ISSN: 0973-7510
E-ISSN: 2581-690X
The optimization of β-glucan production by Ophiocordyceps dipterigena BCC 2073 was carried out. For the three-step optimization performed in 250-mL Erlenmeyer flasks, at the first step, the maximum β-glucan production of 16.48±0.60 g/L was obtained using molasses and malt extract. During the second step, we sought to find an alternative carbon source; the highest β-glucan production of 16.96±1.33 g/L was obtained using yeast extract and hydrolyzed cassava starch. The third step involved the optimization of nitrogen sources, and the highest β-glucan content (18.16±0.15 g/L) was obtained with yeast extract. To reduce the use of yeast extract for β-glucan production, a fractional factorial design at 2 levels was applied after the three-step optimization to reduce the concentration of yeast extract by combination with other suitable inorganic nitrogen sources. The medium consisting of 2.5 g/L yeast extract as a sole nitrogen source yielded 24.65±2.21 g/L β-glucan production. At the last step of optimization, the highest β-glucan production of 35.77±3.01 g/L was obtained in the medium consisting of 68.8 g/L hydrolyzed cassava starch, 2.62 g/L molasses and 2.5 g/L yeast extract using central composite design. The evaluation of β-glucan production was carried out in a 5-L bioreactor, and 76.87±0.96 g/L of material was obtained.
Ophiocordyceps dipterigena, β-glucan, Fractional factorial design, Central composite design, Biopolymer.
β-glucans are homopolysaccharides that are composed of glucose and b-glycosidic bond linkages. They are usually found in a variety of species, including in the cell wall and endosperm of plants1; baker’s and brewer’s yeasts of the Saccharomyces genus2; various species of mushrooms such as shiitake (Lentinus edodes)3, maitake (Grifola frondosa)4 and reishi (Ganoderma lucidum)5; fungi such as Schizophyllan (Schizophyllum commune)6, Sclerotinia sclerotiorum7, Cryptococcus neoformans8, and Cordyceps9; bacteria such as Pneumocystis carinii10; etc. The structure of β-glucans from difference sources can vary in their β-glucan structures. β-glucan in baker’s yeast (Saccharomyces cerevisiae) is found in the cell wall and is composed of b-1,3-, b-1,6- and heterogeneous groups of glucose polymers; it consists of a backbone of b-(1,3)-linked b-D-glucopyranosyl units with b-(1,6)-linked side chains of unstable distribution and length11. Differences in structure can result in challenges for extraction and altered biological activities12. Glucans of high molecular weight can activate leukocytes and stimulate phagocytic, cytotoxic, and antimicrobial activities as well as the production of reactive oxygen species (ROS). In contrast, low-molecular weight glucans demonstrate reduced cellular effects, and very short glucans are considered to be inactive13. β-glucan can be used in many applications such as in the pharmaceutical14, 15, medical, food and petroleum industries16. Most β-glucans are cellular components, which require complicated downstream and purification processes, resulting in high cost. Only a few reports of large-scale extracellular β-glucan production exist, and this study is the first report of high extracellular β-glucan production by O. dipterigena BCC 2073 in low-cost media and in large-scale production.
β-glucan produced by O. dipterigena BCC 2073 is an extracellular product and is a potent wound-dressing material due to its appropriate biological and physiological properties. The material is biocompatible and noncytotoxic, with no oral toxicity. Both β-glucan and the fungal mycelium are strong inducers of interleukin-8 (IL-8), a cytokine responsible for enhancing the wound healing process14, 15. The material is also proven to function as a prebiotic in poultry feed17. The exobiopolymer is composed of a (1®3)-b-D-glucan backbone, substituted at O-6 with side chains of (1®6)-b-D-pyranosyl units, and contains 1.86% arabinose, 29.08% mannose, 25.86% galactose and 43.05% glucose (14, 15). The molecular weight of this β-glucan is in the range of 6.3 x 105–7.7 x 105 Da, and the highest reported production yield (41.2 g/L) thus far was obtained using glucose and malt extract18. The generated β-glucan was resistant to hydrolysis by both hydrochloric acid and porcine pancreatic alpha-amylase19. β-glucan from O. dipterigena BCC 2073 is an extracellular major product that is secreted out into the fermentation medium, which enables simpler downstream purification processes. Hence, this study aimed to optimize the use of low-cost media, followed by scale-up in a 5-L bioreactor.
Inoculum preparation
O. dipterigena BCC 2073 was grown initially on potato dextrose agar (PDA) (Difco, Becton, Dickinson and company, MD, USA) at 25°C for 5–7 days. An agar block (1cm3) containing the growing culture was cut into small pieces and transferred to 25 mL of potato dextrose broth (PDB) (Difco, Becton, Dickinson and company, MD, USA) in a 250-mL Erlenmeyer flask. This liquid seed culture was incubated for 5–7 days at 25°C on a rotary shaker at a shaking speed of 200rpm (New Brunswick, NJ, USA).
Cassava starch hydrolysis
The cassava starch used in this study was from the local cassava starch company in Thailand, and two commercial enzymes amylase (GC147) and glucoamylase (GC358; Genencor, Danisco US Inc., USA) were used to hydrolyze a 50% W/V solution of cassava starch in a 4-L (2kg of cassava starch: 2L of distilled water) working volume in a 5-L of fermentor. A volume of 400µL of GC147 was added to 2 L of preheated distilled water (90°C), incubated for 4h at 85°C and agitated at a speed of 300 rpm. Then, the temperature was reduced to 65°C, and 400 µL of GC358 was then added to the mixture, which was incubated for 4h at 65°C and agitated at a speed of 300 rpm. The sugar concentration after hydrolysis was then analyzed by HPLC.
