodium hypochlorite solution were very effective for 4 and 5 minutes treatment (Figure 5) but these time duration were inhibitory for growth of endophytic bacteria (Figure 6). Therefore, 3% sodium hypochlorite solution for 4 and 5 minutes treatment were not used for surface sterilization of bulb. Combinations of 70% ethanol (6 minutes) with 3% sodium hypochlorite solution (1 minute) were found to be very effective for removing surface bacteria without harming growth of endophytic bacteria when these concentration used for surface sterilization then there were more variability of endophytic bacteria (on the basis of colony morphology) in comparison to 70% ethanol (8 minutes) with 3% sodium hypochlorite solution for 1 minute treatment (Table 1 and Figure 7).
Figure 5. Effect of ethanol (70%) and sodium hypochlorite solution (3%) on surface sterilization of bulb of Allium sativum
Figure 6. Effect of ethanol (70%) and sodium hypochlorite solution (3%) on growth of endophytes after surface sterilization of bulb of Allium sativum
Figure 7. Stereomicroscopic image of bacterial endophyte isolated from bulb of Allium sativum showing variation in colony morphology isolated by using 70% ethanol (6min), 3% Sodium hypochlorite solution (1min) followed by 70% ethanol for 30 Sec [Seven different type of colony morphology was observed ranging from white, creamy yellow, mustard yellow (color), irregular, circular, oval (colony margin)]
Result of statistical analysis
Linear Regression establishes a relationship between dependent variable (number of colony in control plate and growth of endophytes 0.1 ml of sample) and one or more independent variables (time of sterilizing agent such as ethanol for 4 min, 6 min, 8 min & 10 min for fixed treatment time for sodium hypochlorite solution 1 min, 2 min, 3 min & 4 min) using a best fit straight line.
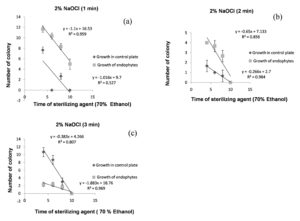
The R2 (coefficient of determination) indicate variation in the dependent variable (number of colony on control plate and growth of endophytes in 0.1 ml of sample), relative to the time of sterilization of ethanol and sodium hypochlorite solution, this is explained graphically with a scatter plot. For surface sterilization of leaf 70% ethanol (6 minutes) and 2% sodium hypochlorite solution for 1 minutes were effective (any colony was not observed on control plate), higher R2 = 0.95 values for growth of endophytes at 95% confidence level indicate that 1 minute treatment of sodium hypochlorite solution is effective for surface sterilization without inhibiting growth of endophytes (Figure 8a). In control plate sodium hypochlorite solution for 2 and 3 minutes treatment was effective and statistically significant (R2 = 0.98 and 0.97 at 99% confidence level) (Figure 8b and Figure 8c). Treatment for 2 and 3 minutes time period were not found to be effective for surface sterilization because this reduced the appearance of endophytes colony on the plate.
Figure 8. Linear regression showing relationship between number of colony in 0.1 ml of sample ( control plate and growth of endophytes) and sterilization time of 70% ethanol for fixed treatment time of 2% NaOCl (1 minutes (a), 2 minutes (b) & 3 minutes (c)) for surface sterilization of leaf of Allium sativum
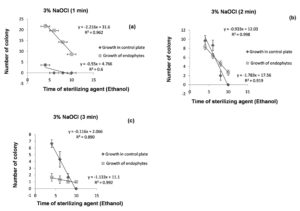
70% ethanol with 2% of sodium hypochlorite solution was not found to be effective for surface sterilization then 3% of sodium hypochlorite solution was used for surface sterilization of bulb (Table 1). The results obtained suggested that for surface sterilization of bulb 70% ethanol (6 minutes) and 3% sodium hypochlorite solution for 1 minute were effective. Statistical analysis suggest that R2 = 0.96 values for growth of endophytes at 95% confidence level indicate that 1minute treatment of sodium hypochlorite solution is effective for surface sterilization without inhibiting growth of endophytes (Figure 9a). In control plate sodium hypochlorite solution were for 2 and 3 minutes treatment were statistically significant (R2 = 0.91 and 0.99 at 95% and 99% confidence level respectively) (Figure 9b and Figure 9c). Treatment for 2 and 3 minutes treatment were not found to be effective because this reduced the appearance of endophytes colony on plate.
