ISSN: 0973-7510
E-ISSN: 2581-690X
Plant growth-promoting rhizobacteria (PGPR) have proved to be an effective solution for enhancing growth of various plant species. Five different bacterial isolates extracted from rhizosphere soil were extensively studied for the production of indole acetic acid (IAA) and among those Bacillus safensis YKS2 strain was found to produce substantial quantities of IAA. B. safensis YKS2 strain was characterized and submitted to National Centre for Biotechnology Information (NCBI) (Gen Bank No. MH539636). Optimization of IAA production with varying pH and temperature revealed that IAA production was maximum at pH 7 and at a temperature of 37°C. The production of IAA was confirmed and quantified by Fourier-transform infrared spectroscopy (FTIR), Thin-layer chromatography (TLC), Gas chromatography-mass spectrometry (GC-MS). The PGPR inoculum showed significant (p<0.05) shoot increase (60.00 – 89.00%) and root increase (30.00 – 90.00%) relative to the controls in Vigna radiata. This study showed that IAA producing ability of B. safensis YKS2 can be used in the large-scale production of IAA for plant growth promotion.
Rhizobacteria, Indole Acetic Acid, Bacillus safensis, Vigna Radiata, PGPR
Plant growth-promoting rhizobacteria (PGPR) play a vital role in farming industries by greatly reducing the use of synthetic fertilizers. Rhizosphere bacteria promote plant growth directly or indirectly through many mechanisms. One of them is by synthesis of plant growth-promoting compounds that are vital for direct plant growth. Phytohormones are chemical messengers that regulate the ability of plants to respond to external environmental stimuli.1-5 Numerous bacterial and fungal species are capable of producing phytohormones. The ability to produce phytohormones is widely distributed with bacterial species linked to soil and plants.6
Indole Acetic Acid (IAA) is an active heterocyclic phytohormone and is referred as plant auxin.7-9 The rhizosphere bacteria that produce IAA synergistically associate with plants by enhancing plant growth; in-return, the plant root exudates are utilized by bacteria as a carbon energy source.10,11 Indole acetic acid actively stimulates plant growth by breaking apical dominance that induces growth of the main stem. It is essential for plant growth and development including cell expansion or division, flowering, fruit abscission, fruit ripening, gravitropic and phototropic responses, root initiation, leaf abscission, leaf senescence and vascular tissue differentiation.12-14
A wide range of bacteria, including species of Arthobacter sp., Alcaligenes sp., Bacillus sp., Enterobacter sp., Klebsiella sp., Pseudomonas sp. and Serratia sp., has been documented to stimulate plant growth. Plant growth promotion involves direct mechanisms, such as nitrogen fixation, iron and phosphorous solubilization from the soil, and production of phytohormones viz., cytokinins, gibberellins and IAA.4 The rhizosphere is a nutrient-rich ecological habitat with diverse microbial species. Analyzing these rhizospheric microbes can provide some vital information on selecting effective PGPR that can be studied as bioinoculants in order to enhance the growth and yield of crops. Klebsiella sp., have been found to be present in the rhizosphere and also reported to exhibit essential PGP characteristics. They have been isolated from the rhizosphere of Kentucky bluegrass, soybean, sugarcane, pearl millet and rice,10,15,16 and investigated extensively for the production of IAA. The present investigation is focused on the secretion and characterization of IAA from rhizosphere soil bacteria Bacillus sp., and to study the effect of pH and temperature on IAA production. The work is also intended to study the effect of IAA produced on root and shoot elongation of Vigna radiata.
