ISSN: 0973-7510
E-ISSN: 2581-690X
Oleoyl-diethanolamide was synthesized through amidification of diethanolamine (DEA) with oleic acid (OA) using immobile lipase as a biocatalyst. Response Surface Methodology (RSM) based on five-level five variable, Central Composite Rotatable Design (CCRD) was developed to study and to optimize the reaction conditions of oleoyl-diethanolamide synthesis under solvent-free system. The influence of the five main variables namely temperature, reaction time, enzyme amount, substrate molar ratio and step of amine added on amide synthesized was analyzed. Results concluded that quadratic polynomial models is in accordance with the data obtained, with the value of R2 is 0.9846. Although the effect of the gradual addition of the amine is not so significant, but it minimize problems of formation of ion pairs amine/fatty acid highly viscous. Oleic acid converted is 61.35% obtained at the optimum conditions 70oC, 24h, enzyme amount 12% (wt/wtOA), three step of amine added and the DEA/OA molar ratio of 7. This is a high ratio of substrate because amine served as a solvent in the reaction. The identity of the oleoyl diethanolamide was confirmed by FTIR. Solvent-free conditions can be used even oleic acid is converted less (61.35%) than synthesis using a solvent (78%).
Alkanolamide, Central Composite Rotatable Design, Response Surface Methodology, Solvent-free, Immobile lipase.
Fatty alkanolamide is a nonionic surfactant with widespread potential applications, because it has a high dispersion and a positive effect on the stability of W/O foam1. It was commercially obtained from the condensation of fatty acids and amines. Due to the nature of the surface active, these surfactants are widely used in several formulations such as detergents, shampoos, antimicrobial agents, antifoaming agents, cosmetics, pharmaceutical chemistry, and lubricants2.
Fatty alkanolamide is usually synthesized using high temperature about 180oC with the presence of chemical catalyst3. The synthesis of alkanolamide from fatty acid with chemical catalysts, generally will arise impurities such as monoesteramines, diesteramines, monoesteramides and diesteramides. Besides that the use of high temperature produces odor and the product colour is less attractive2,4. The application of enzymes in the synthesis of amides has been improved a lot. Enzymatic synthesis has several advantages such as mild reaction conditions, low impurities, low energy needs, minimal waste, product easyly isolated and reusable biocatalyst5,6. Fatty alkanolamide synthesis in organic-base solvent using enzyme have also been reported by some researchers2,7. But so far, there is no report on enzymatic amidification of oleaic acid (OA) and diethanolamine (DEA) in solvent-free system. Further, there is no specific study about the interaction effect of reaction parameters as well as optimal condition reported for this amide.
Althought organic solvent has several benefits on enzymatic reaction, however the use of solvent in the process industry become less than expected. It is due to reason that some organic solvent are a volatile organic compound which may affect the environment and human health, take costs for purification after use, require larger reactors and a more complete equipment, and solvent has an influence to inhibit the enzyme acticity8. Running the reaction in solvent-free condition is potential to solve the problem. Further, high selectivity and high volumetric productivity, increase the product concentration, easy purification step, are some advantages of solvent free system6,9. For economic and pollution reasons, solvent-free system is an interesting way to modernize the classic procedure becomes cleaner, safer and easier to implemented.
In order to optimize fatty acid conversion, Response Surface Methodology (RSM) with Central Composite Rotatable Design (CCRD) has been applied. RSM is a statistical technique that is rapid and beneficial to develop, increase and optimizing the process10,11. It is superior to the traditional approach in which optimization studies are carried out by varying one parameter at a time while keeping others constant. This way has been successful applied for optimization synthesis of ester12,13,14, amide5,15 and gliceride16. In this study, RSM and CCRD have been applied to optimize enzymatic synthesis of oleoyl-diethanolamide from OA and DEA in order to increase the OA conversion and reduces the cost of production in most favorable conditions.
Initial studies in the laboratory showed that the substrate molar ratio, reaction time, temperature, enzyme concentration and step of amine added have a significant influence in the amidation reaction15. Observation made is aimed to examine the possibility of oleoyl-diethanolamide synthesis using lipase in solvent-free system. This study also helps to obtain a better relationship between the main reaction variables against response that is oleic acid conversion, and to determine the optimal conditions fatty alkanolamide synthesis using five levels, five variables, Central Composite Rotatable Design and RSM analysis.
