ISSN: 0973-7510
E-ISSN: 2581-690X
Dunaliella salina has been widely reported in the halophilic environment. The present study first time reveals and reports the presence of green alga D. salina identified by both morphological and molecular identification from Andaman and Nicobar Islands, a rainy tropical environment. Besides, it was adapted for the optimization of total carotenoid production by using Taguchi tool with various combinations of different parameters, like light intensity, pH, salinity, NaNO3 and NaH2PO4 in basal De Walne’s medium of which 40µEm-2s-1 light intensity showed maximum production of 249.24 µg/mL of total carotenoid followed by NaNO3, NaH2PO4, pH, and salinity. Hence, this study proves that D. salina possesses the character of adapting to the extreme habitats with total carotenoid production.
Dunaliella salina; marine algae; halophilic; b-carotene; Taguchi.
The marine ecosystem is an immense source of potential microorganisms and compounds that holds a wide range of biotechnological applications1-3. In earlier decades scientific community had shown considerable interest on taxonomy, physiology, and metabolism of microalgae. Hence, this paved a way to identify the various potential resources from microalgae. In a present scenario, utilization of microalgae are sprouted more in various industries, such as food, feed, fine chemicals, energy, etc. due to its photosynthetic traits, generation time, the presence of high-value compounds, capability in controlling pollution, etc. Dunaliella is unicellular, biflagellate, naked, motile green alga belongs to the family Polyblepharidaceae inhabits the marine environment and commonly seen in hypersaline environments4,5. This is originally first described and reported from the occurrence in saltern brines by Dunal6 and later discovered by Teodoresco in 19057. Dunaliella varies in morphology and it is well known to show polymorphic character since it lacks cell wall. There is also a need for molecular characterization and to examine the level of carotenoid accumulation, especially under different conditions of growth. Dunaliella salina is reported to yield ~400 mg b-carotene/m2 of cultivation area under ideal conditions8. Dunaliella salina is the richest source of carotenoid, b-carotene6. Dunaliella salina can accumulate b-carotene more than 14% of its dry weight besides producing other carotenoids, like a-carotene and xanthophylls like zeaxanthin, cryptoxanthin and lutein9,10. Natural ג-carotene has high antioxidant, anti-cancer and immune regulations properties when compared to synthetic ג-carotene and it is now produced from Dunaliella on a commercial scale in Australia, USA, Israel, China, Chile, Spain, Kuwait and India.
Andaman and Nicobar Islands (A & N) is located in the eastern part of Bay of Bengal, encompasses 572 islands and are heavily inhabited by rich floral communities ranging from small microalgae to highly evolved floral organisms. A & N has a moderate climate with a temperature ranges from 23 to 31°C but with relatively high humidity (70 to 90 %) coupled with heavy rainfall approximately more than 318 cm/year (http://www.imd.gov.in/pages/main.php). A & N island holds 40% of coral reef coverage of entire Indian coastline, provides shelter for diverse marine creatures11. The present study aims at the quantification and optimization of carotenoid production in the marine microalgae D. salina by Taguchi statistical tool for enhancing the total carotenoids production after identification by 18S rDNA sequencing.
The collection, Purification, and Isolation
Five hundred liters of seawater collected from Minnie Bay (Latitude 11°38’39.50’’ N Longitude 92°42’28.15’’ E), A&N Islands, India were filtered in 20µm plankton net. The soup was kept for enrichment in 250 mL Erlenmeyer flask containing 100 mL De Walne’s medium initially for 5 days. After incubation, 0.1 mL from initial enrichment was inoculated in 5mL test tubes containing 1mL De Walne’s medium12 for 30 days to isolate a Dunaliella sp. Above procedure was repeatedly carried out in triplicates for subsequent times to facilitate isolation of Dunaliella species in seawater. Mixed culture was periodically checked for Dunaliella under microscope, while the diatoms existed in enrichment samples were removed by modified method13 using Germanium dioxide (GeO2) at a different concentration from 5 to 25µg/L. Enrichment sample were diluted up to 1 ׳ 10-6 dilution and 0.1 mL was spread on 2% De Walne’s agar medium. Distinct colonies developed on the plates were made axenic by triple antibiotic treatment14 and transferred to De Walne’s medium and incubated at 24 ± 1°C in a thermostatically controlled area, illuminated through cool fluorescent lamps at irradiance of 30 µEm-1 s-1, under 12 h light /12 h dark photoperiod1.
Identification
Identification of the isolated microalga was done by microscopic observations, such as cell size, shape, colour, cell length (L), width (W), flagella length (F) chloroplast arrangement and growth characteristics (the measurements of length and breadth of the cells were calculated from mean value of 100 cells). Besides, it was also identified using 18S rDNA sequence in order to avoid the ambiguity in identification. Amplification of the 18S rDNA and sequencing PCR amplification was performed on cells taken directly from plates. For PCR, the genomic DNA of the Dunaliella sp. was isolated according to Sambrook et al.15, subjected to amplification with the universal eukaryotic forward primers (5’GTCAGAGGTGAAATTGGATTTA’3) and reverse primer (5’AGGGCAGGGACGTAATCAACG’3) performed as described by Rasoul-Amini et al.16. The amplified product partially sequenced (Applied Bio System Instrument (ABI) Prism 310 Genetic) and resulted sequence was submitted to GenBank, NCBI with the accession number (KP635215).
Growth and Screening of Total carotenoids
The isolated Dunaliella salina was studied for its growth and total carotenoids production under laboratory conditions. Initially, 10 mL of log phase culture of Dunaliella isolates were inoculated in 90 mL of basal medium and kept under 30µEm-2s -1 light intensity, 12 h light / 12 h dark cycle and at 24 ± 1°C. Samples were collected and analyzed for the following parameters at every 3 day interval for the period of 30 days. i) Cell number (Cn) using haemocytometer (Thoma) (log10 cells/mL) and ii) concentrations of pigments viz., Chl a, Chl b and total carotenoid of Dunaliella samples extracted were estimated as per17. Also, cell division rates were calculated during the exponential phase as per the method of18.
Optimization of the total carotenoid production by Taguchi method
With the aim to improve total carotenoids production by Dunaliella sp. AT-JP-13, five decisive parameters in De Walne’s basal medium, such as light intensity, medium initial pH and concentrations of NaH2PO4, NaCl and NaNO3 were optimized using Taguchi optimization method19,20. A 32 runs orthogonal array (L32-OA) design was employed to investigate the effect of selected parameters on total carotenoids production. All selected parameters were studied at 4 different levels. In Taguchi’s method, quality is measured by the deviation of a characteristic from its target value and a loss function [L(y)]. The loss function was calculated by the Eq (1).
L(y) = k * (y – m)2 …(1)
Where k = The proportionality constant,
m = Target value and
y = Experimental value obtained for each trial.