Soil Collection and Bacterial Isolation
The rhizosphere soil samples were obtained from Yercaud Hills in Eastern Ghats (11°12’56.62’N to 78°12’36.56’E). Sixteen soil samples from four different locations were obtained with a sampling depth of 10 cm. All the samples were cautiously taken with a clean scoop by scrapping, transferred into sterilized polythene bags kept in an icebox and transported to the laboratory. Each sample was serially diluted in the range 10-3 to 10-10 and spread onto sterile nutrient agar plates followed by incubation at 37°C for 24 h. After incubation, distinct bacterial colonies were identified by morphological analysis.17
Biochemical and Molecular Identification of IAA Producing Bacteria
Indole acetic acid secretion, catalase, citrate utilization and oxidase activities, Methyl Red-Voges Proskauer (MR-VP) and motility tests of the bacterial strains were examined according to the procedure developed by Cappuccino and Sherman.18 All the above-mentioned biochemical assays were used for preliminary identification and characterization of bacterial strains. The genomic DNA of IAA producing bacterial strain was extracted as per the protocol of Kalaimurugan et al.19 Universal primers 27F (5′ AGAGTTTGATCMTGCTCAG 3′) and 1525R (5’AAG GAG GTG ATCCAGCGCAGCA 3′) were used to amplify the partial 16S rRNA gene. The PCR reaction mix was planned for amplification consisting of a total volume of 25 μL comprising 17.3 μL of de-ionized autoclaved water, 2.5 μL of Taq buffer (10x), 1 μL of forward and reverse primers (1 μM/μL), 2 μL of dNTPs (10 mM/μl), 0.2 μL of Taq polymerase (3 U/μL) and 1 μL of DNA template. The isolated bacteria were further described by the use of 16S rRNA gene sequencing following the protocol reported by Kalaimurugan et al.19 The bacterial strains were identified by analyzing the sequences in the BLAST tool (NCBI). Neighbour-joining approach was performed for phylogenetic tree analysis in MEGA 6.0 software.
Screening of Bacterial Isolates for IAA Production
The isolated bacterial cultures were tested for the production of IAA.20 Test inoculated bacterial cultures in LB broth with tryptophan (5mg/ml) were incubated at 28± 2°C for a week. The bacterial cultures after incubation were subjected to centrifugation at 10,000 rpm for 15 min and approximately 2 mL of clear supernatant was combined with 4 mL of Solawaski’s reagent (15 mL H2SO4 + 25 mL of double distilled water + 0.81 g of ferric chloride) and observed at 520 nm for IAA determination.21
Production of IAA
After the screening test, the positive cultures were taken for the production of IAA. The isolated bacteria cultures were inoculated with nutrient broth. Three media such as; LB broth (sodium chloride (NaCl)- 5.00 g/L, tryptone- 10.00 g/L, yeast extract- 5.00 g/L), tryptophan broth (casein enzymic hydrolysate-10.00 g/L, sodium chloride (NaCl)-5.00 g/L, peptone-10.00 g/L), nutrient broth (beef extract- 1.00 g/L, yeast extract- 2.00 g/L, peptone- 5.00 g/L, sodium chloride (NaCl)- 5.00 g/L) were supplemented by tryptophan (5 mg/ml) and incubated at 28±2°C for a week. After incubation, the culture medium was taken for extraction and characterization studies.22
Extraction of IAA
In 100 mL of tryptophan-fortified nutrient broth (1-5 mg/mL), IAA positive bacterial culture was inoculated and incubated at 28±2°C for a week by shaking at 150 rpm in incubator shaker. The bacterial cells were discarded and the supernatant alone was collected by centrifuging the culture at 10,000 rpm for 30 min. The supernatant was acidified from pH 2.5 to 3.0 using 1 N HCl and it was extracted twice with double the amount of ethyl acetate. The fraction extracted from ethyl acetate was evaporated to dryness at 40°C using a rotatory evaporator. Finally, the extract was dissolved with methanol and stored at -20°C for further studies.8
Optimization of IAA Production
The IAA production was optimized by pH and temperature. IAA production with respect to pH range of 4 to 9 and temperature at 25, 37, 40 and 45°C were tested using the respective NB medium. The IAA production was analyzed using a UV-Vis Spectrophotometer at 400-800 nm.