Figure 9. Linear regression showing relationship between number of colony in 0.1 ml of sample (control plate and growth of endophytes) and sterilization time of 70% ethanol for fixed treatment time of 3% NaOCl (1 minutes (a), 2 minutes (b) & 3 minutes (c)) for surface sterilization of bulb of Allium sativum
Effect of culture media on growth of enophytic bacteria
Diversity of bacterial endophytes depends upon the types of different media such as NA, TSA and WA used after surface sterilization process. Three different media were used to isolate endophytic bacteria from bulb and leaf of Allium sativum. The maximum number of endophytes were isolated from bulb and least number of endophytes from leaf of Allium sativum. More growth of endophytic bacteria were obtained on Nutrient Agar whereas Tryptic soya agar showed moderate growth with very less contamination and water agar showed negligible growth of endophytic bacteria (Figure 10).
Figure 10. Recovery percentage of endophytic bacteria on different growth media from bulb and leaf of Allium sativum
MAR index value of test microorganism
Antibiotic resistance is major challenge due to exponential uses of antibiotics, mutation and environmental stress. Antibiotic resistance cause major problem in treatment of infectious disease. Multiple antibiotic resistance of pathogenic microorganism are indicated in Table 2. The MAR value is ratio of the number of effective antibiotic to that of effective antibiotics tested against the different number of microorganisms.
Table (2):
MAR index value of antibiotic resistance microorganism used in present investigation
Clinical isolates |
MAR value |
No. of ineffective antibiotics |
Name of ineffective antibiotics |
|---|---|---|---|
Enterococcus faecalis |
0.5 |
7 |
OX,CD,AMC,E,VA,TEI,CAZ |
Bacillus cereus ATCC 11778 |
0.14 |
2 |
OX, CAZ |
Staphylococcus aureus |
0.07 |
1 |
OX |
Klebsiella pneumoniae |
0.2 |
3 |
AMP, CXM, AMC |
Salmonella typhi |
0.26 |
4 |
AMP, NA, CXM, AMC |
Proteus |
0.26 |
4 |
AMP, CAZ, CXM, AMC |
E.coli |
0.2 |
3 |
AMP,AMC,CXM |
Pseudomonas aeruginosa |
0.5 |
5 |
MZ, AK, CB, CPZ, PI |
Enterobacter |
0.16 |
2 |
AMP,AMC |
Note: multiple antibiotic resistance (MAR) value near to 1 indicates the antibiotics are ineffective; Antibiotics abbreviations: Oxacillin (OX-1µg), Cephalothin (CEP-30µg), Clindamycin (CD-2µg), Amoxyclav (AMC-30µg), Erythromycin (E-15 µg), Penicillin G (P- 10µg), Co-Trimoxazole (COT-25μg), Ampicillin/Sulbactam (A/S-10/10μg), Vancomycin (VA-30 μg), Gentamicin (GEN-10μg), Cefoxitin (CX-30μg), Ofloxacin (OF-5μg), Teicoplanin (TEI-30μg), Ceftazidime (CAZ-30μg), Tobramycin (TOB-10μg), Cefotaxime (CTX-30μg), Ampicillin (AMP-10μg), Nitrofurantoin (NIT-300μg), Amikacin (AK-30μg), Ciprofloxacin (CIP-5μg), Nalidixic acid (NA-30μg), Netillin (NET-30μg), Tetracycline (TE-30 μg), Cefuroxime (CXM-30 μg), Mezlocillin (MZ-75 μg), Carbenicillin (CB-100 μg), Levofloxacin (LE-5 μg), Colistin (CL-10 μg), Cefoperazone (CPZ-75 μg), Piperacillin (PI-100 μg)
Screening of antibacterial activity by agar plug diffusion assay
The pure culture of 86 bacterial endophytes were screened for antibacterial activity against multidrug resistance human pathogen by plug diffusion assay. In present investigation out of 86 bacterial endophytes, none of the bacteria from leaf showed antibacterial activity whereas only 8 bacteria (9%) from bulb of Allium sativum exhibited inhibitory activity against six tested drug resistance tested pathogen (Bacillus cereus ATCC 11778, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Salmonella typhi and Proteus). Among all the tested antibiotic resistance pathogen E. coli, B. cereus ATCC 11778, S. aureus, S. typhi, were most susceptible followed by P. aeruginosa, and Proteus were less susceptible to bacterial endophytes isolated from bulb of Allium sativum. Among all the isolates no bacteria exhibited antibacterial activity against Enterobacter,
E. faecalis and Klebsiella pneumoniae (Table 3). ESS06 and ESS075 where exhibited similar antibacterial activity against four human pathogen (Bacillus cereus ATCC 11778, Staphylococcus aureus, Escherichia coli, Salmonella typhi). ESS072 does not exhibited antibacterial activity any tested human pathogen (Figure 11 and Table 3). ESS074 exhibited exhibit antibacterial activity against five pathogen except Staphylococcus aureus whereas ESS024 showed antibacterial activity against only three tested pathogen (B. cereus, S. aureus, and S. typhi).