Chemicals
Immobile lipase from Candida antarctica supported on an acrylic resin, from Sigma Aldrich (United States). Diethanolamine (DEA) and Olein Acid (OA) were purchased from Merck Chemical Co. (Darmstadt, Germany). Acetone, n-hexane and sodium hydroxide were procured also from Merck Chemical Co. (Darmstadt, Germany). All other chemical used were of analytical grade.
Amidification method and characterization
Immobile lipase acted as a catalyst to execute amidification of diethanolamine and oleic acid. Amide synthesis was performed in stop-pered flasks with a working volume of 200 mL. An appropriate amount of enzyme was added to the flask containing DEA dan OA. The ratio of DEA to OA, temperature, reation time, enzyme concentration, step of amine added are varied as in Table 1. The component reaction is mixed using the Ika-LaboratoryÒ hot plate and the magnetic stirrer at 150 rpm. Temperatures were kept to a stable using a bath containing mineral oil and all samples were taken after a certain time of purification. Reactions that occur are as follows:
CH3(CH2)7CH=CH(CH2)7COOH + HN(CH2CH2OH)2→
(CH2CH2OH)2N-CO-(CH2)7CH=CH(CH2)7CH3 + H2O
oleic acid + diethanolamine → oleoyl-diethanolamide + water
Purification of the product is carried out by dissolving a mixture of products in hexane and separated from the enzyme using a filter paper. Crude amide product separated from its solvent (n-hexane) by rotary evaporator at 90oC. Product that still contain oleic acid and excess diethanolamine then washed with acetone. Amide would be obtained in the bottom layer and residual oleic acid would dissolve with acetone as the top product. The conversion of oleic acid was carried out by the following equation:
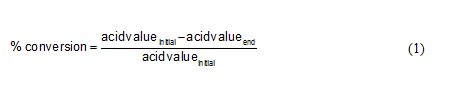
…(1)
The end product was characterized by recording the Fourier Transform-Infra Red (FTIR) spectra of the compound on a FTIR instrument series 1100 from Perkin Elmer.
Experimental design, response surface analysis and optimization
Central composite rotatable design, with five level and five variable was adopted in this study. Rotatability indicates that the variation in the predicted response is constant at a given distance from the center point of the design17. The design consist of 16 factorial points, 10 axial points (2 axial points on the axis of each design variable at a distance of 2 from design center) and 6 center points. Center point is repeated six times to give a good estimate of the experimental error. The parameters and their corresponding ranges selected were : Temperature (45-90oC); Time (18-36h); Enzyme amount (8-14% wt/wtOA); Substrate molar ratio (1-9 molDEA/OA); Step of Amine (1-5 step). High and low levels of each variable were coded as 2 and -2, respectively, and the mean value was coded as zero. Variables and levels developed for the synthesis of of oleoyl-diethanolamide in solvent free system given in Table 1. The employed experimental design is presented in Table 2. All the experiments were performed and duplicated.
Table (1):
Experimental range of five independent variables in terms of coded and actual values.
| Variables | Coded levels of variables | ||||
|---|---|---|---|---|---|
| -2 | -1 | 0 | 1 | 2 | |
| Temperature, X1 (oC) | 30 | 45 | 60 | 75 | 90 |
| Time, X2 (h) | 12 | 18 | 24 | 30 | 36 |
| Enzyme Amount, X3 (% wt/wtOA) | 6 | 8 | 10 | 12 | 14 |
| Substrate Ratio, X4 (mol DEA/OA) | 1 | 3 | 5 | 7 | 9 |
| Step of Amine, X5 (step) | 1 | 2 | 3 | 4 | 5 |
Table (2):
Coded level combination for a five level CCRD and experimental results.