Optimization of β-glucan production by O. dipterigena BCC 2073 in 250-mL Erlenmeyer flasks
In this experiment, we first used the concentrations of 60 g/L of carbon source and 14 g/L of nitrogen source reported by Kocharin et al. (18). The homogenized mixture of liquid seed culture grown in PDB medium and 10% (v/v) seed culture of O. dipterigena BCC 2073 was transferred into a 250-mL flask containing 50 mL of production media in duplicate (60 g/L carbon source, 14 g/L nitrogen source, 0.5 g/L KH2PO4, 0.2 g/L K2HPO4, 0.2 g/L MgSO4×7H2O, 0.14 g/L MnSO4×H2O, 1 mL/L trace element solution (trace elements consisted of 14.3 g/L ZnSO4×H2O, 2.5 g/L CuSO4×5H2O, 0.5 g/L NiCl2×6H2O and 13.8 g/L FeSO4×H2O) and 1 mL/L vitamin solution (Blackmores, NSW, Australia)). In the first step, the carbon sources tested were glucose, hydrolyzed cassava starch and molasses, whereas the nitrogen sources tested were malt extract, yeast extract, ammonium dihydrogenphosphate, diammonium hydrogen phosphate, ammonium nitrate, diammonium sulfate and diammonium hydrogen citrate. The cultures were then incubated for 7 days at 25°C on a rotary shaker at a shaking speed of 200rpm (New Brunswick, NJ, USA). The 60 g/L concentration for hydrolyzed cassava starch was calculated from its glucose composition, and 1L of hydrolyzed cassava starch contained 450g of glucose. The 60 g/L molasses concentration was calculated from its glucose, sucrose, fructose composition, and 1L of molasses contained 86 g of glucose, 263g of sucrose, and 100g of fructose.
In the second optimization step, the sugars in the malt extract were supplemented with 5.88 g/L maltose, 1.26 g/L glucose, 0.14 g/L sucrose and 0.17 g/L fructose, and 6.5 g/L of yeast extract was used as the sole nitrogen source, with either molasses or hydrolyzed cassava starch as the carbon source.
In the 3rd step, the selected carbon source of hydrolyzed cassava starch was tested in combination with 6 nitrogen sources (yeast extract, NH4H2PO4, (NH4)2HPO4, NH4NO3, (NH4)2SO4, and (NH4)2HC6H5O7) with the 7.5 g/L sugar supplement (5.88 g/L maltose, 1.26 g/L glucose, 0.14 g/L sucrose and 0.17 g/L fructose) at a reduced nitrogen source concentration (6.5 g/L).
Optimization of β-glucan production by O. dipterigena BCC 2073 in 250-mL Erlenmeyer flasks using two-level fractional factorial design
In this experiment, the use of a lower concentration of yeast extract in combination with an inorganic nitrogen source (NH4H2PO4, (NH4)2HPO4, NH4NO3, (NH4)2SO4, (NH4)2HC6H5O7, was studied. The best nitrogen source in the previous experiment was yeast extract, but, due to its high cost, we sought to minimize the amount of yeast extract added using two-level fractional factorial design. A two-level fractional factorial design of 2n-1 was applied for the 6 selected factors (g/L) of yeast extract, NH4H2PO4, (NH4)2HPO4, NH4NO3, (NH4)2SO4, (NH4)2HC6H5O7 in the presence of 60 g/L hydrolyzed cassava starch and 7.5 g/L supplemented sugar (5.88 g/L maltose, 1.26 g/L glucose, 0.14 g/L sucrose and 0.17 g/L fructose).
Optimization of β-glucan production by O. dipterigena BCC 2073 in 250-mL Erlenmeyer flasks using a central composite design
Molasses was then substituted by 3 sugars (glucose, 0.14 g/L sucrose and 0.17 g/L fructose), and maltose was substituted by 5.88 g/L hydrolyzed cassava starch. The central composite design was applied at the last step of optimization to maximize β-glucan production. The actual design consisted of -1 actual, +1 coded (i.e., 2.5, 6.5 g/L yeast extract; 6, 12 g/L hydrolyzed cassava starch and 1.5, 7.5 mL/L molasses). Yeast extract, hydrolyzed cassava starch and molasses were selected as the most influential factors. All results were analyzed with Design Expert software (Version 7.0.b1.1, Stat-Ease Inc., Minneapolis, USA).
Evaluation of β-glucan production by O. dipterigena BCC 2073 in a 5-L fermentor
A culture of O. dipterigena BCC 2073 was maintained on potato dextrose agar (PDA). Fifty milliliters of potato dextrose broth (PDB) in a 250-mL Erlenmeyer flask was used for inoculum preparation. The medium used in a 5-L fermentor consisted of 2.5 g/L yeast extract, 68.98 g/L hydrolyzed cassava starch, 2.62 g/L molasses, 0.5 g/L KH2PO4, 0.2 g/L K2HPO4, 0.2 g/L MgSO4×7H2O, 0.14 g/L MnSO4×H2O, 1 mL/L trace element solution (trace elements consisted of 14.3 g/L ZnSO4×H2O, 2.5 g/L CuSO4×5H2O, 0.5 g/L NiCl2×6H2O and 13.8 g/L FeSO4×H2O) and 1 mL/L vitamin solution (Blackmores, NSW, Australia). The culture was agitated at 300 rpm and aerated at 1 vvm, but pH was not controlled. During cultivation, fermentation broths were collected at 0 to 10 days.
Biomass determination
Approximately 30mL of culture broth was centrifuged at 10,000 rpm for 10 min and supernatant was removed. Fresh mycelium was then resuspended in 20 to 30mL of normal saline and filtered through a Whatman No.1 filter paper. The filter cake was washed with distilled water and dried at 105–110°C for 24 to 48h until a stable weight was achieved. Culture filtrate was subjected to sugar analysis and EPS extraction18.
β-glucan purification
The culture filtrate was mixed with four volumes of 95% ethanol, stirred vigorously for 10 to 15 min and stored at 20°C for at least 12h. Precipitated polymer was sedimented at 10,000rpm for 20 min and lyophilized. β-glucan was redissolved in distilled water, and any insoluble material was removed by centrifugation at 10,000 g for 20 min. The supernatant was then dialyzed (2 kDa molecular weight cut off, Spectrum Laboratories, California, USA) against 4L of distilled water for 24h and lyophilized18.
Sugar determination
For the measurement of the sugar content (glucose, fructose, sucrose and maltose), the supernatant was centrifuged at 10,000g for 10min and filtered through 0.22-µm filter paper. The filtrate was subjected to HPLC analysis using a Sugar-Pak column (Waters, MA, USA) at 90°C, with water as the mobile phase at a flow rate of 0.6 mL·min-1. Sugars were detected refractometrically (Waters 410 Differential Refractometer Detector, Millipore Corp., Milford, MA, USA).