Figure 11. Antibacterial activity of bacterial endophytes on Muller Hinton Agar media (MHA) against (a) B. cereus ATCC 11778, (b) S. aureus, (c) P. aeruginosa, (d) Proteus, (e) E. coli and (f) S. typhi
Table (3):
Antibacterial activity of bacterial endophytes against drug resistance human pathogen
| Endophytic bacteria from A. sativum | Zone of inhibition in mm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | ||||||||
| E. faecalis | B. cereus ATCC 11778 | S. aureus | P. aeruginosa | E. coli | Enterobacter | K. pneumoniae | S. typhi | Proteus | |
| Bacillus sp. ESS03 | – | – | 7.66±0.57c | – | – | – | – | – | – |
| B. siamensis ESS06 | – | 2.00±1.00a | 17.66±1.52a | – | 5.66±1.52a | – | – | 17.66±0.57a | – |
| B.paralicheniformis ESS024 | – | 1.66±0.57a | 11.33±0.57b | – | – | – | – | 10.33±0.57c | – |
| B. stercoris ESS025 | – | 1.33±0.57a | – | – | – | – | – | 11.66±0.57bc | – |
| K.quasivariicola ESS072 | – | – | – | – | – | – | – | – | – |
| B. safensis ESS073 | – | 2.00±1.00a | 6.33±1.15c | – | – | – | – | – | – |
| Bacillus velezensis ESS074 | – | 1.66±0.57a | – | 1.00±0.57 | 1.33±0.57b | – | – | 7.66±0.57d | 4.66±0.57 |
| Bacillus velezensis ESS075 | – | 1.66±0.57a | 12.66±0.57b | – | 2.33±0.57b | – | – | 12.66±1.52b | – |
Identification of bacteria
The bacterial endophytes have antibacterial activity were identified 16S rRNA sequencing. The molecular characterization reveled that all the identified bacterial endophytes belong to genus Bacillus sp. except ESS072 which belong to Klebsiella sp. Eztaxon BLAST analysis details bacterial endophytes with their accession number obtained from NCBI Genbank are showed in Table 4.