Characterization of IAA
Thin Layer Chromatography
The ethyl acetate fraction was placed on TLC plates (Silica gel 254, thickness 0.25 mm). The silica gel was activated on a hot plate for 5 min before use. The acidic extract was concentrated to dryness and suspended in methanol and spotted in duplicate along with a standard IAA sample. The solvent system used was (heptane: acetone: glacial acetic acid (50:50:1)). Ascending chromatography was performed in a chromatographic glass chamber at room temperature (37°C). After the development of chromatogram, it was sprayed with ferric perchloric nitrate (FPN) solution, which is composed of 5 mL of ferric chloride (5%), 50 mL of nitric acid 50% (v/v) and 45 mL of perchloric acid 20% (v/v).23
GC-MS Analysis of IAA
The secondary metabolites obtained from bacteria were analyzed by GC-MS (QP2010 plus Equipment) using the protocol described by Cheng et al.24 The Polaris Q Ion Trap GC/FID column was used, and the temperature was initially maintained at 50°C for 2 min. It was then slowly increased to 280°C for 2 min. Helium was used as the carrier gas at a flow rate of 0.9 mL /min, and the mass spectrum was measured at a voltage of 1.07 K ionizing. The individual compounds were identified by the analyzer Wiley: Registry of a mass spectral database the NIST (V 3.0). Further, the values of retention time (RT) and retention index for the isolated compounds were identified by comparing several authentic references.25
FTIR Analysis of IAA
Approximately 1 mg of the sample was finely powdered with 10 mg of pure anhydrous potassium bromide crystals (IR grade) and made into a pellet for IR examination. The IR spectrum of the sample was measured at the optimum conditions for Bacillus sp. and compared with pure IAA.26
Response to Plant Growth
Vigna radiata seeds from the local market were chosen for the experiment. The seeds were soaked in tap water overnight prior to study to enhance the germination rate. About 25 mL of bacterial suspension was inoculated in each experimental unit. The experimental plants were grown in the greenhouse garden in earthen pots filled with 2 kg of sterilized soil along with 5 different concentrations of IAA in triplicates. After 7 weeks, the plant growth was examined and the total plant height, root length and shoot length were measured using Imagin tool 3.0 software in centimeter.27
Statistical Analysis
The data collected were analyzed using SPSS (V 16.0) software and the comparison of treatment means was done by Duncan’s Multiple Range Test (DMRT) (P<0.05).
Isolation and Screening of Bacterial Isolates for IAA Production
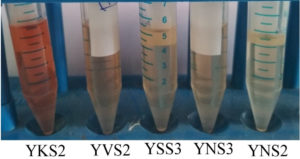
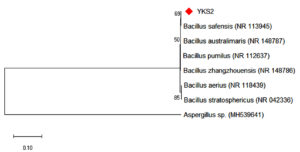
Bacterial species that produce IAA were isolated from the rhizosphere soil sample. The bacterial isolates were cultured at 37°C for 24 h. After incubation, the samples containing the bacterial cells were subjected to various morphological, biochemical, and molecular tests. Five strains were isolated from the rhizosphere soil to determine IAA production. Salkowski reagent was used to identify the secretion of IAA by the bacterial cultures. A colour change from pale yellow to pink is an indication of IAA production by the capability of bacterial isolates. The intensity of the colour confirms high concentration of IAA (Figure 1) present in the sample. This is due to the IAA produced by bacteria is released into the medium at a continuous slow phase. The Bacillus strain was then subjected to various morphological and biochemical tests (Table 1). The presence of Bacillus safensis strain YKS2 (Gen Bank No. MH539636) was confirmed by 16S rRNA gene sequence analysis, which shared 99- 100% similarity with Bacillus safensis. The phylogenetic tree was constructed by neighbour-joining approach (Figure 2) using Kimura 2-parameter (K2P) model by MEGA 6.0 software.
Table (1):
Biochemical tests for Bacillus strain.
| Biochemical Test | Observations |
|---|---|
| Strain YKS2 | |
| Methyl red test | Negative |
| Voges Proskauer test | Positive |
| Citrate utilization test | Positive |
| Catalase test | Positive |
| Oxidase test | Positive |
Figure 1. Bacterial strains of Bacillus sp., isolated from rhizosphere soil having the ability to produce IAA in high quantities.