Three-step optimization of media for β-glucan production by O. dipterigena BCC 2073 in 250-mL Erlenmeyer flasks
For the first optimization step, we sought select optimal carbon and nitrogen sources at the concentrations of 60 g/L and 14 g/L, respectively.18 Two other alternative carbon sources (molasses and hydrolyzed cassava starch) were investigated in this study. Six alternative nitrogen sources (yeast extract, NH4H2PO4, (NH4)2HPO4, NH4NO3, (NH4)2SO4, (NH4)2HC6H5O7) were evaluated in addition to malt extract (Table 1). The maximum β-glucan production of 16.48±0.60 g/L was obtained using molasses and malt extract, while the maximum biomass of 16.32±3.96 g/L was obtained with hydrolyzed cassava starch and NH4H2PO4. A β-glucan production yield of 15.69±0.01 g/L was obtained using hydrolyzed cassava starch as the sole carbon source and malt extract as the sole nitrogen source. Alternative carbon sources for β-glucan production by other fungi in previous studies using molasses, potato starch waste hydrolysate, soy molasses and sugar cane have been found to result in lower yields.9,20-24 The optimization of β-glucan production by fungi given inorganic and organic nitrogen sources of peptone, yeast extract, malt extract soybean powder, bran, ammonium sulfate, ammonium phosphate, ammonium tartrate, ammonium chloride, ammonium nitrate sulfate ammonium, urea, glycine and glutamic acid have also been reported.24,25 Furthermore, Grifola frondosa was observed to stimulate enzyme metabolism of soy molasses and sugar cane responsible for β-glucan production.22 Ganoderma lucidum exhibited a-phosphoglucomutase activity that affected β-glucan accumulation by influencing flux from glucose 6-phosphate to β-glucan during the synthesis process.26 In this study, the alternative carbon sources of molasses and hydrolyzed cassava starch were investigated, and the best alternative carbon for β-glucan production was molasses (16.48±0.60 g/L). The best nitrogen source was malt extract, but its use resulted in β-glucan of a brownish color. This study demonstrated that hydrolyzed cassava starch and NH4H2PO4 enhanced biomass production of O. dipterigena BCC 2073 (16.32±3.96 g/L). A similar result has been reported for β-glucan production by Lyophyllum decastes, with the study authors demonstrating that various carbon sources differentially affected mycelial growth and β-glucan production;24 entomopathogenic fungi and mushrooms can grow on a wide range of carbon sources.27 The results indicated that malt extract was the best nitrogen source for β-glucan, which may have been because malt extract is composed of maltose, glucose, sucrose, and fructose, all of which can serve as extra carbon sources for fungal growth and β-glucan production. However, the maximum yields of mycelium and β-glucan were dependent on the carbon source and varied from species to species. Exopolysaccharides from mushrooms contain a wide variety of types of sugars of differing molecular weights.14
Table (1):
β-glucan production by O. dipterigena BCC 2073 using different carbon and nitrogen sources in 250-mL Erlenmeyer flasks (50 mL of medium).
| Carbon source (60 g/L) | Nitrogen source (14 g/L) | β-glucan (g/L) | Biomass (g/L) |
|---|---|---|---|
| Glucose (control) | Malt extract (control) | 19.66±1.28 | 6.67±0.17 |
| Molasses | Malt extract | 16.48±0.60 | 5.04±0.35 |
| Yeast extract | 1.26±0.28 | 5.34±0.45 | |
| NH4H2PO4 | 0 | 4.90±0.26 | |
| (NH4)2HPO4 | 0 | 4.32±0.25 | |
| NH4NO3 | 1.15±0.03 | 6.61±0.18 | |
| (NH4)2SO4 | 1.81±0.11 | 5.88±0.06 | |
| (NH4)2HC6H5O7 | 1.63±0.24 | 5.07±0.09 | |
| Hydrolyzed cassava starch | Malt extract | 15.69±0.01 | 4.01±2.84 |
| Yeast extract | 5.81±0.00 | 8.82±1.29 | |
| NH4H2PO4 | 6.08±0.03 | 16.32±3.96 | |
| (NH4)2HPO4 | 3.61±0.03 | 7.74±1.64 | |
| NH4NO3 | 1.00±0.00 | 4.99±1.24 | |
| (NH4)2SO4 | 1.89±0.00 | 6.31±2.80 | |
| (NH4)2HC6H5O7 | 5.3±0.01 | 11.45±4.14 |
For the second optimization step, although malt extract was found to be the best nitrogen source for growth and β-glucan production by O. dipterigena BCC 2073, malt extract is a high-cost nitrogen source, so this optimization step was performed using yeast extract as a sole nitrogen source to replace malt extract. Many studies have been performed using organic nitrogen sources for mycelial growth and exobiopolymer production by fungi and mushrooms.9,28-31 In this experiment, the nitrogen sources were reduced from 14 to 6.5 g/L and supplemented with 7.5 g/L of sugars present in malt extract (maltose 5.88 g/L, glucose 1.26 g/L, sucrose 0.14 g/L and fructose 0.17 g/L). Approximately 60 g/L of molasses or hydrolyzed cassava starch was used as the carbon source. The results comparing β-glucan production by O. dipterigena BCC 2073 fed molasses or hydrolyzed cassava starch were analyzed and are shown in Table 2.
Table (2):
Optimization of the second step in β-glucan production by O. dipterigena BCC 2073 using different carbon sources supplemented with extra sugars in 250-mL Erlenmeyer flasks (50 mL of medium).