Table (4):
Molecular identification of Bacterial endophytes from Allium sativum
S.No |
Bacterial endophytes |
Accession number received from NCBI Genbank |
Microorganism found with identity (ExTaxon) |
Percentage of similarity |
|---|---|---|---|---|
1 |
ESS03 |
OR668589 |
Bacillus sp. |
93.82% |
2 |
ESS06 |
OR603129 |
Bacillus siamensis KCTC 13613 |
99.86% |
3 |
ESS024 |
OR603135 |
Bacillus paralicheniformis KJ-16 |
99.66% |
4 |
ESS025 |
OR603142 |
Bacillus stercoris JCM 30051 |
100.00% |
5 |
ESS072 |
OR603146 |
Klebsiella quasivariicola KPN1705 |
100.00% |
6 |
ESS073 |
OR642760 |
Bacillus safensis subsp.safensis FO-36b |
99.65% |
7 |
ESS074 |
OR668591 |
Bacillus velezensis CR-502 |
99.24% |
8 |
ESS075 |
OR668590 |
Bacillus velezensis CR-502 |
99.20% |
Large number of microbes resides in environment and everyone is aware of the fact that they are cosmopolitan in habit. These microbes are successful in nature because of huge metabolic potential as well as capability to make interactions. Among the various criteria used for classification, one is on the basis of site of interaction, whether outside (rhizospheric) and inside (endophyte). In order observe the diversity of endophytes, one has to completely sterilize the outer surface for isolation of endophytes. The surface sterilization process is most crucial aspect of endophyte isolation, if the surface is not properly sterilized the isolates will be the mixture of endophtyes and microorganisms present on surface and the results obtained will be confusing. Therefore, it is must to optimize the surface sterilization process with various disinfectant concentration and time period. Present investigation was conducted to evaluate the potential of ethanol and sodium hypochlorite on surface sterilization process and to assess the effect of these sterilizing agents on isolation of endophytes from leaves and bulb of Allium sativum. Mostly two concentrations 96% and 70% of ethanol is widely used for sterilizing non-living surface. On other hand, due to the highly dehydrating activity of 96% ethanol, most of the surface sterilization protocol used 70% to 80% ethanol due to highest sterilizing activity. Ethanol mostly affect plasma membrane, dissolve lipids and denature protein that result in leakage cytoplasm and cell organelles, ultimately leading to cell death.36 Only ethanol treatment of most plant tissues is insufficient to remove all the microorganisms especially spores so ethanol treatment followed by sodium hypochlorite treatment. Ethanol treatment is less effective in penetrating protein rich layer (https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfectionmethods/chemical.html). Sodium hypochlorite, an oxidizing agent and used for surface sterilization of concentration ranging from 0.5%–5%. Sodium hypochlorite acts by three process: saponification, neutralization, and chloramination that produces hypochlorous acid (HOCI) and hypochlorite ions (–OCl) that, suppress enzyme function, degrade amino acids, damage plasma membrane and DNA, and also inhibit membrane transport.37-39 The antimicrobial spectrum of Sodium hypochlorite is determined by the amount of HOCl in the solutions and HOCl penetrates cell wall and the cell membrane of microorganism.40 On the other hand, the germicidal activity of a concentrated sodium hypochlorite solution is based on its high pH (–OH action) and –OCl oxidation.41
Only a few reports are available on optimization of surface sterilization process for isolation of bacterial endophytes.20-23,42 There are several reports available on endophytic microbes from Allium sativum bulb exhibiting antimicrobial activity.43-48 Optimization of sterilization process is not only crucial for removal of microbes from surface of plant but for isolation of endophytes, because the sterilizing agent have toxic effect on endophytic population. Concentration and time duration of sterilizing agents for all plant surfaces varies with plant and their parts. Chemical disinfectant ethanol (70%) and sodium hypochlorite (2% and 3%) found to be significant when used in combination. 2% sodium hypochlorite solution effective for surface sterilization of leaf while 3% were observed to be effective for surface sterilization of bulb of Allium sativum. The study on endophytic bacteria is relatively least attend as surface sterilization of the samples are very critical and tedious for successful isolation of endophytic bacteria that may enhance the possibility to discover novel endophytes and metabolites. Few reports are available using ethanol and sodium hyochlorite for surface sterilization of Nephelium lappaceum L., Hypericum perforatum and Ziziphora capitata, Teucrium polium L., and Curcuma longa L.49-52 Burkholderia stabilis and Paenibacillus polymyxa SK1 were isolated from ginseng root and Lilium lancifolium respectively by using 15 minutes and 20 minutes of sodium hypochlorite treatment but in present investigation treatment of sodium hypochlorite for 4 and 5 minutes are inhibitory for growth of endophytes.53,54 Endophytes were isolated from Aloe vera and Arachis hypogaea by using 3% sodium hypochlorite as surface sterilizing agent having antimicrobial properties.55,56