The goal is to accomplish the higher carotenoid production from isolated algae. Such case ‘bigger-is-better’ was chosen. When the ‘bigger-is-better’ quality characteristics the loss function can be written as
L(y) = k * (1/Y2)…(2)
To explore the obtained results, analysis of variance (ANOVA) test was performed. Further conformational experiments were performed in order to validate the optimum conditions. Qulitek-4® software, Nutek Inc. was used for the designing and analysis of experiments21.
Initial screening studies reveals that most of the sample soup contains diatoms, Chlorella and Tetraselmis species, few of them had a trace amount of biflagellate species. Therefore, whichever the samples having biflagellate were taken for enrichment in De Walne’s medium under thermostatically controlled room. In the beginning period of enrichment, medium was mainly dominated by Tetraselmis sp. growth in addition to that of diatoms. Later, it was diminished once it attained 15th day. After 20 days of incubation, the microscopic observation revealed consistent growth of Dunaliella sp. with trace diatoms. Diatoms were effectively removed at 10µg/L of Germanium dioxide. Further, 10-4 dilution has shown the maximum of Dunaliella sp. and colonies were well separated in De Walne’s agar medium. The colonies were picked and transferred to 1 mL of basal medium and further scaled up. The unialgal culture was axenized before taking to optimization study. The cultures were made axenic by triple antibiotic treatment as described previously14.
The isolated cells from the pure culture of Dunaliella sp. were naked, oblong, with two smooth equal long flagella inserted apically; chloroplast shifted towards the basal region, cells with tapering apical region; cell measuring average of 9.85±0.59 (9.5 to 12.0µm) long and 8.19±0.74 (7.0 to 9.5µm) wide respectively (Fig.1). Whereas, flagella length measuring average of 12.86±0.64 (12.0 to 14.0µm) (Fig.1). The organism showed a maximum growth rate of 6.298 log10 cells/mL on 21st day and the division rate was 1.008 per day (Fig. 2). Maximum concentrations of Chl – a, Chl – b, and total carotenoids were 4.42, 2.24 and 6.17µg/mL, respectively, as recorded on 15th, 15th and 30th day, respectively (Fig. 2).
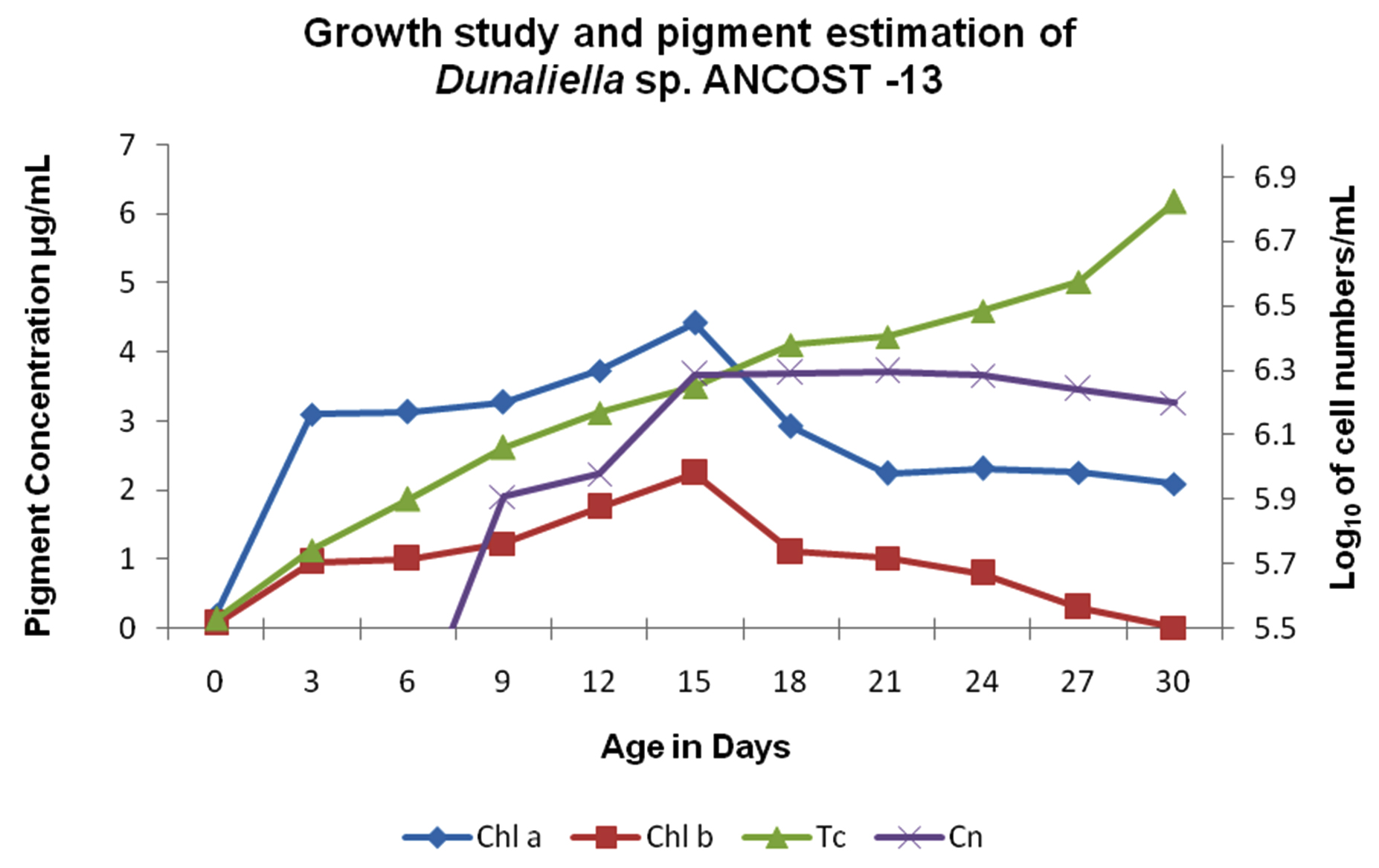 Fig. 2. Growth study and pigment estimation of Dunaliella salina (AT-JP-13). Chl – a: Chlorophyll – a; Chl – b: Chlorophyll – b; Tc: Total carotenoids; Cn: Cell numbers
Fig. 2. Growth study and pigment estimation of Dunaliella salina (AT-JP-13). Chl – a: Chlorophyll – a; Chl – b: Chlorophyll – b; Tc: Total carotenoids; Cn: Cell numbers The PCR amplification of chromosomal DNA of the microalgae with forward and reverse primers revealed efficient amplification. A single band of amplified DNA product of ~700 – bp was recorded by gel documentation system. The amplified product fragment was further purified by PCR purification kit (Fermentos, Spain) and sequence generated was published in NCBI databases. The partial sequence of the isolate AT-JP-13 (KP635215) was 99.13% identical to the consensus sequence of D. salina CCAP 19/12 (KJ756842) D. salina KMMCC 1428 (JQ315781) and D. salina UTEX LB 1644 (DQ009765).