Carbon source |
Nitrogen source |
β-glucan (g/L) |
Biomass (g/L) |
|---|---|---|---|
Glucose (control) |
Malt extract (control) |
14.79±0.62 |
4.68±0.94 |
Molasses* 60 g/L |
Yeast extract
6.5 g/L |
10.81±0.21 |
15.04±0.38 |
Hydrolyzed cassava starch* 60 g/L |
Yeast extract
6.5 g/L |
16.96±1.33 |
30.41±1.49 |
*Supplemented sugar: 5.88 g/Lmaltose, 1.26 g/L glucose, 0.14 g/L sucrose and 0.17 g/Lfructose
The highest β-glucan production of 16.96±1.33 g/L was obtained using yeast extract and hydrolyzed cassava starch. β-glucan production yields of 14.79±0.62 g/L and 10.81±0.21 g/L were obtained with glucose (control) and molasses, respectively. The biomass production yields of 30.41±1.49, 15.04±0.38 and 4.68±0.94 g/L were obtained for hydrolyzed cassava starch, molasses and glucose, respectively. Hence, hydrolyzed cassava starch was the best alternative carbon source for β-glucan biosynthesis by O. dipterigena BCC 2073, which might be because glucose present in hydrolyzed cassava starch is the main precursor of β-glucan production. Although, yeast extract, hydrolyzed cassava starch and other four sugars (maltose 5.88 g/L, glucose 1.26 g/L, sucrose 0.14 g/L and fructose 0.17 g/L) resulted in the highest β-glucan production, yeast extract is not a low-cost nitrogen source. Thus, the third optimization step investigated the use of lower cost nitrogen sources. In the comparison of organic and inorganic nitrogen sources for the selection of low-cost nitrogen sources, hydrolyzed cassava starch supplemented with four additional sugars were used as the carbon source. The production media were composed of 60 g/L hydrolyzed cassava starch, 6.5 g/L nitrogen source (yeast extract, NH4H2PO4, (NH4)2HPO4, NH4NO3, (NH4)2SO4, or (NH4)2HC6H5O7), four extra sugars (5.88 g/L maltose, 1.26 g/L glucose, 0.14 g/L sucrose and 0.17 g/L fructose). The highest β-glucan yield of 18.16±0.15 g/L was obtained with yeast extract (Table 3).
Table (3):
Third optimization of β-glucan production by O. dipterigena BCC 2073 using different nitrogen sources in 250-mL Erlenmeyer flasks (50 mL of medium).
| Carbon source | Nitrogen source | β-glucan (g/L) | Biomass (g/L) |
|---|---|---|---|
| Glucose (control) | Malt extract (control) | 11.61±0.24 | 3.34±0.06 |
| Hydrolyzed* cassava starch
60 g/L |
Yeast extract | 18.16±0.15 | 17.19±0.28 |
| NH4H2PO4 | 0 | 27.23±0.15 | |
| (NH4)2HPO4 | 0 | 6.13±0.08 | |
| NH4NO3 | 0 | 23.32±0.12 | |
| (NH4)2SO4 | 0 | 6.89±0.10 | |
| (NH4)2HC6H5O7 | 10.62±0.02 | 24.79±0.56 |
*Supplemented sugar: 5.88 g/L maltose, 1.26 g/L glucose, 0.14 g/L sucrose and 0.17 g/L fructose
Yeast extract favored β-glucan production by O. dipterigena BCC 2073, and we also found that inorganic nitrogen sources of (NH4)2HC6H5O7 yielded β-glucan production at 10.62±0.02 g/L, while biomass production values of 27.23±0.15, 24.79±0.56, 23.32±0.12, 17.19±0.28, 6.89±0.10, 6.13±0.08 and 3.34±0.06 g/L were obtained using NH4H2PO4, (NH4)2HC6H5O7, NH4NO3, yeast extract, (NH4)2SO4 (NH4)2HPO4 and malt extract, respectively. NH4H2PO4 provided the highest mycelial growth among all of the inorganic nitrogen sources used in this study, but the maximum β-glucan production was obtained using yeast extract. This finding is similar to that of Fan et al32, who reported that yeast extract is a better nitrogen source for β-glucan production (0.189 g/L) by Agaricus brasiliensis than (NH4)2SO4, KNO3, urea or peptone. Furthermore, yeast extract resulted in higher β-glucan but lower mycelial growth, whereas NH4H2PO4 generated higher mycelial growth but not higher β-glucan production. He et al.28 reported that, compared to inorganic nitrogen sources, organic ones yielded relatively lower mycelial growth but higher β-glucan production. The third optimization step results indicated that yeast extract is a better nitrogen source for β-glucan production by O. dipterigena BCC 2073, which may be because yeast extract is composed of growth factors and other materials that support the growth of and β-glucan production by the fungus.
Optimization of β-glucan production in 250-mL Erlenmeyer flasks using two-level fractional factorial design
The three-step optimization of β-glucan production by O. dipterigena BCC 2073 in 50 mL of media revealed that yeast extract and hydrolyzed cassava starch were the best nitrogen and carbon sources, respectively. To reduce the use of yeast extract for β-glucan production, a fractional factorial design at 2 levels were then applied to replace yeast extract with combinations of other suitable inorganic nitrogen sources. The influence of 6 quantitative factors (NH4H2PO4, (NH4)2HPO4, NH4NO3, (NH4)2SO4, (NH4)2HC6H5O7 and yeast extract) on β-glucan production and biomass production of O. dipterigena BCC 2073 were evaluated using a fractional factorial design at 2 levels (coded -1 and +1) with a center point (coded 0) (Table 4). A total of 33 conditions with a control were tested for their ability to support β-glucan production by O. dipterigena BCC 2073. From the results, modified medium No. 1 yielded β-glucan production comparable to the control medium (24.65±2.21 and 26.30±1.63 g/L, respectively). Medium No. 1 contained 2.5 g/L of yeast extract as a sole nitrogen source. The second best modified medium was medium No. 4, which contained a combination of the three nitrogen sources NH4H2PO4, (NH4)2HPO4, and yeast extract, and yielded 20.67±0.76 g/L of β-glucan. Media No. 12, 18, 25 and 26 produced β-glucan at 7 to 9 g/L, and the lowest levels of β-glucan production were obtained using media 6, 17, 20, 21, 24 and 30. For the determination of accuracy of the results, variations of the two responses were evaluated with 32 run duplicates and 3 at the center (67-run data are shown in Table 5). For β-glucan production, the mean was 4.17, standard deviation was 0.9 and coefficient of variation was 21.54%. These results indicate that the coefficient of variation was low and that the accuracy was high. A statistical analysis of the results allowed the determination of their significance and of the experimental equation that associated the variables with the results. The complete factorial analysis allowed evaluation of each variable (A, B, C, D, E, F) and also of their interactions (AB, AC, AD, AE, AF, BC, BD, BE, BF, CD, CE, CF, DE, DF, EF). The following equation gives the mean, the coefficient of influence for each factor and the interaction effect coefficients. The model for β-glucan production with different factors using two-level fractional factorial design is shown in equation 1.