Time to time various endophytic microbes have been isolated from various plants and were found to have prominent bioactivity, and isolated by using various surface sterilization procedures. Rummelibacillus genus (L14) an endophytic bacteria from Handroanthus impetiginosus (pink lapacho plant) exhibiting multiple plant growth promoting activity was isolated from host plant by using 70% ethanol and 2.5% sodium hypochlorite.57 Penicillium polonicum AUMC14487 is a new and potent Taxol producer (90.53 mg/l) were isolated from Ginkgo biloba plant by using ethanol and sodium hypochlorite.58 Among the various plants such as thar deseart plant, Ocimum basilicum, Cordia dichotoma exhibited the capability of production of several bioactive compound.59,60 On other hand endophytic bacteria Streptomyces sp. AE170020 was isolated from root tissues of pine tree and endophytic fungus Xylaria feejeensis from Geophila repens was reported to produce nematicidal compound aureothin, alloaureothin and eremophilane sesquiterpenoids respectively.61,62 Similarly Aspergillus niveus were isolated from root of Foeniculum vulgare was found to produce fifty-eight metabolites among them important ones are asterriquinones, citrinin analogs, citrinin dimers and varioxiranol G that are Inhibitors of cholinesterase.63 Ageratina adenophora an endophytic fungi exhibited antimicrobial activity against Methicillin resistant Staphylococcus aureus (MRSA).64 The overall discussion finally report that the process of surface sterilization decides “what to grow” (endophytes) and “what not to grow” (epiphytes).65
The concentration of sterilizing agent and time can be selected in such a way that it will give maximum growth and variability of endophytes. So, in present study treatment with ethanol (70%) for 6 minute followed by sodium hypochlorite solution (2%) for one minute then 30 second treatment of 70% ethanol was found to be effective for sterilization of Allium sativum leaves. On the other hand ethanol (70%) for 6 min followed by sodium hypochlorite solution (3%) for 1 minute and then 30 second treatment of 70% ethanol was found to be effective for surface sterilization of bulbs. Present investigation clearly reveals that different treatments are required for surface sterilization of different parts of plants for observation of endophytic diversity in them. The present investigation revealed that endophytic bacteria isolated from A. sativum have potential to produce antimicrobial compound and antimicrobial nature of Allium sativum may be due to the reverberation production of bioactive metabolites by endophytic bacteria.
In order to isolate endophytic bacteria from Allium sativum, microbes present on the plant surface have to be fully eliminated first. The present study reveals the comprehensive surface sterilization strategy for recovery of maximum endophytic bacteria with complete elimination of the rhizospheric present on the surface of explants. In conclusion, the present study enables the development of an efficient and effective procedure for optimization of time and concentration of sterilizing agent. Perhaps it might be first comprehensive report from India for surface sterilization of different parts of Allium sativum for isolation of bacterial endophytes. The combination of 70% ethanol for 6 min and 2% sodium hypochlorite for 1 min and 3% sodium hypochlorite for 1 min were found to be effective for bulb with 30 second additional treatments with 70% ethanol. Furthermore, bulb of Allium sativum have many layers so choice of concentration and its exposure time has correlation with tissue type and innovation in sterilization method lead to successful isolation of potential endophytic bacteria. The results revealed that endophytic bacteria from Allium sativum have excellent source of potential antimicrobial compound. In future, experiments for extraction of bioactive compound and their identification are in progress.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Biotechnology and Microbiology for providing the necessary lab facilities for carrying out the research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RS and PS conceptualized and designed the study. AKS designed the experiments and provided lab facility for specialized work. PS designed figures and tables and wrote the manuscript. SPT edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This research work was funded by Women Scientist-A Scheme, Department of Science & Technology, Government of India (File no. SR/WOS-A/LS-11/2018).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Compant S, Clement C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospect for utilization. Soil Biol Biochem. 2010;42(5):669-678.
Crossref - Kandel SL, Joubert PM, Doty L. Bacterial endophyte colonization and distribution within plants. Microorganism. 2017;5(4):77.
Crossref - Lodewyckx C, Vangronsveld J, Porteou F, et al. Endophytic bacteria and their potential applications. Crit Rev Plant Sci. 2002;21(6):583-606.
Crossref - Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67(4):491-502.