De Walne’s basal medium is an efficient medium for Dunaliella growth and carotenoid production. However the production medium of organism is varying from one species to another species as well as their native conditions. Since this isolated Dunaliella was different from other reported species, the optimization of environmental and nutritional parameters was required in order to increase the total carotenoid productions. Keeping this in view, a 32 runs Taguchi experimental design was employed to optimize the medium as well to understand the interaction and influence of selected parameters.
Table 1 depicts the 5 selected parameters at 4 different levels. Analysis of data yields the individual as well as interaction effect of selected parameters on total carotenoids production. The experimental data revealed that there is a significant variation in carotenoids production observed along with experimental runs. Minimum and maximum carotenoids production values were observed to be 48 and 232.61µg/mL, respectively, under selected conditions of the medium. The observed variation positively shows the imperative role of optimization on all related factors in achieving the best possible yields.
Table (1):
L-32 orthogonal design along with the obtained carotenoids produced by D. salina (AT-JP-13)
S. No |
Light intensity (µE/ m2/S) |
NaH2PO4 concentration (µM) |
NaCl concentration (M) |
pH |
NaNO3 concentration (µM) |
Total carotenoids (µg/ml) |
Log10 of cell numbers/mL |
|---|---|---|---|---|---|---|---|
1 |
20.00 |
0.50 |
0.50 |
7.00 |
0.50 |
48.00 |
5.562 |
2 |
20.00 |
0.75 |
0.75 |
7.50 |
1.00 |
90.46 |
5.672 |
3 |
20.00 |
1.00 |
1.00 |
8.00 |
1.50 |
60.92 |
6.612 |
4 |
20.00 |
1.25 |
1.50 |
8.50 |
2.00 |
108.92 |
6.703 |
5 |
30.00 |
0.50 |
0.50 |
7.50 |
1.00 |
216.00 |
6.12 |
6 |
30.00 |
0.75 |
0.75 |
7.00 |
0.50 |
223.38 |
6.42 |
7 |
30.00 |
1.00 |
1.00 |
8.50 |
2.00 |
134.76 |
6.701 |
8 |
30.00 |
1.25 |
1.50 |
8.00 |
1.50 |
173.53 |
6.412 |
9 |
40.00 |
0.50 |
0.75 |
8.00 |
2.00 |
158.76 |
6.214 |
10 |
40.00 |
0.75 |
0.50 |
8.50 |
1.50 |
195.69 |
6.726 |
11 |
40.00 |
1.00 |
1.50 |
7.00 |
1.00 |
227.07 |
6.705 |
12 |
40.00 |
1.25 |
1.00 |
7.50 |
0.50 |
179.07 |
6.155 |
13 |
50.00 |
0.50 |
0.75 |
8.50 |
1.50 |
168.00 |
5.812 |
14 |
50.00 |
0.75 |
0.50 |
8.00 |
2.00 |
116.30 |
5.962 |
15 |
50.00 |
1.00 |
1.50 |
7.50 |
0.50 |
131.07 |
6.164 |
16 |
50.00 |
1.25 |
1.00 |
7.00 |
1.00 |
153.23 |
6.120 |
17 |
20.00 |
0.50 |
1.50 |
7.00 |
2.00 |
48.00 |
5.550 |
18 |
20.00 |
0.75 |
1.00 |
7.50 |
1.50 |
57.23 |
4.996 |
19 |
20.00 |
1.00 |
0.75 |
8.00 |
1.00 |
86.76 |
4.871 |
20 |
20.00 |
1.25 |
0.50 |
8.50 |
0.50 |
116.30 |
5.136 |
21 |
30.00 |
0.50 |
1.50 |
7.50 |
1.50 |
177.23 |
6.616 |
22 |
30.00 |
0.75 |
1.00 |
7.00 |
2.00 |
136.61 |
6.743 |
23 |
30.00 |
1.00 |
0.75 |
8.50 |
0.50 |
68.30 |
6.456 |
24 |
30.00 |
1.25 |
0.50 |
8.00 |
1.00 |
145.84 |
6.552 |
25 |
40.00 |
0.50 |
1.00 |
8.00 |
0.50 |
217.84 |
6.740 |
26 |
40.00 |
0.75 |
1.50 |
8.50 |
1.00 |
232.61 |
6.686 |
27 |
40.00 |
1.00 |
0.50 |
7.00 |
1.50 |
134.76 |
6.612 |
28 |
40.00 |
1.25 |
0.75 |
7.50 |
2.00 |
114.46 |
6.799 |
29 |
50.00 |
0.50 |
1.00 |
8.50 |
1.00 |
204.92 |
6.495 |
30 |
50.00 |
0.75 |
1.50 |
8.00 |
0.50 |
158.76 |
6.314 |
31 |
50.00 |
1.00 |
0.50 |
7.50 |
2.00 |
73.84 |
6.588 |
32 |
50.00 |
1.25 |
0.75 |
7.00 |
1.50 |
57.23 |
6.476 |
The average effect of factors at the assigned levels on carotenoids production by Dunaliella salina AT-JP-13 is shown in Fig. 3. At individual level the highest effect on carotenoids production was noticed with light intensity at 3rd level (182.53) followed by concentration of NaNO3 at 2nd level (169.61), NaCl concentration at 4th level (157.14) and NaH2PO4 concentration at 1st level (154.84). Among all parameters pH has the least effect on the carotenoids production at 4th level (153.68). The selected variable preferences are in the following order:
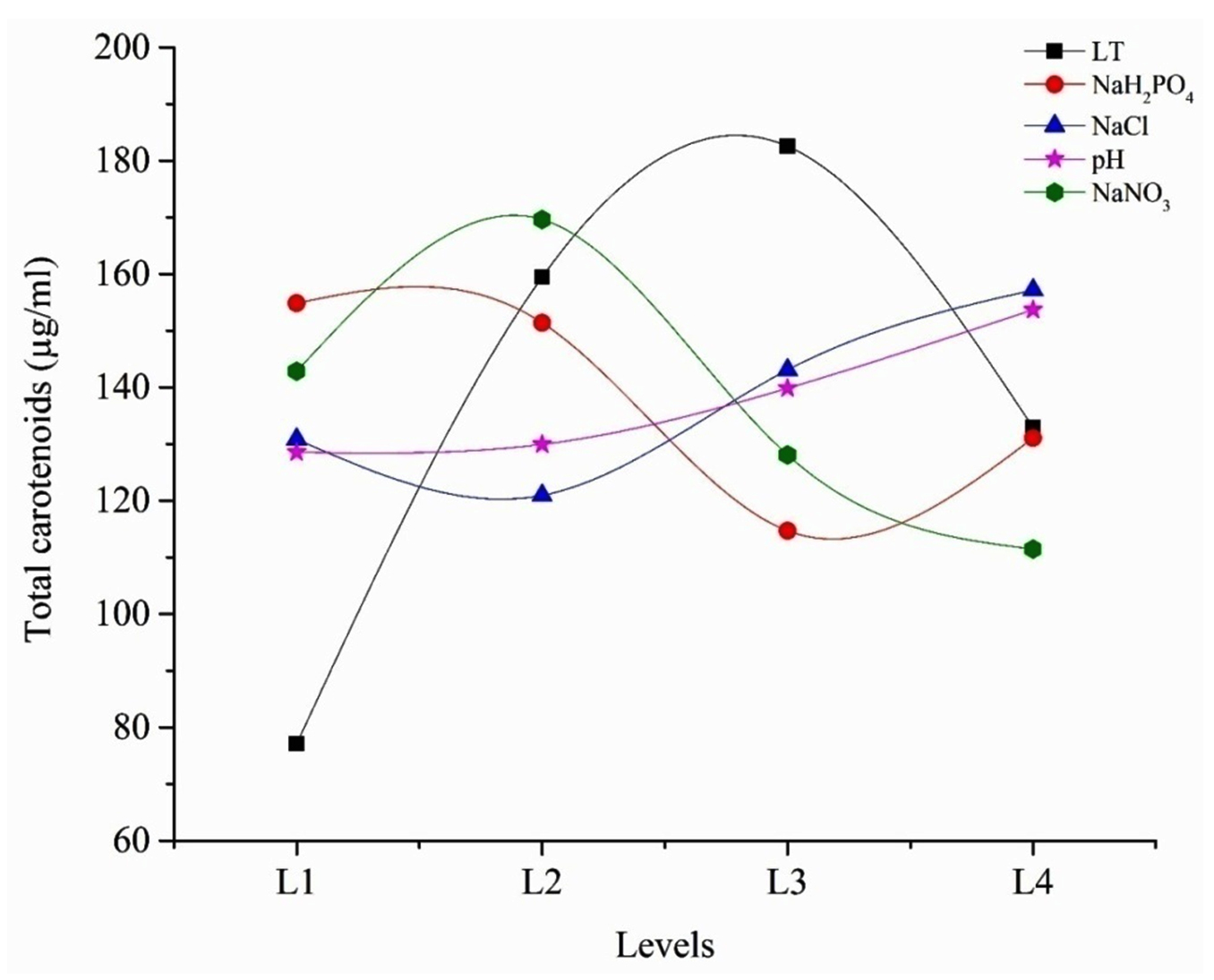 Fig. 3. Influence of the selected parameters at different levels on carotenoids production by isolated algae. (LT = Light intensity)
Fig. 3. Influence of the selected parameters at different levels on carotenoids production by isolated algae. (LT = Light intensity) Light intensity > NaNO3 > NaCl > NaH2PO4 > pH
In Taguchi method, the interaction between two factors was represented by severity index (SI). Table 2 represents the interaction matrix of various factors, calculated by Qualitek-4 program. Table 2 depicts that interaction between NaH2PO4 concentration and pH has highest SI (76.66 %) as compared with others. Least SI was observed with interaction between light intensity and pH (2.77%). The light intensity and NaNO3 concentration have a highest individual effect on carotenoids production; however, their interaction has lesser SI (8.37%).
Table (2):
Interaction influence, ANOVA and optimized conditions for selected factors for carotenoids production by isolated algae (DoF = Degree of freedom; SS = sum of squares)
| LT | NaH2PO4 | NaCl | pH | NaNO3 | others/Error | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interactions | LT | 0 | 12.77 | 12.16 | 2.77 | 8.37 | ||||||||||||
| NaH2PO4 | 12.77 | 0 | 11.37 | 76.66 | 47.53 | |||||||||||||
| NaCl | 12.16 | 11.37 | 0 | 57.55 | 45.67 | |||||||||||||
| pH | 2.77 | 76.66 | 57.55 | 0 | 22.25 | |||||||||||||
| NaNO3 | 8.37 | 47.53 | 45.67 | 22.25 | 0 | |||||||||||||
| ANOVA | DoF | 3 | 3 | 3 | 3 | 3 | 16 | 31 | ||||||||||
| SS | 49450.74 | 8434.556 | 5883.371 | 3234.804 | 14606.38 | -0.001 | 81609.851 | |||||||||||
| Variance | 16483.58 | 2811.518 | 1961.123 | 1078.268 | 4868.792 | -0.001 | ||||||||||||
| F-Ratio | 10.44255 | 1.04405 | 1.08002 | 0.91206 | 2.26866 | |||||||||||||
| Percent contribution | 60.594 | 10.335 | 7.209 | 3.963 | 17.897 | 0.002 | 100 | |||||||||||
| Optimum conditions | Level | 3 | 1 | 4 | 3 | 2 | ||||||||||||
| Level Description | 40 | 0.5 | 1.5 | 8 | 1 | |||||||||||||
| Contribution | 44.537 | 16.848 | 19.153 | 1.843 | 31.615 | 0 | 113.996 | |||||||||||
| Total contribution from all factors 113.996 | ||||||||||||||||||
| Current grand average of performance 137.995 | ||||||||||||||||||
| Expected Results at optimum condition 251.991 | ||||||||||||||||||
To determine the influence of each factor on the production of carotenoids by isolated algae, ANOVA approach was used. Factor with interactions are shown in Table 2. Among all selected factors light intensity has the highest contribution (60.59 %) on overall process. After light intensity, a concentration of NaNO3 and NaH2PO4 has highest effect of 17.89 and 10.33%, respectively. Among all studied parameters, pH has a least contribution on the carotenoids production.
Optimum conditions of selected parameters and their performance in terms of contribution for achieving higher carotenoids yield are shown in table 2. The optimum conditions predicted by the software are light intensity of 40 µEm-1 s-1, 0.5 mM NaH2PO4, 1.5 M NaCl, 1 mM NaNO3 concentration and pH of 8. The predicted carotenoids yield at these optimum conditions is 251.991µg/mL with total contribution from all the factors being 113.996 µg/mL with grand average performance of 137.995 µg/mL. After validating at the predicted conditions, carotenoids production of 249.24µg/mL was achieved. The obtained yield was nearer to the predicted value, which indicates the robustness of employed design.