Figure 2. Neighbour-Joining Tree represents the phylogenetic placement of the YKS2 strain IAA with similar reference strains.
Optimization of IAA Production
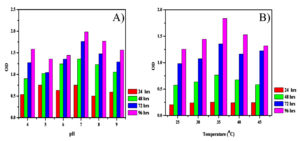
Production of IAA by B. safensis strain YKS2 was optimized at various pH (4 to 9) values, and temperatures (25, 37, 40 and 45°C) to find the maximum production conditions. From the results, it was inferred that the optimal pH was 7.00 and the optimal temperature was 37°C (Figure 3). The IAA produced by B. safensis incubated at 37°C in an orbital shaker at 150 rpm after 24, 48, 72 and 96 h was quantified. After incubation, the samples were taken from flask and centrifuged at 8000 rpm for 15 min. The concentration of IAA was determined by Salkowski reagent.
Figure 3. Effects of different parameters A) pH and B) temperature on the IAA producing YKS2 strain.
Extraction of Crude IAA
Solvent extraction technique was used to extract IAA from the medium. Ethyl acetate was found to be the best performing extraction solvent. The dry crystals were then dissolved in methanol and stored for further analysis.
Characterization of IAA
Thin Layer Chromatography (TLC) Analysis
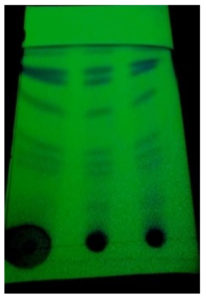
Ethyl acetate extract of IAA was subjected to TLC analysis using methanol: chloroform mixture at various ratios such as 9:1, 8:2 and 7:3 as a solvent system. In the Iodine vapor saturated tank, the 7:3 ratio solvent system formed a simple band separation clearly visible under UV light. The Rf value was determined for the separated fractions (Fraction 1- Rf = 3.4/5.7 = 0.59649 cm, Fraction 2- Rf = 4.8/5.5 = 0.87273 cm and Fraction 3- Rf = 3.8/5.6 = 0.67857 cm). The fractions were classified as phenolic (Rf value = 0.54 and 0.74) compounds based on the obtained Rf values (Figure 4).
Figure 4. TLC to analyze the band separation in the solvent system of chloroform: methanol (ratio of 9:1, 8:2 and 7:3).
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
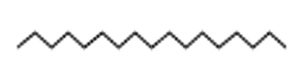
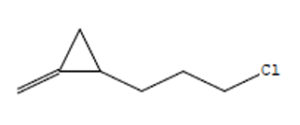
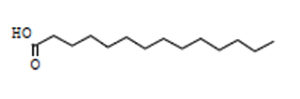
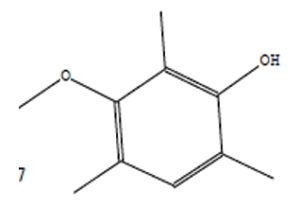
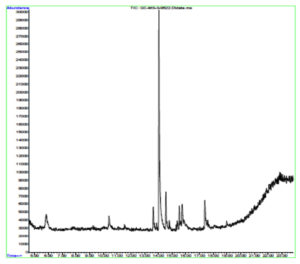
Indole acetic acid is crucial for plant growth. Its additional supply from rhizosphere bacteria can enhance a plant’s defence systems against abiotic as well as biotic stresses. The GC-MS chromatogram of chloroform extracts of IAA produced seven peaks and showed the existence of thirty-six phytochemical compounds. Out of them, five were identified as major compounds in heptadecane. The results showed that B. safensis strain YKS2 produced 85.70±3.55 µg/mL IAA in the culture medium (Figure 5 and Table 2).
Table (2):
Major compounds identified by Gas Chromatography-Mass Spectrometry (GC-MS).
