ISSN: 0973-7510
E-ISSN: 2581-690X
Soil samples were collected from the feather dumped area where Bacillus pumilus was isolated and used for keratinase production and keratinolytic activity. In the optimization study, optimal condition for enzyme production was observed at 144 h, pH 7, temperature 37°C. The organism was utilized for feather degradation study. The maximum degradation of 57% was obtained at 37°C, pH 7 and 6 days incubation. The size of keratinase was determined by SDS- PAGE and was observed as 52 KDa.
Keratinase, feather, keratinolytic bacteria, SDS-PAGE
As a by-product of poultry waste, million tons of feathers are produced annually from the poultry Industries1. Due to improper handling more than 5 million tons of Feather wastes are dumped into baren lands which leads a negative impact to environment thereby leading to various environmental pollutions2. Since Feather is made of rich source of protein called keratin, such keratin cannot be used directly so physical and chemical treatment are required to destroy certain amino acids for better digestibility and get converted into a digestible dietary protein for animal feed3,4.
Feather pollution can be controlled by alternate use of feather wastes5. But the draw back behind these methods is they are high energy consuming and also destructs certain other amino acids such as methionine, lysine and tryptophan5,6 which affects protein quality7. Many researchers isolated microorganisms like fungi, actinomycetes, and several bacterial species such as Bacillus sp., Pseudomonas sp., which are able to produce keratinase which indicates that they are capable of utilising the keratin found in the feather with a metabolic reaction8-11.
Microbial degradation of feather improves the nutritional quality of the feather meal and it is a good alternative for Physio chemical treatment12,13. Amongst all the proteolytic enzymes Keratinase has gained attention in last few years due to its potential application mainly in hydrolysis of keratin. Keratin and keratinases play various applications in biotechnology like feed for cattles, removal of hairs and feathers in leather and poultry industries, to generate natural gas, in textile industries for shrink proofing of wool and for removing blockages in sewage system14-16. So the present study was conducted to use Bacillus pumilus isolated from the feather contaminated soil, it was optimized for production of keratinase enzyme and the organism was used for effective degradation of feather.
Collection of soil
Soil sample was collected from feather dumped area from the depth of 5-10 cm and transferred to the air tight containers. After serial dilution, the sample was transferred into Bacillus Isolation agar. The isolated organism was subjected to Gram’s staining and biochemical test based identification.
Processing of feather
Chicken feathers were collected from Broiler chicken from a slaughterhouse nearby Alathur, Kerala, India. Stains present in feathers were washed under tap water and kept for drying. After drying feathers are boiled in water for 30 min at 100°C and further dried at 60°C in hot air oven. The ground powder was obtained from the dried feather. The powder collected was used as a keratin source in this study.
Optimization of growth on feather containing medium
Incubation period optimization
The suitable media was designed by dissolving 0.1g of dried feather powder, 0.5g of K2PO4, 0.04g of K2HPO4, 0.2g of MgSO4, at pH – 7.0 in 100 mL distilled water and sterilization was done for 15min at 121°C in autoclave. Media were added with 1 mL 24 h old bacterial culture and incubated at 37°C for 48- 168 h.
pH and temperature optimization
The optimum pH at 37°C was observed by incubating the cells in aforementioned media in optimal incubation time at different ranges of pH from 4- 9. The optimum temperature was estimated ranging from 30, 35, 37, 40 and 42°C respectively at optimal incubation time and pH. Effect of heavy metal in the production was carried in the presence of heavy metals (Lead, Mercury and Copper) were also studied in the optimal condition obtained from the above parameters.
Enzyme assay
Enzyme assay was performed by taking 1mL of 50 mM Tris HCl buffer at pH 8.0 as solvent in which 5mg keratin azure powder was dissolved. 1mL of (crude enzyme) culture supernatant obtained after centrifugation at 10000 rpm was added with the suspension and kept for 30 min at 50°c, then 0.4 M TCA of volume 2 ml was added for stopping the reaction. The mixture was centrifuged for 40 min at 5000 rpm and collected supernatant was measured spectrophotometrically and the optical density (OD) was read at 280nm. Enzyme activity was enzyme activity in Unit (U)’ as decribed earlier 3.