β-glucan (g/L) = 1.17 + 0.27A – 0.25B – 1.12C + 0.51D – 1.51E + 0.68F + 0.18AB -0.08AC + 0.55AD + 0.75AE + 0.5AF + 1.2BC + 0.84BD – 0.24BE + 0.25BF + 0.41CD + 0.6 CE + 0.9DE + 0.40DF + 0.08EF …(1)
where A=NH4H2PO4, B=(NH4)2HPO4, C=NH4NO3, D=(NH4)2SO4, E=(NH4)2HC6H5O7 and F= yeast extract.
Table (4):
β-glucan and biomass production by O. dipterigena BCC 2073 using a two-level fractional factorial design with 6 factors (2n-1).
| No. | A | B | C | D | E | F | β-glucan (g/L) | Biomass (g/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 | 2.5 | 24.65±2.21 | 30.29±3.18 |
| 2 | 7.5 | 0 | 0 | 0 | 0 | 6.5 | 5.43±0.59 | 25.32±1.06 |
| 3 | 0 | 7.5 | 0 | 0 | 0 | 6.5 | 5.72±0.18 | 23.21±0.35 |
| 4 | 7.5 | 7.5 | 0 | 0 | 0 | 2.5 | 20.67±0.76 | 20.67±1.31 |
| 5 | 0 | 0 | 7.5 | 0 | 0 | 6.5 | 3.29±0.20 | 21.48±0.66 |
| 6 | 7.5 | 0 | 7.5 | 0 | 0 | 2.5 | 0 | 20.50±0.62 |
| 7 | 0 | 7.5 | 7.5 | 0 | 0 | 2.5 | 4.66±0.021 | 21.44±1.33 |
| 8 | 7.5 | 7.5 | 7.5 | 0 | 0 | 6.5 | 3.94±0.51 | 23.64±0.61 |
| 9 | 0 | 0 | 0 | 7.5 | 0 | 6.5 | 5.50±0.44 | 18.88±0.02 |
| 10 | 7.5 | 0 | 0 | 7.5 | 0 | 2.5 | 4.45±0.16 | 19.74±1.38 |
| 11 | 0 | 7.5 | 0 | 7.5 | 0 | 2.5 | 5.68±0.68 | 21.70±0.74 |
| 12 | 7.5 | 7.5 | 0 | 7.5 | 0 | 6.5 | 8.98±1.44 | 23.01±0.45 |
| 13 | 0 | 0 | 7.5 | 7.5 | 0 | 2.5 | 2.61±0.20 | 22.90±0.69 |
| 14 | 7.5 | 0 | 7.5 | 7.5 | 0 | 6.5 | 3.56±0.10 | 24.87±2.53 |
| 15 | 0 | 7.5 | 7.5 | 7.5 | 0 | 6.5 | 5.17±0.32 | 24.51±1.43 |
| 16 | 7.5 | 7.5 | 7.5 | 7.5 | 0 | 2.5 | 5.11±0.85 | 26.81±3.32 |
| 17 | 0 | 0 | 0 | 0 | 7.5 | 6.5 | 0 | 20.48±0.58 |
| 18 | 7.5 | 0 | 0 | 0 | 7.5 | 2.5 | 7.76±0.10 | 23.48±0.72 |
| 19 | 0 | 7.5 | 0 | 0 | 7.5 | 2.5 | 1.66±01.4 | 25.55±1.10 |
| 20 | 7.5 | 7.5 | 0 | 0 | 7.5 | 6.5 | 0 | 24.54±1.26 |
| 21 | 0 | 0 | 7.5 | 0 | 7.5 | 2.5 | 0 | 21.36±0.23 |
| 22 | 7.5 | 0 | 7.5 | 0 | 7.5 | 6.5 | 3.43±0.24 | 26.53±0.23 |
| 23 | 0 | 7.5 | 7.5 | 0 | 7.5 | 6.5 | 1.04±0.30 | 27.08±0.41 |
| 24 | 7.5 | 7.5 | 7.5 | 0 | 7.5 | 2.5 | 0 | 24.56±0.62 |
| 25 | 0 | 0 | 0 | 7.5 | 7.5 | 2.5 | 7.78±0.48 | 30.6±1.94 |
| 26 | 7.5 | 0 | 0 | 7.5 | 7.5 | 6.5 | 6.98±0.35 | 31.64±0.35 |
| 27 | 0 | 7.5 | 0 | 7.5 | 7.5 | 6.5 | 1.23±0.28 | 23.51±4.84 |
| 28 | 7.5 | 7.5 | 0 | 7.5 | 7.5 | 2.5 | 5.99±1.32 | 21.64±0.84 |
| 29 | 0 | 0 | 7.5 | 7.5 | 7.5 | 6.5 | 1.98±0.02 | 19.44±0.56 |
| 30 | 7.5 | 0 | 7.5 | 7.5 | 7.5 | 2.5 | 0 | 21.08±2.19 |
| 31 | 0 | 7.5 | 7.5 | 7.5 | 7.5 | 2.5 | 2.14±0.52 | 22.99±1.41 |
| 32 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 6.5 | 4.07±3.69 | 25.61±2.20 |
| 33 | 3.75 | 3.75 | 3.75 | 3.75 | 3.75 | 4.5 | 0.49±0.84 | 26.22±0.68 |
| 60 g/L glucose, 14 g/L malt extract | 26.30±1.63 | 14.03±3.25 | ||||||
A: ammonium dihydrogenphosphate, B: diammonium hydrogen phosphate, C: ammonium nitrate, D: diammonium sulfate, E: diammonium hydrogen citrate, F: yeast extract
Table (5):
Analysis of variance (ANOVA) for two-level fractional factorial design (2n-1) of β-glucan production by O. dipterigena BCC 2073.