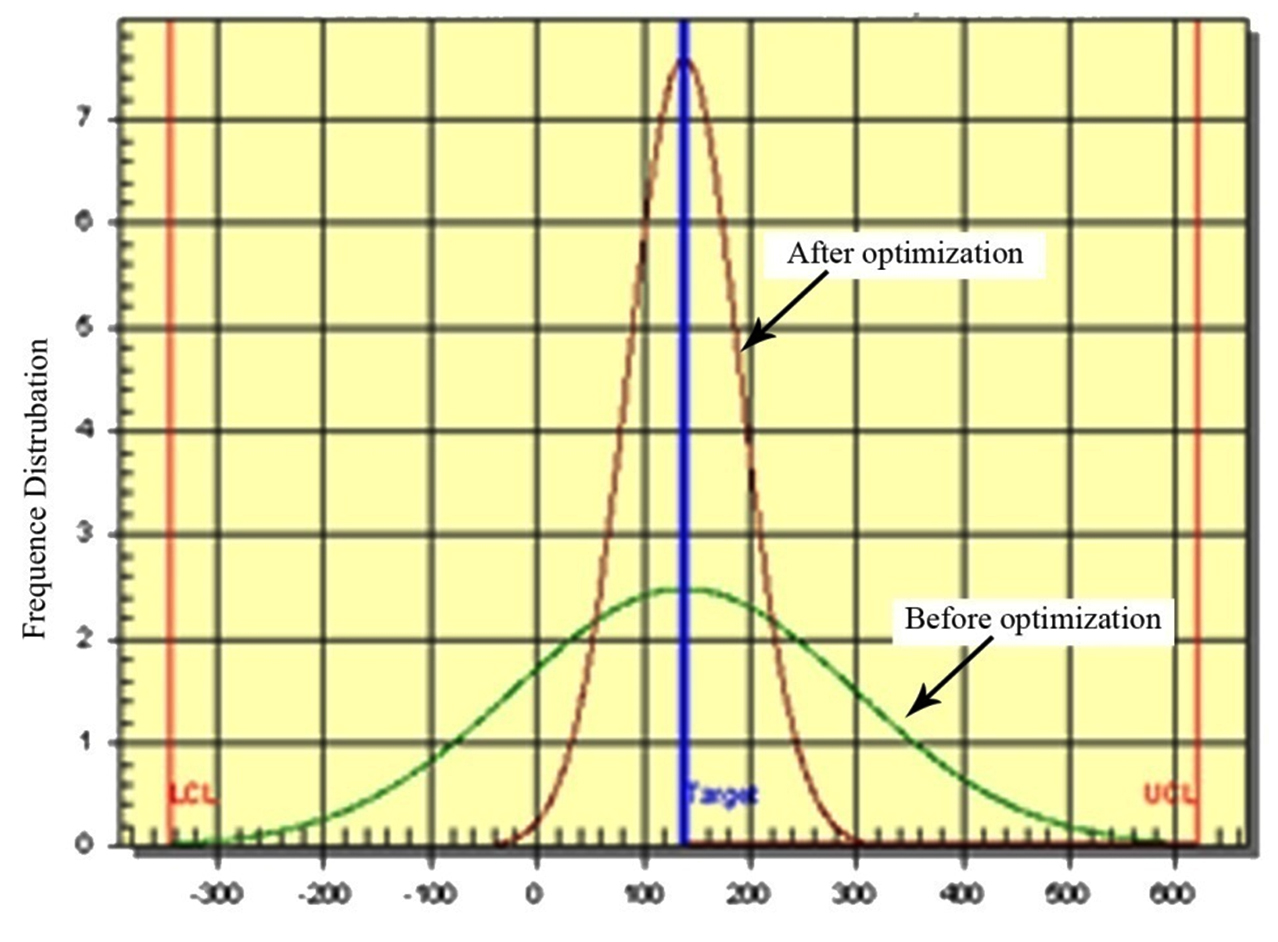
Fig. 4 shows the variation reduction achieved with optimized conditions. The sharp curve indicates the improvement on overall carotenoids production by this algal strain. The improved Signal / Noise ratio at optimum condition depicts the reduction in standard deviation before optimization.
The validation data revealed that with the help of L-32 Taguchi experimentation the culture condition were modified. A 4.2 fold (from 48.00 to 249.24µg/mL) enhancement in the total carotenoids production was observed with altered culture conditions.
Identification of novel species and strains from natural habitats is the primary purpose in the path of obtaining superior productive strains22. Hence, in this study, an attempt was made to isolate a superior strain of Dunaliella from seawater. A&N islands are one of the hot spot of marine biodiversity in the world. Besides, it is not much studied in the context of microalgal and their potentialities because of the wide geographic distribution of saline systems. Marine microorganisms such as bacteria require unique adaptations to survive in these chemically and physically diverse environment leading to extensive species diversity than the terrestrial environment3,23-25. Same way, microalgae also tend to have distinctive adaptations characteristics with unique metabolites for survival26. It is a challenge to isolate the Dunaliella salina from seawater rather than from the samples of salt pans. Various studies conducted on D. Salina so far were mainly collected from hypersaline environments4,27,28.
A large volume of seawater was filtered several times and consistent microscopic observations have been made for effective isolation. During initial period of enrichment up to 15 days, the periodical inspection revealed that the Tetraselmis sp. was found to grow in large number than that of Dunaliella and diatoms. Later the growth was started diminishing because of the high salt concentration in the medium which supported the growth of Dunaliella and avoiding Tetraselmis growth. The optimum growth conditions reported earlier for Tetraselmis were: 20–35% of salinity and temperature ranges from 19 to 21°C29. Several early studies on the effect of germanium dioxide inhibited the growth of mature Chlorophyta and Phaeophyta species13 apart from diatoms; hence the germanium dioxide was taken with limited concentrations in order to prevent the inhibition of target isolate. The present isolate D. salina withstands all studied concentrations except with minimum inhibition at the higher range (25µg/mL). After 20 days of enrichment, Dunaliella occurrence was observed because of their slow adaptation to the medium. Some of the species belongs to this Genera has the tendency to adapt to various environmental conditions like salinity, temperature, heavy metals and pesticides30. The present results once again proved the nature of its adaptations to extreme environments like A & N islands, a rainy tropical climate.
The Present isolate, D. salina that has been isolated from seawater in the salinity ranging from 28-30 ppt (0.5 M), also adapted up to 87.66 ppt (1.5 M) NaCl concentration. An extensive study made by Borowitzka and Siva30 in the taxonomy of this genus revealed that the authors segregated the species D. salina based on the optimum salinity range of > 6%. It is contradictory to the results of the present work. However, it is justified by its extreme adaptability to various environments. In addition, some other species was also isolated during isolation task (data not shown) and considered to be euryhaline species. This was well correlated by the study of Oren7 citing Lerche31 who reported earlier that some of the species of this genus were typically marine organisms and those were never reported in hypersaline environments.
Ambiguity always exists in its identification and physiological characteristics due to its quick adaptation to various environmental conditions and thus the present isolate has its ability to adapt to the different type of environment in A & N islands ecosystem. Influence of salinity on carotenoid biosynthesis and growth pattern in Dunaliella shows different characters in different media32. The different growth patterns exhibited by geographically distinct strains confirm the hypothesis that these algae do not adapt to a specific saline condition, but can tolerate a wide range of salinities33.