Crossref - Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278(1):1-9.
Crossref - Tamez-Guerra P, Gomez-Flores R, Orozco-Flores AA, Rodriguez-Padilla C, Gonzalez-Ochoa G, Tamez-Guerra P. Bioactive products from plant-endophytic Gram positive. Front Microbiol. 2019;10:463.
Crossref - Koskimaki JJ, Pirttila AM, Ihantola EL, Halonen O, Frank AC. The intracellular scots pine shoot symbiont Methylobacterium extorquens DSM13060 aggregates around the host nucleus and encodes eukaryote-like proteins. M Bio. 2015;6(2):e00039-00015.
Crossref - Cankar K, Kraigher H, Ravnikar M, Rupnik M. Bacterial endophytes from seeds of Norway spruce (Picea abies L. Karst). FEMS Microbiol Lett. 2005;244(2):341-345.
Crossref - de Melo-Pereira GV, Magalhaes KT, Lorenzetii E R, Souza TP, Schwan RF. A multiphasic approach for the identification of endophytic bacterial in strawberry fruit and their potential for plant growth promotion. Microb Ecol. 2012;63(2):405-417.
Crossref - Compant S, Mitter B, Colli-Mull JG, Gangl H, Sessitsch A. Endophytes of grapevine flowers, berries, and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb Ecol. 2011;62(1):188-197.
Crossref - Yu H, Zhang L, Li L, et al. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol Res. 2010;165(6)437-449.
Crossref - Mohamad OAA, Li L, Ma JB, et al. Evaluation of the Antimicrobial Activity of Endophytic Bacterial Populations from Chinese Traditional Medicinal Plant Licorice and Characterization of the Bioactive Secondary Metabolites Produced by Bacillus atrophaeus Against Verticillium dahliae. Front Microbiol. 2018;9:924.
Crossref - Photolo MM, Mavumengwana V, Sitole L, Tlou MG. Antimicrobial and Antioxidant Properties of a Bacterial Endophyte, Methylobacterium radiotolerans MAMP 4754, Isolated from Combretum erythrophyllum Seeds. Int J Microbiol. 2020;9483670.
Crossref - Bafana A. Diversity and metabolic potential of culturable root-associated bacteria from Origanum vulgare in sub-Himalayan region. World J Microb Biot. 2012;29(1):63-74.
Crossref - FROeHLICH J, Hyde KD, Petrini O. Endophytic fungi associated with palms. Mycolog Res. 2000;104(10):1202-1212.
Crossref - Hyde K, Soytong K. The fungal endophyte dilemma. Fungal Divers. 2008;33(163):e173.
Crossref - Schulz B, Boyle C. The endophytic continuum. Myco Res. 2005;109(6):661-686.
Crossref - Schulz B, Guske S, Dammann U, Boyle C. Endophyte-host interactions. II. Defining symbiosis of the endophyte-host interaction. Symbiosis. 1998;25:213-227.
- Anjum N, R Chandra. Endophytic bacteria:optimization of isolation procedure from various medicinal plants and their preliminary characterization. Asian J Pharm Clin Res. 2015;8 (4):233-238.
- Khanam B, Chandra R. Optimization of Surface Sterilization Process of Selected Dye-Yielding Plants for Isolation of Bacterial Endophytes. Appl Biotechnol Sust Dev Springer. 2017:45-50.
Crossref - Shukla S, Wahla C. Influence of different sterilizing methods on isolation endophytic bacteria from Rauvolfia serpentina. Pharm Innov J. 2019;8(1):38-41.
Crossref - Waheeda K, Shyam K. Formulation of novel surface sterilization method and culture media for the isolation of endophytic actinomycetes from medicinal plants and its antibacterial activity. J Plant Pathol Microbiol. 2017;8(2):1000399.
Crossref - Bayan L, Koulivand PH, Gorji A. Garlic:a review of potential therapeutic effects. Avicen J phytomed. 2014;4(1):1-14.
- Srivastava P, Tiwari SP, Sharma R. Optimization of surface sterilization process for isolation and and cultivation of bacterial endophytes from Allium sativum. Mishra G, Dhawan, Gupta MK (Eds), In: Pro Int Conf Ultra Mat Sci Adv Technol, Narosa Publisher, New Delhi. 2019:189-194.