Retention Time |
Molecular formula |
Molecular weight |
Compound name |
Structure |
|---|---|---|---|---|
23.70 |
C17H36 |
240 |
Heptadecane |
|
30.98 |
C7H11Cl |
130 |
1-(3-Chloropropyl)-2-Methyle |
|
36.86 |
C14H28O2 |
228 |
Tetradecanoic Acid |
|
20.50 |
C10H14O2 |
166 |
Phenol, 3-Methoxy-2,4,6-Trime |
|
16.17 |
C13H13N3O |
227 |
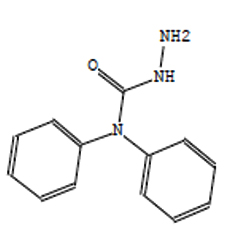
Hydrazinecarboxamide,N,N- |
|
12.17 |
C14H25F3O2 |
282 |
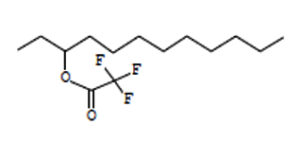
3-Trifluoroacetoxydodecane |
|
8.38 |
C14H28 |
196 |
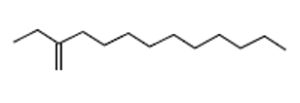
1-Dodecene, 2-Ethyl- |
Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
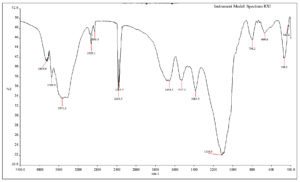
Indole acetic acid produced by B. safensis strain YKS2 was extracted, subjected to FTIR analysis and compared with the spectrum of pure indole acetic acid. Peaks corresponding to C=C, NH, C=O and C-OH vibrations were observed in the IR spectrum of the sample (Figure 6). The characteristic N-H (indole) stretching was found at 3855 cm-1. The bending and wagging vibrations of the N-H bond were detected at 1654 cm−1 and 669 cm-1, respectively. Indole ring stretching (C-H) bond was recorded at 1110 cm-1. The C=O stretching peak was observed around 1700 cm-1. The peak at 1384 cm-1 is due to the C-OH stretching. Thus, the FTIR spectrum confirms the presence of IAA.
Response to Plant Growth
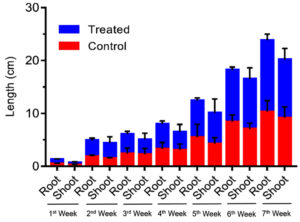
The plant Vigna radiata was exposed to different concentrations of IAA. The initial and the final growth readings were recorded at concentrations of 250, 500, 750 and 1000 ppm. The results showed that IAA enhanced plant growth (Figure 7 and 8) in terms of the shoot length (23.3 cm) and root length (7.4 cm). Thus, it can be concluded that IAA plays a major role in promoting plant growth.
Figure 7. Effect of different concentrations of B. safensis on plant growth before drought stress (upper panel) and (lower panel) 7 weeks and effect of IAA on plant growth in terms of root and shoot elongation.
Indole acetic acid is a vital member of the plant hormone group and is generally regarded as a significant natural auxin.28 It acts as an essential signal molecule for plant growth regulators, cell proliferation, cell differentiation, cell division and gene regulation.29 Various endophytic bacteria are capable of producing the plant hormone IAA and they develop auxins in the presence of active precursors like L-tryptophan. In the present study, thirteen bacterial isolates from fifteen plant root samples have been analyzed for IAA production capability.
In the present investigation, the bacteria that produce IAA were isolated from rhizosphere soil from Yercaud hills. The effect of B. safensis strain YKS2 isolate on the root elongation was analyzed using various concentrations of IAA extracts. Indole acetic acid was also identified to be generally released either on the surfaces or within the plant tissues due to the presence of bacterial growth. Tryptophan is a key intermediate in IAA biosynthesis by microorganisms used as a precursor for IAA production.27,30 The Bacillus sp., isolated in the study produced high amount of IAA. The results correlate well with earlier studies on plant growth following inoculation with IAA producing PGPB.