The isolate obtained from different environments apart from its native environment necessitates the need to study its growth characteristics and carotenoid production. The cells were shown considerable growth up to 24th day and started to decline and remained green in color and were never shown any carotenogenic phase during the growth study and isolation. Cell size was comparatively larger than the other strain mentioned earlier. Pigments, such as Chl-a and Chl-b were dominant up to 15th day, while the total carotenoids were steadily increased up to 30th day of experiment. It clearly indicates that chlorophyll had contributed in its growth, whereas the total carotenoids showed steady increase even at cessation of growth and protected the cell during the nutrient depletion condition5,34.
As per previous reports, the alga typically did not show any carotenogenic phase until the present study (Fig. 2). It was also subjected to molecular identification to know up to their species level. Moreover, the taxonomy of Dunaliella, particularly the carotenogenic strains, has a history of controversy as stated by Borowitzka9. The author considered that D. bardawil35 as a nomennudum of D. salina Teod, and that D. salina was probably of D. parva. In the present study, the amplified product size obtained by using universal primer suggests that these primers are well conserved for Dunaliella isolated from the natural habitat. The results acquired in the present study are similar to the observations made by16.
Optimization of process and nutrient parameters are essential for enhancing the carotenoids production. Hence L-32 orthogonal array design (Taguchi DOE) was employed. A significant variation of total carotenoid production was observed during experimental runs, indicating the influence of selected parameters and their quantitative requirement for carotenoids production.
Severity Index is a measurement which indicates the percentage of interaction between two factors. Though pH and NaH2PO4 concentration have least individual effects, their interaction has a highest significant effect (SI= 76.66 %) on the carotenoids production. The pH was noticed in both higher and lower interactions (table 2) indicating that pH is one of the important factors which affects the carotenoid production either synergistic way along with other parameters. The interaction data reveals that the production of carotenoids by
- salina, AT-JP-13 depends on the individual as well as the interaction effect of selected parameters.
The analysis of data reveals that light intensity has more contribution (60.59%) on overall carotenoids production suggesting that alteration of light intensity has significant changes in the carotenoids production. Light is the vital factor regulating the rate of synthesis of ג-carotene in Dunaliella under favorable growth conditions36,37. As per the preliminary investigation and in Taguchi experiment the total carotenoid was found to be higher on the 30th day (initial decline phase). This may be due to the high light intensity, lack of cell wall and the cell swelling which accumulate a large amount of carotenoids irrespective of growth. Likewise, the swelling of D. salina cells is a typical response to various extreme conditions such as high light intensity and/or low temperature38. D. salina cell size has been observed with seven-fold increase in the incident light intensity39. Moreover, the high light intensity does not support the cell growth in the present study, instead, it indirectly reflects on the total carotenoids despite its high performance in Taguchi. This is evidently proved the D. salina reported to die due to reduced chlorophyll levels under very high-light illumination when it was growing at a slow rate35.
Next to light intensity, NaNO3 concentration has shown the highest influence on total carotenoids production. According to Milko40, the best source of nitrogen for maximum growth of D. salina, D. tertiolecta and D. viridis was NaNO3. Perhaps the present basal medium also inherently contains the NaNO3. Saha et al.41 observed that the best stress conditions include high-light illumination in depleted nitrogen coupled to micronutrients such as Zn, Mn and Fe in medium for enhancement of carotenoid production. In addition, Mojaat et al.42 observed the accumulation of high quantities of b-carotene (35 pg/cell) in the nitrate-free culture. These values drastically dropped to 24 and 12pg/cell at the initial nitrate concentration of 1.2 and 6.0 mM, respectively. Usually, nitrate limiting conditions enhance the carotenoid production in the cells, whereas, it is contradictory to the above authors view since the design used in the present study is well correlated with each other. Even at higher concentration, the total carotenoid was increased, while the lower concentration diminishes the carotenoid production, which may be because of other factors used.
The effects of salinity on growth and total carotenoids production by the microalga D. salina is most important since it was isolated from the normal seawater and not from the hypersaline area. As per the previous reports, D. salina had shown the maximum growth at 1 M NaCl concentration7. Likewise, in the present study also, the organism preferred the lesser range of NaCl between 0.5 to 1.5 M concentrations. In General, of the various ranges taken the maximum carotenoids accumulated in higher ranges than the lesser ranges. Besides, it was well adapted to all the salinity tested. It is well correlated with the following reports narrated by the various researchers. Salt tolerance and growth experiments showed for their isolate could grow at different salinity levels ranging from 0.5 to 4.0 M NaCl, thereby elevating the carotenoid content of the cells, which was cultivated at the high salinity of 4M NaCl22. Fazeli et al.32 found that the productivity of total carotenoids on the cellular basis to be very high at extreme salt concentrations (3 M NaCl). D. salina is the widely studied model alga for its incredible ability to acclimatize to a wide range of salinity43.
Phosphate is an essential nutrient that plays a vital role in structural and functional components of a living system44. Similarly, an investigation was made on the effect of NaH2PO4. Phosphate also exhibited the same condition but phosphate deprivation had little effect due to intracellular phosphate. In the present study, the phosphate showed less impact on the total carotenoid production by isolated D. salina. The concentration of 0.5 mM NaH2PO4 was found to be optimum for higher carotenoid production. Usually, the optimum phosphate concentration for the growth of D. salina and D. viridis was 0.002 to 0.025 gL-1 of K2HPO440,45, while higher concentrations (>5gL-1) inhibited their growth. The ratio of accessory phosphor protective pigments (a and b-carotene) to Chlorophyll-a improved under restrictive levels of nitrogen and phosphate46,47.
The Taguchi results reveal that pH has a least contribution on the carotenoid production by isolated D. Salina among all studied parameters. However, it gives a tremendous synergetic effect to other parameters to enhance the carotenoids production. Most of the microalgae chose the suitable pH ranges from 6 to 9. Keeping this in mind, the present microalga was tested within the range of 7.0 to 8.5. The alga used in this study exhibited normal growth up to 30th day and showed an ample amount of total carotenoids production at the higher pH (pH 8.0). The obtained results are contradicting to the report of Ying et al48. The investigators observed that once after 9 days of inoculation, the pH barely increased and growth was inhibited. This may be prevented in this case by combination of appropriate nutrients supplementation. Shariati49 reported that the optimum pH range for D. salina, D. pseudosalina and D. parva isolated from Iran was between pH 7.0 to 8.0. Thakur et al.50 reported that a biologically pure culture of D. salina ARL 5 exhibited a wide pH tolerance within the range of 5-10, a wide temperature tolerance within the range of 18 to 55°C and salinity tolerance up to 1M KCl concentration.
On the whole, it has been already pointed out that the accumulation of b-carotene in D. salina depends upon the factors, which include high light intensity51,52, high salinity53-55, extreme temperatures49, and deprivation of mineral nutrients like nitrate, phosphate and sulphate34,36,56,57. Hence, the present study effectively concludes for the first time that the alga D. salina, an extreme salt lover isolated from seawater could be optimized for total carotenoid production by the use of Taguchi tool to know the best synergistic condition of the nutrients available in the medium.