| Source | Sum of Squares | df | Mean Square | F-Value | Probability (p) > F |
|---|---|---|---|---|---|
| Model* | 1311.64 | 32 | 40.989 | 50.89 | < 0.0001 |
| Pure Error | 27.38 | 34 | 0.805 | ||
| Corrected Total | 1339.02 | 66 | |||
R2 = 0.98, Adj. R2 = 0.96, Std. Dev = 0.9, C.V.%= 21.54, *significant
The quantity of yeast extract (A) was the strongest positive influence on β-glucan production and ranged from 2.5 to 6.5 g/L. The interactions observed in the experimental domain (from -1 to +1) were limited, except for the negative interaction between the quantity of yeast extract and 5 nitrogen sources on β-glucan production. A similar experiment reported that yeast extract was the best material for β-glucan production (8.68 g/L) by Agaricus at low concentration (3 g/L) using Plackett-Burman design for optimization (32). The optimal media component for exopolysaccharide gellan gum (43.6 g/L) production by Sphingomonas paucimobilis ATCC 31461 was also reported to be a low concentration of 0.25 g/L yeast extract, as determined using an L16-orthogonal array (33). Additionally, the maximum yield for exopolysaccharide (3.64 g/L) production by Boetus spp. ATCC 50328 was obtained with a high concentration of yeast extract (14 g/L) combined with 2.7 g/L (NH4)2SO4 using Plackett-Berman design analysis (34). Optimal exopolysaccharide production (3.4 g/L) by Cordyceps militaris NG3 at a low concentration of nitrogen source (1.03 g/L corn steep powder) using Plackett-Burman design has also been reported35.
Optimization of β-glucan production by O. dipterigena BCC 2073 using central composite design (CCD) in 250 mL Erlenmeyer flasks
In this experiment, optimizations were performed for yeast extract concentration; molasses was substituted with three extra sugars (glucose 1.26 g/L, sucrose 0.14 g/L and fructose 0.17 g/L); and hydrolyzed cassava starch was used to replace the 5.88 g/L maltose found in the malt extract. Central composite design (CCD) was used to identify the optimal concentrations of these significant factors and to understand the relationship between the various factors and
β-glucan production by O. dipterigena BCC 2073. A set of CCD was 16 runs and 6 replicates of center points, and all experiments were carried out in triplicate. The CCD was applied to the concentrations of nitrogen sources and carbon source optimization for β-glucan production by O. dipterigena BCC 2073 (Table 6), and the production media contained 60 g/L hydrolyzed cassava starch. A β-glucan production yield of 25.70±0.85 g/L and biomass production of 15.42±0.38 g/L were obtained with the control medium. Medium 9, composed of 1.87 g/L yeast extract, 9 g/L hydrolyzed cassava starch and 4.5 g/L molasses, gave the highest β-glucan production of 35.77±3.01 g/L and the highest biomass of 21.94±0.42 g/L, and no β-glucan production was obtained using media 5, 6, 7, 8 or 14. At low concentration of 1.87 g/L , yeast extract yielded the highest β-glucan production. The ANOVA results for the model were highly significant (Table 7), as evidenced by the very elevated model F-value (123.64) but very low p-value (p <0.0001). The optimal conditions in this experiment for β-glucan production by O. dipterigena BCC 2073 were 2.5 g/L yeast extract, 68.98 g/L hydrolyzed cassava starch and 2.62 g/L molasses, which were selected for further evaluation in a 5-L bioreactor. The model of β-glucan production with different factors using central composite design is shown in equation 2.
β-glucan (g/L) = 28.93-4.18A+0.17B-9.71C+0.44AB+0.33AC-0.35BC+0.77A2+1.15B2-9.33C2 …(2)
where A=yeast extract, B=hydrolyzed cassava starch and C=molasses.
Table (6):
β-glucan and biomass production by O. dipterigena BCC 2073 using central composite design (CCD).
| No. | A (g/L) | B (g/L) | C (mL/L) | β-glucan (g/L) | Biomass (g/L) |
|---|---|---|---|---|---|
| 1 | 2.5 | 6 | 1.5 | 23.60±3.88 | 24.28±1.17 |
| 2 | 6.5 | 6 | 1.5 | 20.54±0.50 | 27.96±0.65 |
| 3 | 2.5 | 12 | 1.5 | 23.23±0.66 | 24.0±1.06 |
| 4 | 6.5 | 12 | 1.5 | 23.70±1.44 | 23.27±1.63 |
| 5 | 2.5 | 6 | 7.5 | 0 | 1.89±0.15 |
| 6 | 6.5 | 6 | 7.5 | 0 | 1.23±0.22 |
| 7 | 2.5 | 12 | 7.5 | 0 | 1.47±0.73 |
| 8 | 6.5 | 12 | 7.5 | 0 | 1.67±0.29 |
| 9 | 1.87 | 9 | 4.5 | 35.77±3.01 | 21.94±0.42 |
| 10 | 7.13 | 9 | 4.5 | 24.76±0.96 | 18.72±0.59 |
| 11 | 4.5 | 5.05 | 4.5 | 30.70±3.37 | 20.84±0.26 |
| 12 | 4.5 | 12.95 | 4.5 | 31.16±4.21 | 20.88±1.08 |
| 13 | 4.5 | 9 | 0.6 | 25.56±3.48 | 31.85±2.59 |
| 14 | 4.5 | 9 | 8.4 | 0 | 2.30±0.43 |
| 15 | 4.5 | 9 | 4.5 | 29.19±2.02 | 24.42±0.98 |
| 16 | 4.5 | 9 | 4.5 | 28.68±2.00 | 22.24±1.52 |
| 17 | 60 g·L-1 glucose, 14 g·L-1 malt extract | 25.70±0.85 | 15.42±0.38 | ||
A: yeast extract, B: hydrolyzed cassava starch, C: molasses
Table (7):
Analysis of variance (ANOVA) for central composite design of β-glucan production by O. dipterigena BCC 2073.