Percentage of degradation of chicken feather
Feather degradation percentage by the organism was found by sterilizing 10 g of feather to the above-mentioned media along with 10% of 24 h old Bacillus pumilus culture. Degradation percentage of dried feather was observed at regular interval.
Column chromatography
Culture grown in aforementioned medium in the optimal condition was centrifuged, supernatnant was subjected for Column chromatography to purify the keratinase enzyme. Stationary phase was prepared with DEAE sephadox A – 50 and was allowed to set for 5 minutes and the sample was loaded on the top of the column and the 2 mL fraction was collected at 10 min interval and analyzed by taking the OD at 280 nm using spectrophotometer.
Characterization of enzyme
Molecular weight of the enzyme was identified by Polyacrylamide gel electrophoresis (PAGE). Casting plate was assembled and plate was sealed using space aid. The separating gel was prepared with 1.5 M tris buffer, 30% of acrylamide, 10% ammonium per sulfate (APS), 10% SDS, TEMED and kept undisturbed for 2-5 minutes at room temperature for polymerization. 1M tris buffer was used instead of 1.5M tris buffer for the preparation of Stacking gel. The comb was fixed immediately and kept for 5- 10 min for polymerization. The casting tray was kept in the electrophoretic unit after removing the comb, and SDS buffer solution was added to electrophoretic unit. Prepared sample consists of 10µl of 2x SDS solution and 10 µL of sample and incubated for 10 min at 90°C was loaded to the well along with marker. Electrophoresis was carried out for 4 hours at 50V, until the bromophenol blue dye reaches the bottom of the separating gel. After separating gel from the casting plate it was stained with 0.5% coomassie brilliant blue in water, acetic acid, methanol in the ratio 25:10:45 and kept in shaker at 60 – 70 rpm for overnight followed by the process of destaining (water : acetic acid : methanol – 25:10:4) and the result was estimated using gel documentation and the molecular weight was estimated.
Keratin degrading microbiata load are normally observed in the feather dumped soil, as the feather is rich in keratin17,18 and poultry waste samples were reported to have keratinolytic bacteria19,20. The organism isolated from the soil after it grew on Bacillus isolation agar, the organisms was identified as Bacillus pumilus based on Gram’s staining and biochemical test as follows – the orgainsm was Gram positive rod and the biochemical test is listed in Table 1. Abdel-Fattah et al.21, also isolated Bacillus licheniformis from soil which was found to be keratinolytic.
Table (1):
Biochemical testing of isolated organism.
Sl No |
Biochemical test |
Optical Density(nm) |
|---|---|---|
1 |
MR |
Positive |
2 |
VP |
Negative |
3 |
TSI agar |
Acid butt, acid slant and gas production, No H2S production |
4. |
Mannitol utilization |
Positive |
Optimization of keratinase production
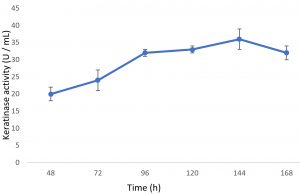
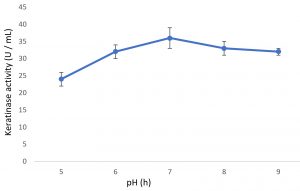
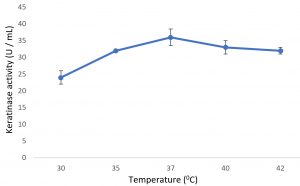
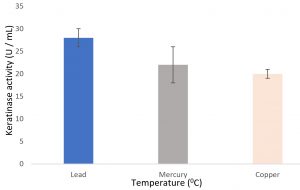
Growth of the organism on the designed media, showed the maximum production of keratinase on 6th day (Fig. 1). Optimal pH and temperature was found to be pH 7 and 37°C respectively (Fig. 2 and 3). Effective keratinase production was observed at 37°C (Fig. 3). Dhiva et al.5 found Pseudomonas aeruginosa SU-1 to produce keratinase at 30°C. The presence of heavy metal in medium was having negative influence on the enzyme production, it might be the impact of heavy metal on the growth pattern of the organism (Fig. 4).