- McInroy JA, Kloepper JW. Studies on indigenous endophytic bacteria of sweet corn and cotton. Molecular Ecology of Rhizosphere Microorganisms: Biotechnology and the Release of GMOs. 1994;19-28.
Crossref - Jasim B, Jimtha CJ, Jyothis M, Radhakrishnan EK. Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul. 2013;71:1-11.
Crossref - Mahaffee W, Kloepper J. Temporal changes in the bacterial communities of soil, rhizosphere, and endorhiza associated with field-grown cucumber (Cucumis sativus L.). Microb Ecol. 1997;34:210-223.
Crossref - Zinniel DK, Lambrecht P, Harris NB, et al. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol. 2002;68(5):2198-2208.
Crossref - Tiwari R, Kalra A, Darokar MP. et al. Endophytic bacteria from Ocimum sanctum and their yield enhancing capabilities. Curr Microbial. 2010;60(3):167-171.
Crossref - Pleban S, Fanya I, Illan C. Control of Rhizoctonia solani and Sclerotium rolfsii in the greenhouse using endophytic Bacillus spp. Europ J Plant Pathol. 1995;101:665-672.
Crossref - Shishido M, Loeb BM, Chanway CP. External and internal root colonization of lodgepole pine seedlings by two growth-promoting Bacillus strains originated from different root microsites. Canadian J Microbiol. 1995;41(8):707-713.
Crossref - Marcellano JP, Collanto AS, Fuentes RG. Antibacterial activity of endophytic fungi isolated from the bark of Cinnamomum mercadoi. Pharmaco J. 2017;9(3). 405-409
Crossref - Karuppiah P, Rajaram S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens Asin. Paci. J Trop Biomed. 2012;2(8):597-601.
Crossref - Edwards U, Rogall T, Blocker H, Emde M, Bottger EC. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic acids Res. 1989;17(19):7843-7853.
Crossref - Kim OS, Cho YJ, Lee K, et al. Introducing EzTaxon-e:a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol. Microbiol. 2012;62(Pt3):716-721.
Crossref - Madigan MT, Martinko JM, Dunlap PV, Clark DP. Brock biology of microorganisms. 12th ed. San Francisco: Benjamin Commings. 2008.
- Estrela C, Estrela CR, Barbin EL, Spano JC, Marchesan MA, Pecora JD. Mechanism of action of sodium hypochlorite. Braz Dent J. 2002;13(2):113-7.
Crossref - Dukan SA, Touati D. Hypochlorous acid stress in Escherichia coli:resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178(21):6145-6150.
Crossref - Dukan S, Belkin S, Touati D. Reactive oxygen species are partially involved in the bacteriocidal action of hypochlorous acid. Arch Biochem Biophys. 1999;367(2):311-316.
Crossref - Le Dantec C, Duguet JP, Montiel A, Dumoutier N, Dubrou S, Vincent V. Chlorine disinfection of atypical mycobacteria isolated from a water distribution system. Appl Environ Microbiol. 2002;68(3):1025-1032.
Crossref - Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11(4):147-57.
Crossref - Deepak H, Virk V. Optimization of surface sterilization method for the isolation of endophytic fungi associated with Curcuma longa L. and their antibacterial activity. J Adv Biotechnol Exp Ther. 2022;5(2):334-346.
Crossref - Costa Jr PSP, Cardoso FP, Martins AD, et al. Endophytic bacteria of garlic roots promote growth of micropropagated meristems. Microbiol Res. 2020;241:126585.
Crossref - da Rosa Leoncio M, Botelho, GR. Isolation and characterization of plant growth promoting bacteria isolated from garlic (Allium sativum). Scientia Agraria. 2017;18(3):95-106.
Crossref - Sayed AA, Eraky AMI, Abd-El-Rahman TM, Abd-El-Razik AA. Endophytic fungi associated with Allium Plants and their antagonistic activity against Fusarium oxysporum f. sp. Cepae. J Sohag Agri Sc. 2021;6(1):1-7.