Source |
Sum of Squares |
df |
Mean Square |
F-Value |
Probability (p) > F |
|---|---|---|---|---|---|
Model* |
8114.59 |
14 |
579.61 |
123.64 |
< 0.0001 |
Pure Error |
154.7 |
33 |
4.69 |
||
Corrected Total |
8269.29 |
47 |
R2 = 0.98, Adj. R2 = 0.97, Std. Dev = 2.17, C.V.%= 11.67, *significant
Production of β-glucan by O. dipterigena BCC 2073 in a 5-L fermentor
The medium used in the 5-L fermentor consisted of 68.98 g/L hydrolyzed cassava starch; 2.62 g/L molasses; 2.5 g/L yeast extract; and 0.5 g/L salts, minerals and vitamins. The culture was agitated at 300rpm and aerated at 1 vvm, but pH was not controlled. The time profile of β-glucan production by O. dipterigena BCC 2073 in the 5-L fermentor is shown in Figure 1. A maximum β-glucan production yield of 76.87±0.96 g/L and biomass production of 25.6±3.68 g/L were obtained at 288 h. In comparison, Kocharin et al. (18) obtained a maximum β-glucan production of 41.2 g/L at 377h when the fungi was grown on 60 g/L glucose and 14 g/L malt extract. The higher β-glucan content may have been affected by the high viscosity of the medium; sugar uptake may have occurred at a slower rate over the longer cultivation time, creating difficulties in mixing that resulted in a low rate of oxygen transfer (data not shown). The production medium in this experiment generated a higher amount of β-glucan (76.87±0.96 g/L) than that predicted from CCD, which may be due to aeration and temperature control in the fermentor supporting β-glucan production, compared to growth in the 250mL Erlenmeyer flask. This finding was similar to that of Park et al. (36), who demonstrated the effects of the aeration rate on mycelial morphology and obtained maximum exopolysaccharide production (14.5 g/L) by C. militaris using an aeration rate of 2 vvm. An exopolysaccharide production of 29 g/L by Aureobasidium pullulans was obtained at 84 h in a 1-L batch fermentation at high concentration (165.73 g/L) of sucrose combined with low (0.71 g/L) Ashbya gossypii extract37
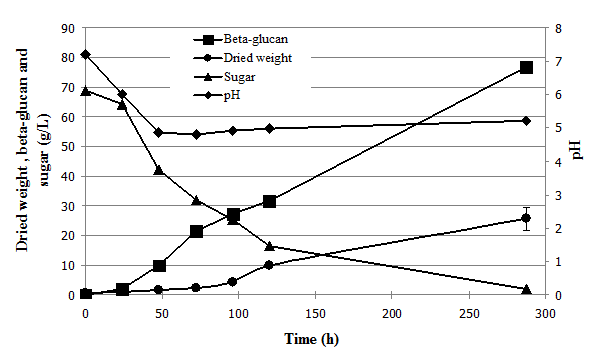 Fig. 1.Time profile of growth and β-glucan production by O. dipterigena BCC 2073 in a 5-L fermentor.
Fig. 1.Time profile of growth and β-glucan production by O. dipterigena BCC 2073 in a 5-L fermentor. After the implementation of three-step optimization, two-level fractional factorial design and central composite design for β-glucan production optimization by O. dipterigena BCC 2073 in low-cost media, we obtained β-glucan production yields 1.9-fold higher than those of a previous study and 4.5-fold higher than that obtained after the first optimization step, while also reducing the production time from 377 to 288h. The cost of media was lowered from $30–40 to $2 per kg of dried product, compared with glucose- and malt extract-based media. This high level of extracellular β-glucan production facilitates the use of downstream processing and avoids the need for extraction from cells. This is the first report of high-yield extracellular β-glucan production by O. dipterigena BCC 2073 obtained using low cost media and with subsequent β-glucan characterization. The material’s structure was identified as (1, 3)-b-D-glucan with 0-6 side chains, high branching, and high molecular weight in the range of 6.3 x 105–7.7 x 105 Da, making this β-glucan suitable for use in a wide range of food, agricultural, medical, pharmaceutical, cosmetic and petrochemical applications in the near future.
ACKNOWLEDGMENTS
This research work was financially supported by the Agricultural Research and Development Agency (ARDA), Ministry of Agriculture and Cooperatives (MOAC), Thailand.
- Donot, F., Fontana, A., Baccou, J. C., Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym., 2012, 87, 951-962.
- Manners, D. J., Fleet, G. H. Isolation and composition of alkali soluble glucan from cell walls of Saccharomyces cerevisiae. J. Gen. Microbiol., 1976, 94, 180-192.
- Shida, M., Matsuda, K. Studies on polysaccharides from Lentinus edodes (Shii-ta-ke). Mushroom Sci.1974, 9, 531-539.
- Lei, H., Ma, X., Wu, W. Anti-diabetic effect of a-glucan from fruiting body of maitake (Grifola frondosa) on KK-Ay mice. J. Pharm. Pharmacol., 2007, 59, 575-582.
- Liu, Y., Zhang, J., Tang, Q., Yang, Y., Guo, Q., Wang, Q., Wu, D., Cui, S. W. Physicochemical characterization of a high molecular weight bioactive beta-D-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydr. Polym., 2014, 101, 968-74.
- Zhang, Y., Kong, H., Fang, Y., Nishinari, K., Phillips, G. O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioact. Carbohydr. Dietary Fibre., 2013, 1, 53–71.
- Hashimoto, K., Suzuki, I., Yadomae, T. Oral administration of SSG, a ² -glucan obtained from Sclerotinia sclerotiorum, affects the function of Peyer’s patch cells. Int. J. Immunopharmacol., 1991, 13, 437-442.
- James, P. G., Cherniak, R., Jones, R. G., Stortz, C. A. Cell-wall glucans of Cryptococcus neoformans CAP 67. Carbohydr. Res., 1990, 198, 23-38.
- Kim, H.O., Yun, J. W. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J. Appl. Microbiol., 2005, 99, 728-38.
- Vassallo, R., Standing, J. E., Limper, A. H. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol., 2000, 164, 3755-3763.
- Aimaniada, V., Clavaud, C., Simenel, C., Fontaine, T., Delepierre, M., Latage, J. P. Cell wall (1®6)-b-D-glucan of Saccharomyces cerevisiae – structural characterization and in situ synthesis. J. Biol. Chem., 2009, 284, 13401-13412.