Test Run |
Temp. (oC) |
Time (h) |
Enzyme Amount (%wt/ wtOA) |
Substrate Ratio (mol DEA/OA) |
Step of Amine (Step) |
Oleic Acid Conversion (%) |
|---|---|---|---|---|---|---|
1 |
-1 |
-1 |
-1 |
-1 |
1 |
1.51 |
2 |
1 |
-1 |
-1 |
-1 |
-1 |
6.52 |
3 |
-1 |
1 |
-1 |
-1 |
-1 |
1.53 |
4 |
1 |
1 |
-1 |
-1 |
1 |
1.55 |
5 |
-1 |
-1 |
1 |
-1 |
-1 |
11.56 |
6 |
1 |
-1 |
1 |
-1 |
1 |
59.32 |
7 |
-1 |
1 |
1 |
-1 |
1 |
11.55 |
8 |
1 |
1 |
1 |
-1 |
-1 |
57.62 |
9 |
-1 |
-1 |
-1 |
1 |
-1 |
32.01 |
10 |
1 |
-1 |
-1 |
1 |
1 |
80.42 |
11 |
-1 |
1 |
-1 |
1 |
1 |
86.49 |
12 |
1 |
1 |
-1 |
1 |
-1 |
76.54 |
13 |
-1 |
-1 |
1 |
1 |
1 |
65.37 |
14 |
1 |
-1 |
1 |
1 |
-1 |
62.65 |
15 |
-1 |
1 |
1 |
1 |
-1 |
81.52 |
16 |
1 |
1 |
1 |
1 |
1 |
69.92 |
17 |
-2 |
0 |
0 |
0 |
0 |
36.56 |
18 |
2 |
0 |
0 |
0 |
0 |
73.82 |
19 |
0 |
-2 |
0 |
0 |
0 |
27.25 |
20 |
0 |
2 |
0 |
0 |
0 |
56.48 |
21 |
0 |
0 |
-2 |
0 |
0 |
28.65 |
22 |
0 |
0 |
2 |
0 |
0 |
73.48 |
23 |
0 |
0 |
0 |
-2 |
0 |
1.65 |
24 |
0 |
0 |
0 |
2 |
0 |
97.24 |
25 |
0 |
0 |
0 |
0 |
-2 |
62.68 |
26 |
0 |
0 |
0 |
0 |
2 |
64.65 |
27 |
0 |
0 |
0 |
0 |
0 |
62.75 |
28 |
0 |
0 |
0 |
0 |
0 |
55.25 |
29 |
0 |
0 |
0 |
0 |
0 |
55.32 |
30 |
0 |
0 |
0 |
0 |
0 |
62.60 |
31 |
0 |
0 |
0 |
0 |
0 |
62.75 |
32 |
0 |
0 |
0 |
0 |
0 |
62.75 |
For generating response surface, the experimental data obtained based on the above design was fitted to a second order polynomial equation of the form:
![]()
…(2)
where Y = oleic acid conversion (%) ; X1 = temperature (oC); X2 = reaction time (h); X3 = enzyme amount (% wt/wtOA); X4 = substrate molar ratio (mol DEA/OA); X5 = step of amine, b0 = intercept (constant); bi = linear coefficient; bii = quadratic coefficients; bij = cross product coefficient. The regression analysis, statistical significance and response surfaces were done using Minitab 17® trial version software package. The optimum value of percent conversion of oleic acid obtained by analyzing response contour plot.
Model development and analysis of variance
In this study, range of selected variables based on preliminary carried out15. Optimal value from the previous study would be the middle value on studies using solvent-free system. Diethanolamine concentration varied at fixed oleic acid and amide formed was expressed with respect to conversion of oleic acid. The actual conversion has been obtained experimentally then analyzed for generating response surface model. Final equation that predicted is given as follow:
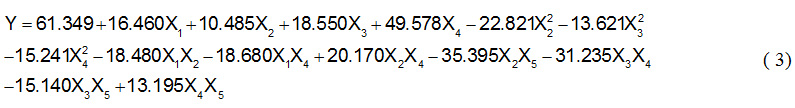
Table 3 shows the regression coefficients and P value for each factor. The ‘t’ test showed that most of the linear coefficients and quadratic coefficients were highly significant (log P < 0.05) except the linear coefficients for the step addition of amines (log P = 0.113) and quadratic for the step addition of amines (log P = 0.820) and temperature (log P = 0.053). Of ten cross product term it found three insignificant term namely cross product coefficients for temperature and enzyme (log P = 0.157), temperature and step of amine added (log P = 0.226) and also time and enzyme log P = 0.335). The coefficient of determination (R2) of the model is 0.9846, which indicates that the model created is appropriate to represent the real relationship between parameters selected.