Acknowledgements
We are thankful to Dr. M. A. Atmanand, Director, ESSO – National Institute of Ocean Technology (NIOT), for the constant encouragement to conduct this work. We thank Dr. M. Vijayakumaran (Consultant) and Prof. T. Subramoniam, for their critical review of this manuscript. We also thank all the scientific and supporting staffs of ANCOST, ESSO – NIOT, for their invaluable assistance in field and laboratory for completing this research work.
Conflict of Interest
The authors declares that there is no conflict of interest.
Authors’ Contribution
JKR, ST, VNV, DG and KR involved in designing the research and experiment. JKR, BB and DPS involved in carrying out the designed experimental procedure. JKR, ST, BB, VNV, DG and KR involved in analysis the data. JKR and BB wrote the manuscript with the input of co-authors. All the authors agreed with the final version of manuscript.
Funding
The authors are thankful for the financial support given by the Earth System Sciences Organization, Ministry of Earth Sciences, Government of India, to carry out this research.
Data Availability
The datasets are available from the corresponding author on reasonable request.
Ethics Statement
Algal sample collection was carried out with a proper approval from the appropriate institutions as per the guidelines of ministry of earth sciences, Government of India.
- Ganesh Kumar A., Baskar B., Santhanakumar J., Vinithkumar N.V., Vijayakumaran M., Kirubagaran R. Diversity and functional properties of intestinal microbial flora of the spiny lobster Panulirus versicolor (Latreille, 1804). J. Mar. Biol. Ass. India, 2010; 52(2): 282-285.
- Vasanthi L.A., Muruganandam A., Revathi P., Baskar B., Jayapriyan K., Baburajendran R., Munuswamy N. The application of histo-cytopathological biomarkers in the mud crab Scylla serrata (Forskal) to assess heavy metal toxicity in Pulicat Lake, Chennai. Mar. Pollut. Bull., 2014; 81(1): 85-93
Crossref - Balakrishnan B., Sahu B.K., Ranishree J.K., Lourduraj A.V., Nithyanandam M., Packiriswamy N., Panchatcharam P. Assessment of heavy metal concentrations and associated resistant bacterial communities in bulk and rhizosphere soil of Avicennia marina of Pichavaram mangrove, India. Environ. Earth Sci., 2017; 76(1): 58.
Crossref - Jayappriyan K.R., Rajkumar R., Rengasamy R. Unusual occurrence of non carotenogenic strains of Dunaliella bardawil and Dunaliella parva in India. J. Basic. Microbiol., 2011; 51: 473–483.
Crossref - Jayappriyan K.R., Rajkumar R., Venkatakrishnan V.S., Nagaraj R., Rengasamy R. In vitro anticancer activity of natural ג-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomed. Preventive. Nutria., 2013; 3: 99-105.
Crossref - Dunal F. Extrait dun memoire sur les algues qui colorant en rouge certains eaux des marais selants mediterraneens. Ann. Sc. Nat. Bot. Ser., 1838; 9: 172.
- Oren A. A hundred years of Dunaliella research:1905-2005. Sal. Sys., 2005; 1: 2.
- Finney K.F., Pomeranz Y., Bruinsma B.L. Use of algae Dunaliella as a protein supplement in bread. Food. Additives., 1984; 61: 402–406.
- Borowitzka M.A., Borowitzka L.J. Dunaliella. In: Borowitzka M.A., Borowitzka L.J. (Eds.), Microalgal Biotechnology, Cambridge University Press, Cambridge, 1988.
- Gomez P.I., Barriga A., Cifuentes A.S., Gonzalez M.A. Effect of salinity on the quantity and the quality of carotenoids accumulated by (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) Chlorophyta. Biol. Res., 2003; 36: 185–192.
Crossref - Jha D.K., Vinithkumar N.V., Marimuthu N., Baskar B., Sahu B.K., Das A.K., Kirubagaran R. Field abd GIS based post-tsunami assessment of Scleractinian coral cover in the Aerial Bay group of islands, North Andaman, India. J. Coast. Conserv., 2013; 17: 671-677.
Crossref - Orest S., Young A. Low temperature induced synthesis of a- carotene in the microalga Dunaliella salina (Chlorophyta). J. Phycol., 1999; 35: 520–527.
Crossref - Shea R., Chopin T. Effects of Germanium dioxide, an inhibitor of diatom growth, on the microscopic laboratory cultivation stage of the kelp, Laminaria saccha rina. J. App. Phycol., 2007; 19: 27-32.
Crossref - Droop M.R. A procedure for routine purification of algal culture with antibiotics. Br. Phycol. Bull., 1967; 3: 295–297.
Crossref - Sambrook K.T., Frisch E.F., Maniatis T. Molecular Cloning: a laboratory manual. Cold Spring Harbor Laboratory, 1989.
- Rasoul-Amini S., Ghasemi Y., Morowvat M.H., Mohagheghzadeh A. PCR amplification of 18S rRNA, single cell protein production and fatty acid evaluation of some naturally isolated microalgae. Food Chem., 2009; 116: 129–136.
Crossref - Lichtenthaler H.K. Chlorophylls and carotenoids: pigments of photosynthetic membrances. Meth. Enzymol., 1987; 148: 350–382.
Crossref - Guillard R.R.L. Division rates. In Stein J. (Ed), Handbook of Phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge, London, 1973.
- Taguchi G. Quality through engineering design. ; Elsevier Science Publisher, Netherlands, 1993.
- Prakasham R.S., Sathish T., Brahmaiah P. Biohydrogen production process optimization using anaerobic mixed consortia: A prelude study for use of agro-industrial material hydrolysate as substrate. Biores. Tech., 2010; 101: 5708–5711.
Crossref - Balakrishnan B., Ranishree J.K., Thadikamala S., Panchatcharam P. Purification, characterization and production optimization of a vibriocin produced by mangrove associated Vibrio parahaemolyticus. Asian Pac. J. Trop. Biomed., 2014; 4(4): 253–261.
Crossref - Hejazi M.A., Barzegari A., Gharajeh N.H., Hejazi M.S. Introduction of a novel 18S rDNA gene arrangement along with distinct ITS region in the saline water microalga Dunaliella. Saline Systems, 2010; 6: 1-4.
Crossref - Baskar B., Prabakaran P. Isolation and characterization of copper resistant Exiguo-bacterium strains isolated from rhizosphere soil of Avicennia marina. Res. J. Pharm. Biol. Chem. Sci., 2011; 2(4): 640-646.
- Baskar B., PrabaKaran B. Characterization of mangrove associated nitrogen fixing halophilic bacterium Panibacillus sp. Int. J. Curr. Res., 2011; 3(9): 065–067.