Degradation of feather
Feather degradtion at different incubation time
Degradation of keratin was observed at 37°C, where 57% of degradation was observed in 6 days (result not shown here). Degradation of keratin was due to keratinase enzyme produced by Keratinolytic organisms. Similar work was reported by Intagun et al.19 and Lakshmi et al.22 .
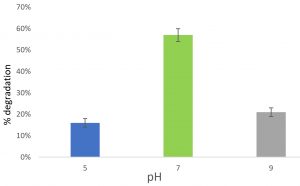
Feather degradation at different pH
At pH 5, 16% of feather was degraded after incubated for 6 days. At pH 7, 57% feather was degraded after incubated for 6 days. At pH 9, 21% feather was degraded after incubated for 6 days. Maximum feather degradation was observed at pH 7. So, pH 7 is the optimum pH for the activity of keratinase enzyme (Fig. 5).
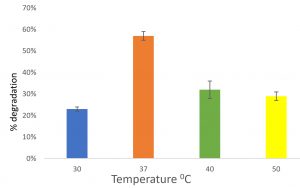
Feather degradation at different temperature
At temperature 30°C, 23% of feather was degraded after incubated for 6 days. At temperature 37, 40 and 50°C, 57%, 32% and 29% respectively was degraded after incubated for 6 days (Fig. 6). Vigneshwaran et al.23, reported Bacillus licheniformis to degrade feathers in 7 days at 40°C. Abirami et al.4 also found Lichtheimia corymbifera AS1 to degrade cattle hooves efficiently.
Column chromatography
When the fraction obtained through column chromatography was subjected for absorbance at 280 nm, the highest absorbance was recorded in the B6 fraction where the absorbance was found to be 0.06 (Table.2). It states that the highest protein was obtained at that time.
Table (2):
OD value of fractions obtained using column chromatography.
Sl No |
Tube Name |
Optical Density(nm) |
|---|---|---|
1 |
B1 |
0.024 |
2 |
B2 |
0.022 |
3 |
B3 |
0.042 |
4 |
B4 |
0.049 |
5 |
B5 |
0.060 |
6 |
B6 |
0.029 |
7 |
B7 |
0.015 |
8 |
B8 |
0.013 |
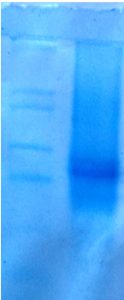
SDS- PAGE
The enzyme was characterized by SDS- PAGE. The organism used in this study produced enzyme of 52 KDa (Fig. 7). The produced keratin enzyme was 58 KDa in size from the organism Bacillus sp. CBNRBT25.
In this study, Bacillus pumilus was isolated from soil and used for keratinase production. The optimized conditions were determined as Temperature 37°C, pH 7 and incubation time 6 days. The estimated size of keratin was 52KDa. The organism was capable of degrading 57% of feather on 6 days.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript
- Azeredo L, Lima M, Coelho R Freire D. Thermophilic protease production by Streptomyces sp. 594 in submerged and solid-state fermentations using feather meal. J Appl Microbiol. 2006;100:641-647.
Crossref - Han MW, Luo Q, Gu, Yu X. Isolation and characterization of a keratinolytic protease from a feather – degrading bacterium pseudomonas aeruginosa c11. Afr J Microbiol Res. 2011;6:2211-2221.
Crossref - Riffel A, Brandelli A. Keratinolytic bacteria isolated from feather waste. Braz, J. Microbiol. 2006;37:395-399.
Crossref - Abirami S, Ragavi R, Samrot AV. Utilization of Keratinolytic Lichtheimia corymbifera AS1 for Degradation of Cattle Hoove-a Slaughter House Waste to Use in Plant Growth. Biointerface Res Appl Chem. 2020;10(5):6417-6426.
Crossref - Dhiva S, Sreelakshmi R, Sruthi S, et al. Optimization of Biomass of Keratinase Producing Bacillus sp CBNRBT2 to Utilize in Whole Cell Immobilization for Feather Degradation. Letters in Applied NanoBioScience. 2020;9(3):1339-1347.