Crossref - Lestari AR, Wonorahardjo S, Suharti S. Analysis of total acidity toward bacterial and endophytic fungi profile dur-ing black garlic processing from garlic (Allium sativum l.) and shallot (Allium ascalonicum l.). Makara J Sci. 2021;25(3):188-194.
Crossref - Ibrahim E, Zhang M, Zhang Y, et al. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat fusarium head blight pathogen fusarium graminearum. Nanomaterials. 2020;10(2):219.
Crossref - Wang J, Shi L, Wang D, et al. White rot disease protection and growth promotion of garlic (Allium sativum) by endophytic bacteria. Plant Pathol. 2019;68(8):1543-1554.
Crossref - Suhandono S, Kusumawardhani MK, Aditiawati P. Isolation and molecular identification of endophytic bacteria from Rambutan fruits (Nephelium lappaceum L.) cultivar Binjai. Hayati J Biosci. 2016;23(1):39-44.
Crossref - Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol. 2017;8:199.
Crossref - Kumar A, Singh R, Yadav A, Giri DD, Singh PK, Pandey KD. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech. 2016;6:1-8.
Crossref - Hassan SE-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res. 2017;8(6):687-695.
Crossref - Kim H, Rim SO, Bae H. Antimicrobial potential of metabolites extracted from ginseng bacterial endophyte Burkholderia stabilis against ginseng pathogens. Biol Cont. 2019;128:24-30.
Crossref - Khan MS, Gao J, Chen X, et al. Isolation and Characterization of Plant Growth-Promoting Endophytic Bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. BioMed Res Int. 2020;8650957.
Crossref - Akinsanya MA, Goh JK, Lim SP, Ting ASY. Diversity, antimicrobial and antioxidant activities of culturable bacterial endophyte communities in Aloe vera. FEMS microbiol Lett. 2015;362(23):fnv184.
Crossref - Chen L, Shi H, Heng J, Wang D, Bian K. Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol Res. 2019;218:41-48.
Crossref - Yarte ME, Gismondi MI, Llorente BE, Larraburu EE. Isolation of endophytic bacteria from the medicinal, forestal and ornamental tree Handroanthus impetiginosus. Environ Technol. 2022;43(8):1129-1139.
Crossref - Abdel-Fatah SS, El-Batal AI, El-Sherbiny GM, Khalaf MA, El-Sayed AS. Production, bioprocess optimization and g-irradiation of Penicillium polonicum, as a new Taxol producing endophyte from Ginko biloba. Biotechnol Reports. 2021;30:e00623.
Crossref - Sharaf MH, Abdelaziz AM, Kalaba MH, Radwan AA, Hashem AH. Antimicrobial, Antioxidant, Cytotoxic Activities and Phytochemical Analysis of Fungal Endophytes Isolated from Ocimum Basilicum. Appl Biochem Biotechnol. 2022;194(3):1271-1289.
Crossref - Sharma M, Mallubhotla S. Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced From Cordia dichotoma L. Front Microbiol. 2022;13:879386.
Crossref - Kang MK, Kim JH, Liu MJ, et al. New discovery on the nematode activity of aureothin and alloaureothin isolated from endophytic bacteria Streptomyces sp. AE170020. Sci Rep. 2022;12(1):3947.
Crossref - Rajendran S, Robertson LP, Kosgahakumbura L, et al. Antibacterial eremophilane sesquiterpenoids from Xylaria feejeensis, an endophytic fungi of the medicinal plant Geophila repens. Fitoterapia. 2023;167:105496.
Crossref - Morris GM, Lim-Wilby M. Molecular docking. Methods Mol Biol. 2008;443:365-382.
Crossref - Wen J, Okyere SK, Wang J, et al. Endophytic Fungi Isolated from Ageratina adenophora Exhibits Potential Antimicrobial Activity against Multidrug-Resistant Staphylococcus aureus. Plants. 2023;12(3):650.
Crossref - Sahu PK, Tilgam J, Mishra S, et al. Surface sterilization for isolation of endophytes:Ensuring what (not) to grow. J Basic Microbiol. 2022;62(6):647-668.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.