The growth regulatory effects of indole acetic acid (IAA) have been examined at various concentrations and under different circumstances.31 In the results presented here, IAA treatment at different concentrations has shown different levels of plant growth in both root and the shoot. In some cases, IAA treatment has shown reduced plant growth in a number of plant species, including flax. Ethylene biosynthetic pathway might be one of the primary suspect,32 in these cases. Xylem or outer tissue radii of the plant has shown significant improvement due to IAA treatment. These results are in correlation with some reported cases where exogenous IAA treatments stimulate xylem expansion, including one study in flax.33,34 Treatment conditions such as stems decapitation and defoliation to impair endogenous hormone biosynthesis also plays a role in plant growth promotion. Indole acetic acid has shown significant increase in xylem fiber cell wall thickness. Therefore, the IAA extracts at various concentrations used in this study have shown significant biological responses. Preliminary separation and identification of phenolic compounds, flavonoids, tannins and saponins in alcoholic and water extracts of IAA were performed using chromatography.
A previous study15 demonstrated that the inoculation of Rhodobacter sp., strongly improved the morphological features of cucumber plants due to the production of IAA by the bacterial species. A report by Ahmad et al.35 stated that the Azotobacter sp., and Pseudomonas sp., produced high IAA levels when they grow in a nutrient broth amended by tryptophan with 2 and 5 mg/mL. Azospirillum sp., is one of the most examined producers of IAA among the PGPR species. The bacterial species belonging to various genera such as Aeromonas sp., Azotobacter sp., Bacillus sp., Burkholderia sp., Pseudomonas sp., and Rhizobium sp.,36-40 have been isolated from rhizosphere soils. The IAA-producing PGPR bacterial inoculation promotes stem germination and increases root biomass.
The bacteria associated with plants play a vital role in regulating plant physiology. Plant growth promoting bacteria can offer growth-enhancing properties leading to high production and ensuring food and environmental safety by minimizing the application of harmful agrochemicals.41 The synthesis of IAA in the rhizosphere by bacteria is an important factor in plant development.7 In this study, tryptophan (5 mg/mL) was used as a precursor for IAA production. One of the most valuable compounds is IAA, which is a key plant-growth regulator and signaling molecule in the plant response/adaptation to environmental stresses. IAA is produced by the interplay of enzymes that mediate multiple interconnected biosynthesis pathways. The results demonstrated that the plant growth regulators provided by Bacillus sp., may also play an important role in promoting plant development.42,43
The rhizosphere based bacterial IAA production is an essential property that impacts on the growth of plants. Therefore, the present investigation aimed to evaluate the IAA production by B. safensis strain YKS2 was confirmed to produce regulators for plant growth which can also play a crucial role in promoting plant growth.
In this present study, the maximum IAA yield was obtained (85.70±3.55 µg/mL) at pH 7 (83%) and temperature 37°C (91%) by isolated Bacillus safensis strain YKS2. It is also evident that B. safensis strain YKS2 has emerged as a novel alternate source for the production of IAA, which could aid the development of root and shoot biomass in crops. Therefore, it can also be used as an effective bio-inoculant for promoting plant growth. More research is needed to further examine the rhizosphere-based bacterial IAA production and to study its effect on numerous crop plants that are exposed to both the biotic and abiotic factors in the field.
ACKNOWLEDGMENTS
The authors would like to thank Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia for their support and encouragement in conducting the research and publishing this report.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RL and DK conceptualized the study. DK and PS wrote the original draft. MMP, KP and SV reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Dhungana SA, Itoh K. Effects of Co-Inoculation of Indole-3-Acetic Acid-Producing and-Degrading Bacterial Endophytes on Plant Growth. Horticulturae. 2019;5(1):17.
Crossref - Chauhan A, Shirkot CK, Kaushal R, Rao DLN. Plant growth-promoting rhizobacteria of medicinal plants in NW Himalayas: current status and future prospects. In: Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants. Springer, Switzerland. 2015; 381-412.
Crossref - Dawwam GE, Elbeltagy A, Emara HM, Abbas IH, Hassan MM. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann Agric Sci. 2013;58(2):195-201.