Identification of amide product
The FTIR spectrum of oleoyl-diethanolamide shown in Fig. 1 where the visible absorption peak on the wave number 3365.6 cm-1 which indicates the presence of OH groups. The presence of OH is supported by bending OH at 1366.31 cm-1. vibrations of CH sp3 appears in the wave number 2924.57 cm-1 and 2853.70 cm-1 supported by the appearance of absorption at the wave number 1466.83 cm-1 which shows the bending vibrations of CH sp3. Type text or a website address or The spectrum shows the peak vibration on the wave number 721.3 cm-1 which is a rocking vibration (CH2)n for n>4. Vibration group C=O (carbonil) appears in the wave number 1621.99 cm-1 and C-N at 1563.51 cm-1 which is a typical group of the N-C = O amide. C-N bond also expressed the C-N stretching mode at 1068.41 cm-1.
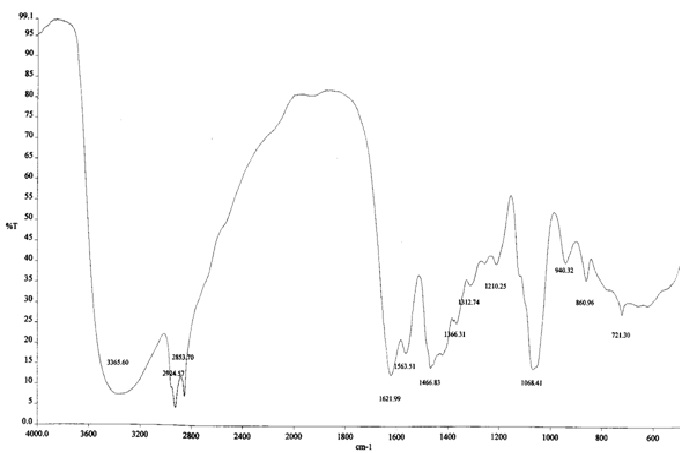
Fig. 1. The FTIR spectrum of oleoyl-diethanolamide from oleic acid and diethanolamine
Response contour plots
The reactants used in this study is oleic acid and diethanolamide. Both reactants are in the liquid phase, so it can be used in solvent-free reaction conditions. Type text or a website address or translate a document. Due to perform enzymatic reactions at Type text or a website address or the conditions without solvent, at least one of the reactants are in the liquid phase. In this condition Type text or a website address or temperature is a crucial parameter Type text or a website address or which must be selected by considering the melting point and the solubility of the reactants.
Contour plots are very helpful for interpreting the main effects of reaction parameters and their mutual interaction14 Figure 2 until Fig. 7 are showing the contour plots interaction results between two variable against response namely oleic acid conversions. Other variables are constant at their center points and the number inside the contour plots indicate conversion (%) of the amide.
Results contour response plot in Fig. 2 between variables molar ratio and temperature, Type text or a website address or indicates that at this solvent-free conditions a linier increase in amide production with increase in molar ratio has been observed. If the molar ratio of DEA/OA is large, it shows the use of excess DEA. This is necessary because in solvent free systems, one substrate is generally used in a large excess over another in order to act as a solvent for other reactants6.
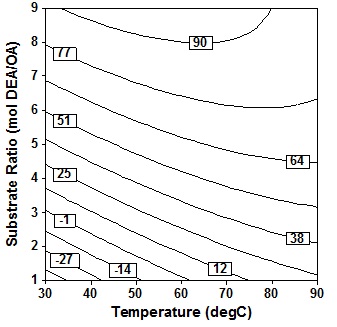 Fig. 2. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of substrate ratio (X4) and temperature (X1), at X2 = 24 h, X3 = 10% (wt/wtOA), X5 = three step
Fig. 2. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of substrate ratio (X4) and temperature (X1), at X2 = 24 h, X3 = 10% (wt/wtOA), X5 = three step However, increase in temperature end with decrease in proportionally with oleic acid conversion. The c Type text or a website address or on version is in the range of 80% both at low and high reaction temperature, provide that the molar ratio of the substrate (DEA/OA) is in the ratio of 7 to 9. It is noted that to work at a molar ratio DEA/OA lower than 7, it would be beneficial to employ a high temperature in the range of 70″90oC for amidation. The contour response is also consistent with results of analysis of variance which states that temperature is a significant variable for this solvent-free system (P = 0.009).
S Type text or a website address or election of immobile lipase is based on the observation of15,17, which generally concluded that lipase from C. antarctica shows a very high tolerance rather than lipase from R. miehei, Lipozyme TL IM and Lipozyme RM IM. Immobile lipase from Candida antarctica therefore used for the entire observation. Type text or a website address or The concentration of enzyme or enzyme amount declared by actual quantity of enzyme present against acyl donor (acid) as the requirement of enzyme is likely a function of acid concentration.