Crossref - Gurav R, Nalavade V, Aware C, et al. Microbial degradation of poultry feather biomass in a constructed bioreactor and application of hydrolysate as bioenhancer to vegetable crops. Environ Sci Pollut Res Int. 2020;27:2027-2035.
Crossref - Zerdani I, Faid M, Malki A. Feather waste digestion by new isolated strains Bacillus spp. in Morocco. Afr J Biotechnol. 2004;3:67-70.
Crossref - Cai C, Zheng X. Medium optimisation for keratinase production in hair substrate by a new Bacillus subtilis KD-N2 using response surface methodology. J Ind Microbiol Biotechnol. 2009;36:875-883.
Crossref - Rieger TJ, De Oliveira CT, Pereira JQ, Brandelli A, Daroit DJ. Proteolytic system of Bacillus sp. CL18 is capable of extensive feather degradation and hydrolysis of diverse protein substrates. Br Poult Sci. 2017;58:329-335.
Crossref - Daroit DJ, Correa APF, Brandelli A. Keratinolytic potential of a novel Bacillus sp. P45 isolated from the Amazon basin fish Piaractus mesopotamicus. Int Biodeter Biodegr. 2009;63:358-363.
Crossref - Chaturvedi V, Bhange K, Bhatt R, Verma P. Production of kertinases using chicken feathers as substrate by a novel multifunctional strain of Pseudomonas stutzeri and its dehairing application. Biocatal Agric Biotechnol. 2014;3:167-174.
Crossref - Forgacs G, Alinezhad S, Mirabdollah A. Feuk-Lagerstedt, E, Horvath IS. Biological treatment of chicken feather waste for improved biogas production. Int J Environ Sci. 2011;23:1747-1753.
Crossref - Reddy MR, Reddy KS, Chouhan YR, Bee H, Reddy G. Effective feather degradation and keratinase production by Bacillus pumilus GRK for its application as bio-detergent additive. Bioresource Technology. 2017;243:254-263.
Crossref - Brandelli A. Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioproc Tech. 2008;1:105-116.
Crossref - Gupta R, Sharma R, Beg QK. Revisiting microbial keratinases: next generation proteases for sustainable biotechnology. Critical Reviews in Biotechnology. 2013;33:216-228.
Crossref - Sharma R, Devi S. Versatility and commercial status of microbial keratinases: a review. Rev Environ Sci Biotechnol. 2018; 17: 19-45.
Crossref - Tesfaye T, Sithole B, Ramjugernath D, Chunilall V. Valorisation of chicken feathers: Characterisation of chemical properties. J Waste Manag. 2017;68:626-635.
Crossref - Li Q. Progress in microbial degradation of feather waste. Front Microbiol. 2019;10:2717.
Crossref - Intagun W, Kanoksilapatham W. A review: biodegradation and applications of keratin degrading microorganisms and keratinolytic enzymes, focusing on thermophiles and thermostable serine proteases. Am J Appl Sci. 2017;14:1016-1023.
Crossref - Wang T, Liang C, Sun Y, Gao W, Luo X, Gao Q, Yuan H. Strategical isolation of efficient chicken feather-degrading bacterial strains from tea plantation soil sample. Int. Microbiol. 2019;22:227-237.
Crossref - Abdel-Fattah AM, El-Gamal MS, Ismail SA, Emran MA, Hashem AM. Biodegradation of feather waste by keratinase produced from newly isolated Bacillus licheniformis ALW1. J Genet Eng Biotechnol. 2018;16(2):311-318.
Crossref - Jeevana Lakshmi P, Kumari Chitturi CM, Lakshmi VV. Efficient degradation of feather by keratinase producing Bacillus sp. Int J Microbiol. 2013;2013.
Crossref - Vigneshwaran C, Shanmugam S, Kumar TS. Screening and characterization of keratinase from Bacillus licheniformis isolated from Namakkal poultry farm. Researcher. 2010;2:89-96
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.