Crossref - Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr. 2013;13(3):638-649.
Crossref - Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31(4):425-448.
Crossref - Mujahid TY, Subhan SA, Wahab A, Masnoon J, Ahmed N, Abbas T. Effects of different physical and chemical parameters on phosphatesolubilization activity of plant growth promoting bacteria isolated fromindigenous soil. J Pharm Nutr Sci. 2015;5(1):64-70.
Crossref - Silpa D, Brahmaji Rao P, Kranthi Kumar G, Raghu Ram M. Plant Growth Promoting Characteristics of Bacillus Licheniformis DS3 Isolated from Agriculture Field Soil. IJSRST. 2018;4:2395-6011.
- Chandra S, Askari K, Kumari M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J Genet Eng Biotechnol. 2018;16(2):581-586.
Crossref - Li M, Guo R, Yu F, et al. Indole-3-acetic acid biosynthesis pathways in the plant-beneficial bacterium Arthrobacter pascens ZZ21. Int J Mol Sci. 2018;19(2):443.
Crossref - Das S, Nurunnabi TR, Parveen R, et al. Isolation and Characterization of Indole Acetic Acid Producing Bacteria from Rhizosphere Soil and their Effect on Seed Germination. Int J Curr Microbiol Appl Sci. 2019;8(3):1237-1245.
Crossref - Karnwal A, Dohroo A. Effect of maize root exudates on indole-3-acetic acid production by rice endophytic bacteria under influence of L-tryptophan. F1000 Research. 2018;7:112.
Crossref - Susilowati DN, Riyanti EI, Setyowati M, Mulya K. Indole-3-acetic acid producing bacteria and its application on the growth of rice. AIP Conference Proceedings. 2018;1:020016.
Crossref - Nutarata P, Monprasit A, Srisuk N. High-yield production of indole-3-acetic acid by Enterobacter sp. DMKU-RP206, a rice phyllosphere bacterium that possesses plant growth-promoting traits. 3 Biotech. 2017;7:305.
- Mujahid MD, Sasikala CH, Ramana CV. Production of indole-3-acetic acid and related indole derivatives from L-tryptophan by Rubrivivax benzoatilyticus JA2. Appl Microbiol Biotechnol. 2011;89(4):1001-1008.
Crossref - Kang SM, Shahzad R, Bilal S, et al. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019;19(1):80.
Crossref - Liu WH, Chen FF, et al. Indole-3-Acetic Acid in Burkholderia pyrrocinia JK-SH007: Enzymatic Identification of the Indole-3-Acetamide Synthesis Pathway. Front Microbiol. 2019;10:2559.
Crossref - Bertrand H, Nalin R, Bally R, Marel JCC. Isolation and identification of the most efficient plant growth promoting bacteria associated with canola. Biol Fertil Soils. 2001;33(2):152-156.
Crossref - Cappuccino JG, Sherman N. Instructor’s Guide for Microbiology: a laboratory Manual. Benjamin/ Cummings Publishing Company. 1996.
- Kalaimurugan D, Balamurali krishnan B, Durairaj K, et al. Isolation and characterization of heavy-metal-resistant bacteria and their applications in environmental bioremediation. Int J Environ Sci Technol. 2020;17(1):1455-1462.
Crossref - Loper JE, Schroth MN. Influence of bacterial source of indole-3-acetic acid of root elongation of sugar beet. Phytopathology. 1986;76(4):386-389.
Crossref - Sarwart M, Arshad M, Matens DA, Frankenberger WT. Tryptophan-dependent biosynthesis of auxins in soil. Plant and Soil. 1992;147(2):207- 215.
Crossref - Brick JM, Bostock RM, Silverstones SE. Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol. 1991;57(2):535-538.
- Bentley JA. Analysis of plant hormones. Methods Biochem Anal. 1962;9:75-125.
Crossref - Cheng H, Chen J, Chen S, Wu D, Liu D, Ye X. Characterization of aroma-active volatiles in three Chinese bayberry (Myrica rubra) cultivars using GC-MS-olfactometry and an electronic nose combined with principal component analysis. Food Research International. 2015;72:8-15.