The observation in Fig. 3 shows that increasing the amount of enzyme and temperature will simultaneously increase the percent conversion of oleic acid. The increase was marginal beyond 10% (wt/wtOA) enzyme amount and temperature 60oC. Conversion were maximum at higher enzyme amount (12″14% wt/wtOA) and maximum reaction temperature (80″90oC). The results were also in line with results that obtained by Lee et al6, which stated that the effect of temperatur is more significant at higher amount of enzyme. The presence of higher amount of enzyme provides more et al active sites for the acyl-enzyme complex formation and also increases the probability of enzyme substrate collition and subsequent reaction8. Thus, also concluded that as well as the results of the analysis in Table 3, reaction temperature, enzyme amount and also substrate molar ratio were the most important parameters on percentage molar conversion (P = 0.000). However, although the reaction is faster when working at high temperatures, but it ends with the increase of enzyme deactivation. High temperatures change the conformation of enzymes that alter the free energy of the system, potentially affecting substrate binding capacity and reducing the yield of the reaction18.
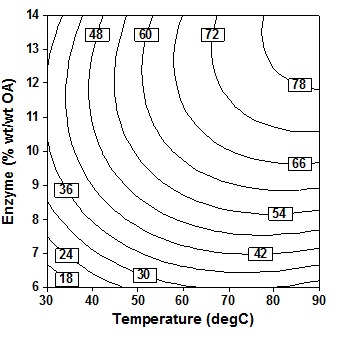 Fig. 3. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of enzyme amount (X3) and temperature (X1), at X2 = 24 h, X4 = 5 (mol DEA/OA), X5 = three step
Fig. 3. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of enzyme amount (X3) and temperature (X1), at X2 = 24 h, X4 = 5 (mol DEA/OA), X5 = three stepTable (3):
The estimated regression coefficients and P value for each factor.
| Term | Coef | SE Coef | T | P | |
|---|---|---|---|---|---|
| Constant | 61.349 | 2.366 | 25.927 | 0.000 | |
| Temperature (oC) | 16.460 | 2.422 | 6.796 | 0.000 | |
| Time (h) | 10.485 | 2.422 | 4.329 | 0.001 | |
| Enzyme Amount (% wt/wtOA) | 18.550 | 2.422 | 7.659 | 0.000 | |
| Substrate Ratio (mol DEA/OA) | 49.578 | 2.422 | 20.471 | 0.000 | |
| Step of Amine (Step) | 4.177 | 2.422 | 1.725 | 0.113 | |
| Temp. (oC)* Temp. (oC) | -9.496 | 4.381 | -2.167 | 0.053 | |
| Time (h)* Time (h) | -22.821 | 4.381 | -5.209 | 0.000 | |
| Enzyme Amount (% wt/wtOA)* Enzyme Amount (% wt/wtOA) | -13.621 | 4.381 | -3.109 | 0.010 | |
| Substrate Ratio (mol DEA/OA)* Substrate Ratio (mol DEA/OA) | -15.241 | 4.381 | -3.479 | 0.005 | |
| Step of Amine (Step)* Step of Amine (Step) | -1.021 | 4.381 | -0.233 | 0.820 | |
| Temp. (oC)* Time (h) | -18.480 | 5.932 | -3.115 | 0.010 | |
| Temp. (oC)* Enzyme Amount (% wt/wtOA) | 9.005 | 5.932 | 1.518 | 0.157 | |
| Temp. (oC)* Substrate Ratio (mol DEA/OA) | -18.680 | 5.932 | -3.149 | 0.009 | |
| Temp. (oC)* Step of Amine (Step) | -7.605 | 5.932 | -1.282 | 0.226 | |
| Time (h)* Enzyme Amount (% wt/wtOA) | -5.985 | 5.932 | -1.009 | 0.335 | |
| Time (h)* Substrate Ratio (mol DEA/OA) | 20.170 | 5.932 | 3.400 | 0.006 | |
| Time (h)* Step of Amine (Step) | -35.395 | 5.932 | -5.966 | 0.000 | |
| Enzyme (%)* Substrate Ratio (mol DEA/OA) | -31.235 | 5.932 | -5.265 | 0.000 | |
| Enzyme (% wt/wtOA)* Step of Amine (Step) | -15.140 | 5.932 | -2.552 | 0.027 | |
| Molar Ratio (mol DEA/OA)* Step of Amine (Step) | 13.195 | 5.932 | 2.224 | 0.048 | |
S = 5.93248, R-Sq = 98.46%, R-Sq (adj) = 95.65%
Observation of the effect of time and molar ratio at 60oC temperature value, 10% (wt/wtOA) the amount of enzyme and 3 stages the addition of the amine showed in Fig. 4. In general it appears that increase the value of these two variables will increase the percent conversion although the influence of the molar ratio of more apparent in increasing the percent conversion compared to the time variable. It seems that time is not a significant parameter that influence the conversion. Increasing molar ratios or the ratio between DEA to OA, will increase the formation of amide product, because the larger the amount of amine than oleic acid will drive the reaction towards the formation of amide compared the results of the other side namely esters and esteramide. These results are in line with Lee et al.6 which states that increase in acid concentration will increase the formation of ester.