- Baskar B., Prabakaran P. Assessment of nitrogen fixing bacterial community present in the rhizosphere of Avicennia marina. Indian J. Geo-Mar. Sci., 2015; 44(3): 318-322
- Abatzopoulos T.J., Beardmore J.A., Clegg J.S., Sorgeloos P. Artemia: Basic and Applied biology. Kluwer Academic, Dordrecht, 2002.
Crossref - Borowitzka M.A. High value products from algae, Dunaliella salina and Haematococcus pluvialis. In: Thirakhupt V., Boonakijjinda V. (eds.), Mass Cultures of Microalgae. Proceedings of the Research Seminar and Workshop, Silpakorn University, Thailand. UNESCO: Thailand, 1995.
- Mishra A., Jha B. Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Biores. Tech., 2009; 100: 3382–3386.
Crossref - Tawfiq A.S., Al-Musallam L., Al-Shimmari J., Dias P. Optimum production conditions for different high-quality marine algae. Hydrobiologia., 1999; 403: 97–107.
Crossref - Borowitzka M.A., Siva C.J. The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J. Appl. Phycol., 2007; 19: 567–590.
Crossref - Lerche W. Untersuchungen ber Entwicklung und Fortpflanzung in der Gattung Dunaliella. Arch. f. Protistenkd., 1937; 88: 236–268.
- Fazeli M.R., Tofighi H., Samadi N., Jamalifar H. Effect of salinity on ג-carotene production by Dunaliella salina DCCBC26 isolated from the Urmia salt lake, north of Iran. Biores. Tech., 2006; 97: 2453–2456.
Crossref - Garcia F., Freile-Pelegrin Y., Robledo D. Physiological characterization of Dunaliella sp. (Chlorophyta, Volvocales) from Yucatan, Mexico. Biores. Tech., 2007; 98: 1359–1365.
Crossref - Shelly K., Heraud P., Beardall J. Nitrogen limitation in Dunaliella tertiolecta (Chlorophyta) leads to increased susceptibility to damage by UV-B radiation but also increased repair capacity. J. Phycol., 2002; 38: 713–720.
Crossref - Ben-Amotz A., Avron M. Accumulation of metabolites by halotolerant algae and its industrial potential. Annu. Rev. Microbial., 1983; 37: 95–119.
Crossref - Ben-Amotz A. Effect of irradiance and nutrient deficiency on the chemical composition of Dunaliella bardawil, Ben-Amotz and Avron (Eds) (Volvocales, Chlorophyta). J. Plant. Physiol., 1987; 131: 479–487.
Crossref - Harding R.W., Shropshire W. Photo control of carotenoid biosynthesis. Annu. Rev. Plant. Physiol., 1980; 31: 217–238.
Crossref - Cowan A.K., Rose P.D., Horne L.G. Dunaliella salina: A model system for studying the response of plant cell to stress. J. Exp. Bot., 1992; 43: 1535–1547.
Crossref - Lamersa P.P., Janssena M., De Vos R.C., Bino R.J., Wijffels R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotech., 2012; 162: 21– 27.
Crossref - Milko E.S. The effect of various environmental factors upon pigment formation in the alga Dunaliella salina. Mikrobiologiya, 1963; 32: 299–307.
- Saha S.K., Moane S., Murray P. Effect of macro- and micro-nutrient limitation on superoxide dismutase activities and carotenoid levels in microalga Dunaliella salina CCAP 19/18. Biores. Tech., 2013; 14: 723–28.
Crossref - Mojaat M., Foucault A., Pruvost J., Legrand J. Effect of organic carbon sources and Fe2+ ions on growth and ג-carotene accumulation by Dunaliella salina. Biochem. Eng. J., 2008; 39: 177–184.
Crossref - Giordano M., Pezzoni V., Hell R. Strategies for the allocation of resources under sulfur limitation in the green alga Dunaliella salina. Plant Physiol., 2000; 124: 857–886.
Crossref - Daram P., Brunner S., Persson B.L., Amrhein N., Buche M. Functional analysis and cell-specific expression of a phosphate transporter from tomato. Planta., 1998; 206: 225–233.
Crossref - Giobor A. The culture of brine algae. Biological Bulletin Woods Hole., 1956; 3: 223–229.
Crossref - Krom M.D., Brenner S. Phosphorus limitation of primary productivity in the eastern Mediterranean Sea. Limnol. Oceanogr., 1991; 37: 424–432.
Crossref - Geider R.J., Macintry H.L., Graziano L.M., Mckay R.M. Responses of the photosynthesis apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorous limitations. Eur. J. Phycol., 1998; 33: 315–332.
Crossref - Ying K., Gilmour D.J., Shi Y., Zimmerman W.B. Growth Enhancement of Dunaliella salina by Microbubble Induced Airlift Loop Bioreactor (ALB)—The Relation between Mass Transfer and Growth Rate. J. Biomat. Nanobio., 2013; 4: 1-9.
Crossref - Shariati M. Characterization of three species of Dunaliella salina, Dunaliella parva and Dunaliella pseudosalina isolated from salt marshes of Gave Khoni Isfahan-Iran. Iranian J. Sci. Technol., 2003; 27: 185–190.
- Thakur A., Kumar H.D., Cowsik S.M. Effect of pH and inorganic carbon concentration on growth, glycerol production, photosynthesis and dark respiration of Dunaliella salina. Cytobios., 2000; 102: 69-74.
- Massyuk N.P., Radchenko M.I. Comparative chromatographic study of the pigments in some species and strains of Dunaliella. Hydrobiologia., 1970; 6: 51–58.
- Semenenko V.E., Abdullayev A.A. Parametric control of ג-carotene in Dunaliella salina cells under conditions of intensive cultivation. Sov. Plant Physiol., 1980; 27: 22-30.
- Evans R.W., Kates M., Ginzburg M., Ginzburg B.Z. Lipid composition of halotolerant algae Dunaliella parva Lerche and Dunaliella tertiolecta. Biochim. Biophys. Acta., 1982; 712: 186–195.
Crossref - Mortain-Bernard A., Rey P., ElArmani A.E., Lament A. Pyrophosphate inorganique et synthese de carotene Dunaliella salina. Life Sci., 1994; 317: 485–488.
- Henley W.J., Major K.M., Hironaka J.L. Responses to salinity and heat stress in two halotolerant Chlorophyte algae. J. Phycol., 2002; 38: 757-766.
Crossref - Borowitzka L.J., Borowitzka M.A. Industrial production methods and Economics. In Cresswell R.C., Rees T.A.V., Shah N. (Eds.), Algal and Cyanobacterial biotechnology. Longmann Scientific, London, 1989.
- Vorst P., Barrd R.L., Mor L.R., Korthals H.J., Van D. Effect of growth arrest on carotene accumulation photosynthesis in Dunaliella. Microbiol., 1994; 140: 1411–1417.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.