Crossref - Pratheeba T, Ragavendran C, Natarajan D. Larvicidal, pupicidal and adulticidal potential of Ocimum gratissimum plant leaf extracts against filariasis inducing vector. Int J Mosq Res. 2015;2(2):1-8.
- Kumar BS, Prabakaran G. Production of IAA (bioplastics) using bioeffluent as substrate by Alcaligenes eutrophs. Indian Journal of Biotechnology. 2006;5:76-79.
- Khan AL, Halo BA, Elyassi A, et al. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solarium lycopersicum. Electronic Journal of Biotechnology. 2016;19(3):58-64.
Crossref - Kloepper JW, Ryu CM, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus sp. Phytopathology. 2004;94(11):1259-1266.
Crossref - Ryu RJ, Patten CL. Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by TyrR in Enterobacter cloacae UW5. J Bacteriol. 2008;190(21):7200-7208.
Crossref - Barazani OZ, Friedman J. Is Indole Acetic Acid the major root growth factor secreted from plant-growth mediating bacteria. J Chem Ecol. 1999;25:2397-2406.
Crossref - Kizhakedathil MPJ. Rhizoshpheric bacteria isolated from the agricultural fields of Kolathur, Tamilnadu promotes plant growth in mustard plants. Biocatal Agric Biotechnol. 2018;16:293-302.
Crossref - Ayala-Silva T, Akin D, Foulk J, Dodd RB. Effect of two growth regulators on yield and fiber quality and quantity in flax (Linum usitatissimum L.). Plant Growth Regulation Society of America Quarterly. 2005;33:90-100.
- McKenzie RR, Deyholos MK. Effects of plant growth regulator treatments on stem vascular tissue development in linseed (Linum usitatissimum L.). Industrial Crops and Products. 2011;34(1):1119-1127.
Crossref - El Shourbagy MN, Ghaffar A, El Naggar RA. Effect of IAA and GA3 on the anatomical characteristics, straw and fiber yield and quality of flax. Journal of Agronomy and Crop Science. 1995;174(1):21-26.
Crossref - Ahmad F, Ahmad I, Khan MS. Indole acetic acid production by the indigenous isolates of Azotobacter and pseudomonas fluorescent in the presence and absence of tryptophan. Turkish Journal of Biology. 2005;29:29-34.
- Dobbelaere S, Croonenborghs A, Thys A, Vande B, Vanderleyden J. Phytostimulatory effect of Azospirillum brasilence wild type and mutant strains altered in IAA production on wheat. Plant and Soil. 1999;212:153-162.
Crossref - Halda-Alija L. Identification of indole-3-acetic acid producing freshwater wetland rhizosphere bacteria associated with Juncus effuses L. Can J Microbiol. 2003;49(12):781-787.
Crossref - Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163(2):173-181.
Crossref - SwainMR, Naskar SK, Ray RC. Indole-3-acetic acid production and effect on sporuting of yam (Dioscorea rotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol J Microbiol. 2007;56:103-110.
- Shoebitz M, Ribaudo CM, Pardo MA, Cantore ML, Ciampi L, CuraJA. Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lolium perenne rhizosphere. Soil Biol Biochem. 2009;41(9):1768-1774.
Crossref - Ghosh S, Penterman J, Little RD, Chavez R, Glick BR. Three newly isolated plant growth-promoting bacilli facilitate the seedling growth of Canola, Brassica Campestris. Plant Physiol Biochem. 2003;41:277-281.
Crossref - Dhara PS, Chaudhari HG, Kasture VM, Dhavale DD, Chopade BA. Isolation and characterization of indole acetic acid producing Klebsiella pneumonia strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J Exp Biol. 2009;993-1000.
- LuZX, Song W. Research of indole-3-acetic acid biosynthetic pathway of Klebsiella oxytoca SG-11by HPLC and GC-MS. SePu. 2000;18(4): 328.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.