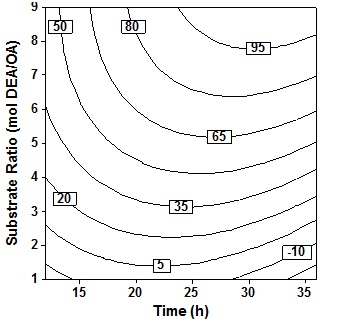 Fig. 4. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of substrate ratio (X4) and time (X2), at X1= 60oC, X3 = 10% (wt/wtOA), X5 = three step
Fig. 4. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of substrate ratio (X4) and time (X2), at X1= 60oC, X3 = 10% (wt/wtOA), X5 = three stepThe effect of enzyme amount and reaction time shown in Fig. 5 at fixed temperatur (60oC), molar ratio 5 molDEA/OA, and 3 step of amine added. An increase in acid conversion with increase in time and enzyme amount was observed. Synthesis of oleic acid with diethanolamine would produce optimum oleoyl-diethanolamide and maximum percent conversion when referring to this plot contour. Where the best results will be obtained when reaction time 23″28h and enzyme amount 11″14% (wt/wtOA). Increased reaction time more than 28h show very small improvement in the conversion. Further increase in the enzyme amount ends with a decrease in the conversion because higher amount of enzyme may cause diffusional restriction and mass transfer limitations. It can be found in the system containing immobilized enzyme and poorly soluble compound19.
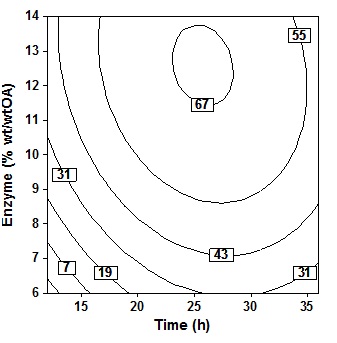
Fig. 5. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of enzyme amount (X3) and time (X2), at X1= 60oC, X4 = 5 (mol DEA/OA), X5= three step
Gradual addition of amines is observed when the reaction starts where each one mole of oleic acid is reacted with only 1/5 to 1/2 of the amine. Each time interval amine is added from 1 to 5 steps Type text or a website address or until all the amine is added. Fig. 6 shows the contour surface plot at various substrate molar ratio and step of amine added at an enzyme amount of 10% (wt/wtOA), temperature 60oC and 24h. Interestingly, Type text or a website address or addition of amine either 2 to 5 stages do not affect oleoyl-diethanolamide formed at the whole range of substrate molar ratio used. This was also verified experimentally and conversion reached only 10% under substrate molar ratio of 2 molDEA/OA. However, at a molar ratio of 7 to 9 molDEA/OA, step of amine added has little effect on the increase in conversion. The results of these observations confirm the results of analysis of variance at Tabel 3, that the observation of the five variables step of amine added, variable phase addition of the amine is the least influential variable to the increase in percent conversion of oleic acid.