- Bohn, J. A., BeMiller, J. N. (1®3)(1®6)-b-D-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr. Polym., 1995, 28, 3-14.
- Akramiene, D., Kondrotas, A., Didziapetriene, J., Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina., 2007, 43, 597-606.
- Madla, S., Methacanon, P., Prasitsil, M., Kirtikara, K. Characterization of biocompatible fungi-derived polymers that induce IL-8 production. Carbohydr. Polym., 2005, 59, 275-280.
- Methacanon, P., Madla, S., Kirtikara, K., Prasitsil, M. Structural elucidation of bioactive fungi-derived polymers. Carbohydr. Polym., 2005, 60, 199-203.
- Kokub, D. Production of biopolymers by indigenous fungal strains of Sclerotium rolfsii and their possible use in petroleum industry. PhD thesis. Arid Agricultural University Rawalpindi, Pakistan. 2008.
- Prathumpai, W., Rachtawee, P., Khajeeram, S. Potential of fungal exopolysaccharide as novel source for prebiotic supplement to broiler chicken diet. Indian J. Anim. Sci., 2015, 85, 1362–1369.
- Kocharin, K., Rachathevee, P., Sanglier, J.J., Prathumpai, W. Exobiopolymer production of Ophiocordyceps dipterigena BCC 2073: optimization, scaling-up, and characterization. BMC Biotechnol., 2010, 10, 51.
- Prathumpai, W., Rachathewee, P., Khajeeram, S., Sanglier, J.J., Tanjak, P., Methacanon, P. Optimization, characterization and in Vitro evaluation of entomopathogenic fungal exopolysaccharides as prebiotic. Adv. Biochem., 2013, 1, 13-21.
- Barnett, C., Smith, A., Scanlon, B., Israilides, C. J. Pullulan production by Aureobasidium pullulans growing on hydrolysed potato starch waste. Carbohydr. Polym., 1999, 38, 203-209.
- Cha, S. H., Lim, J. S., Yoon, C. S., Koh, J. H., Chang, H. I., Kim, S. W. Production of mycelia and exo-biopolymer from molasses by Cordyceps sinensis 16 in submerged culture. Bioresour. Technol., 2007, 98, 165-8.
- Chimilovski, J. S., Habu, S., Teixeira, R. F. B., Soccol, A. T., Noseda, M. D., Medeiros, A. B. P., Pandey, A., Soccol, C. R. Antitumour activity of Grifola frondosa exopolysaccharides produced by submerged fermentation using sugar cane and soy molasses as carbon sources. Food Technol. Biotechnol., 2011, 49, 359–363.
- Cho, E. J., Oh, J. Y., Chang, H. Y. Yun, J. W. Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. J. Biotechnol., 2006, 127, 129-140.
- Pokhrel, C. P., Ohga, S. Submerged culture conditions for mycelial yield and polysaccharides production by Lyophyllum decastes. Food Chem., 2007, 105, 641-646.
- Opens overlay Koichi Hashimoto, Opens overlay Iwao Suzuki, Opens overlay Toshiro Yadomae25. Wu, J., Ding, Z. Y., Zhang, K. C. Improvement of exopolysaccharide production by macro-fungus Auricularia auricula in submerged culture. Enz. Microb. Technol., 2006, 39, 743-749.
- Tang, Y. J., Zhong, J. J. Exopolysaccharide biosynthesis and related enzyme activities of the medicinal fungus, Ganoderma lucidum, grown on lactose in a bioreactor. Biotechnol. Lett., 2002, 24, 1023-1026.
- Yang, L.Y., Huang, W. J., Hsieh, H.G., Lin, C. Y. H1-A extracted from Cordyceps sinensis suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. J. Lab. Clin. Med., 2003, 141, 74-83.
- He, P., Geng, L., Wang, Z., Mao, D., Wang, J., Xu, C. Fermentation optimization, characterization and bioactivity of exopolysaccharides from Funalia trogii. Carbohydr. Polym., 2012, 89, 17-23.
- Mahapatra, S., Banerjee, D. Optimization of a bioactive exopolysaccharide production from endophytic Fusarium solani SD5. Carbohydr. Polym., 2013, 97, 627-634.
- Shrestha, B., Han, S.K., Sung, J.M., Sung, G.H. Fruiting Body Formation of Cordyceps militaris from Multi-Ascospore Isolates and Their Single Ascospore Progeny Strains. Mycobiol., 2012, 40, 100-6.
- Tayuan, C., Tannock, G.W., Rodtong, S. Growth and exopolysaccharide production by Weissella sp. from low-cost substitutes for sucrose. Afr. J. Microbiol. Res., 2011, 5, 3693-3710.
- Fan, L., Soccol, A. T., Pandey, A., Ricardo-Soccol, C. Effect of nutritional and environmental conditions on the production of exo-polysaccharide of Agaricus brasiliensis by submerged fermentation and its antitumor activity. LWT – Food Sci. Technol., 2007, 40, 30-35.
- Bajaj, I. B., Saudagar, P. S., Singhal, R. S., Pandey, A. Statistical approach to optimization of fermentative production of gellan gum from Sphingomonas paucimobilis ATCC 31461. J. Biosci. Bioeng., 2006, 102, 150-156.
- Chen, W., Zhao, Z., Chen, S. F., Li, Y. Q. Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol., 2008, 99, 3187-3194.
- Kim, S.W., Xu, C. P., Hwang, H. J., Choi, J. W., Kim, C. W., Yun, J. W. Production and characterization of exopolysaccharides from an enthomopathogenic fungus Cordyceps militaris NG3. Biotechnol Prog., 2003, 19, 428-35.
- Park, J. P., Kim, S. W., Hwang, H. J., Yun, J. W. Optimization of submerged culture conditions for the mycelial growth and exo-biopolymer production by Cordyceps militaris. Lett. Appl. Microbiol., 2001, 33, 76-81.
- Yoon, S., Hong, E., Kim, S., Lee, P., Kim, M., Yang, H., Ryu, Y. Optimization of culture medium for enhanced production of exopolysaccharide from Aureobasidium pullulans. Bioprocess Biosyst. Eng., 2012, 35, 167-72.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