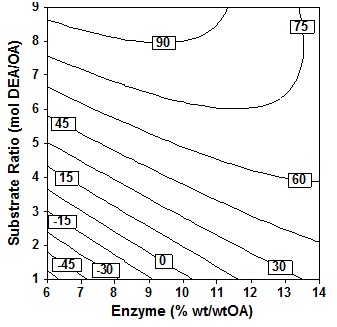
Fig. 6. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of step of amine (X5) and substrate ratio (X4), at X1= 60oC, X2 = 24 h, X3 = 10 % (wt/wtOA)
However, for synthesis in this solvent-free system, still advisable to add the amine gradually. By adding the amine gradually, it will avoid the mixture becomes viscous and mass transfer will be lower. The addition of the amine gradually also can increase the amount of amines that can react, because new amine is added, after the previously added amine has been consumed.
Figure 7 depicts the response contour plot and showing the simultan effects of substrate molar ratio and enzyme amount on oleic acid conversion in the product at fixed temperature 60oC, time 24h and 3 step of amine added. It is seen that the acid conversion rose marginally as enzyme amount increase at fixed substrate molar ratio, increase in substrate molar ratio at fixed enzyme amount also led to a marked increase in acid conversion. The results indicate that even at low enzyme amount provided that high molar ratio levels, high conversion could be achieved, which is relevant from economic view point. The present study shows that conversion of the order of 62% could be achieved only at high substrat molar ratio of the order of 8 to 9 molDEA/OA.
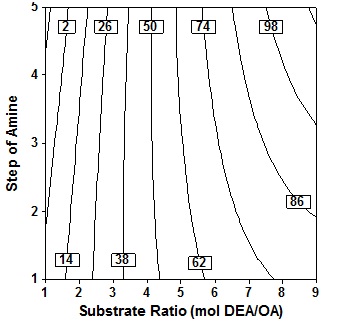 Fig. 7. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of substrate ratio (X4) and enzyme amount (X3), at X1= 60oC, X2 = 24 h, X5 = three step
Fig. 7. Contour plot of oleic acid conversion, during amidification of diethanolamine as function of substrate ratio (X4) and enzyme amount (X3), at X1= 60oC, X2 = 24 h, X5 = three stepThe ratio of the substrate is a crucial variable for this enzymatic reaction. When the OA and DEA reacted, the salt in the form of ion pairs acid/amine will be formed. The salts formed of equivalent amounts of OA and DEA would be very viscous, although the temperature increased up to 100oC. The high viscosity will result in a low mass transfer and Type text or a website address or makes the reaction time will be very long. Therefore the equimolar reaction mixture of acid and amine not suitable for this reaction. Type text or a website address or If excess acid is used, it will be a drastic decrease of the viscosity, and excess acid will act as a solvent for salt6, but the observations made generally found that amidation reaction will be reduced if given excess acid7,12. So it was favored if used in excess amine because amine will act as an solvent.
Optimum condition
From Fig. 2 to 7 and also refers to the results of the data conversion of oleic acid in Table 2, from these contour plots, the best operating conditions may be selected. Some combinations of experimental variables can provide the same acid conversion. As an example, with work on 75oC will result in the conversion of 75%. When the amount of enzyme used 12% (wt/wtOA) and a reaction time of 30h, will also result in the conversion of 75%. Thus, it is posibble to economize on enzyme amount substantially by aiming at acid conversion lower than 70%, which is still relatively high and should be attractive from the view point of process development. So, the optimum operating conditions selected are temperature 70oC, 24h, 12% (wt/wtOA) enzyme amount, three step of amine added and the diethanolamine/oleic acid molar of 7 molDEA/OA, which is expected to result in the conversion of oleic acid to 65%. This result is lower than the oleoyl diethanolamide synthesis using n-hexane as a solvent namely 78% conversion [15].
An optimized enzymatic synthesis of oleoyl-diethanolamide in solvent free system is successfully performed. Central composite rotatable design and respon contour plot can be used as a tool to describe the effect of the interaction of variables against oleic acid conversion. Temperature, time, enzyme amount, substrate molar ratio and step of amine added are the significant process variables that affected the synthesis of oleoyl-diethanolamide. Reaction rate is slower in solvent free system as compared to that in the solution, neverthless for reason of economy and pollution, solvent-free systems are the great interest in order to modernize classical procedures making them more clean, safe and easy to perform.
ACKNOWLEDGMENTS
This work was supported by Universities Sumatera Utara for providing research funds.
- Galilee, U.S., Modesto, T.C., Soma, C. Biocatalytic synthesis of diethanolamide surfactants under mild reaction condition. Philipp. J. Sci., 2009; 138: 49?54.
- Al-Mulla, E.A.J., Wan Yunus, W.M.Z., Ibrahim, N.A., Rahman, M.Z.A. Enzymatic synthesis of fatty amides from palm olein. J. Oleo Sci., 2010; 59: 59-64.
- Hoidy, W.H., Ahmad, M.B., Al-Mulla, E.A.J., Wan Yunus, W.M.Z., Ibrahim, A.A. Chemical synthesis and characterization of N-hydroxy-N-methyl fattyamide from palm oil. Orient. J. Chem., 2010; 26: 369-72.
- Masyithah, Z., Herawan, T., Sembiring, S.B., Alfian, Z. The optimization of enzymatic synthesis for lauoryl-N-methyl glucamide surfactants. Indones. J. Chem., 2011; 11: 223?28.
- Tufvesson, P., Tornvall, U., Carvalho, J., Karlsson, A.J., Hatti-Kaul, R. Toward a cost-effective immobilized lipase for the synthesis of speciality chemicals. J. Mol. Catal. B Enzym., 2011; 68: 200-5.
- Lee, A., Chaibakhsh, N., Rahman, M.B.A., Basri, M., Tejo, B.A. Optimized enzymatic synthesis of luvulinate ester in solvent-free system. Ind. Crops Prod., 2010; 32: 246-51.
- Wang, X., Wang, X., Wang, T. Synthesis of oleoylethanolamide using lipase. J. Agric. Food Chem., 2012; 60: 451-57.
- Tufvesson, P., Ekman, A., Sardari, R.R.R., Engdahl, K., Tufvesson, V. Economic and environmental assessment of propionic acid production by fermentation using different renewable raw materials. Bioresour. Technol., 2013; 149: 556-64.
- Tufvesson, P., Annerling, A., Hatti-Kaul, R., Adlescrentz, D. Solvent-free enzymatic synthesis of fatty alkanolamides. Biotechnol. Bioeng., 2007; 97: 447?53.
- Gaffari-Moghaddam, M., Yekke-Ghasemi, Z., Khajeh, M., Rakhshanipour, M., Yasin, Y. Application of response surface methodology in enzymatic synthesis: a review. Russ. J. Bioor. Chem., 2014; 40: 252?62.
- Shokimi, N.W., Khalil, K.A., Mustafa, S., Yusha, F., Ghazali, M., Zain, M.M., Musa, M. Optimization of time and pH condition for cell autoaggregation of bifidobacterium pseudocatenulatum KAKii using FCCD- response surface methodology. J. Pure Appl. Microbiol., 2015; 9(3):1797-804.
- Pan, Q., Yang, L., Meng, X. Optimization of enzymatic synthesis of tricaprylin in ionic liquids by response surface methodology. J. Am. Oil Chem. Soc., 2013; 90: 501?9.
- Siramon, P., Pusuvon, V., Riengsilchai A. Optimization of lipid production by mortierella isabellina using glycerol, a by-product of biodiesel production as a carbon source. J. Pure Appl. Microbiol., 2016; 10(2): 865-70.
- Arumugam, A., Saravan, M., Harini, S. Bioethanol production by response surface methodology from corn cobs by alkali pretreated. J. Pure Appl. Microbiol., 2016; 10(1): 547-58.
- Masyithah , Z. Optimization Method for the Synthesis of Fatty Amide Surfactants from Oleic Acid Catalyzed by Immobilized Lipase. Orient. J. Chem., 2016; 32(3): 1361-71.
- Singh, A.K., Mukhopadhyay, M. Optimization of lipase-catalyzed glyserolisis for mono and diglyceride production using response surface methodology. Arab J. Sci. Eng., 2013; 39: 2463?74.
- Tufvesson, V., Fu, W., Jensen, V., Woodley, J.M. Process consideration for scale-up and implementation of biocatalysis. Food Bioprod. Process., 2010; 88: 3-11.
- Chau, C.M., Liu, K.J., Lin, C.H. Enzymatic synthesis of sialic acid derivative by immobilized lipase from Candida antarctica. Bioresour. Technol., 2011; 102: 10136?38.
- Rahman, M.B.A., Jarmi, N.I., Chaibakhsh, N., Basri, M. Modeling and optimization of lipase-catalyzed production of succinic acid ester using central composite design analysis. J. Microb. Biotechnol., 2011; 38: 229?34.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


