ISSN: 0973-7510
E-ISSN: 2581-690X
The resin extract of Commiphora myrrh is Widely used in the folk medicine. The studying myrrh resin extract include moisture. minerals such as (Ca, Fe, Mg, Na, Cu and Zn), protein, total fat and crude fiber. In this study used Muffle furnace, Kjeldahl methods Soxlet and atomic absorption. HPLC using to evaluating Polyphenol constituents of myrrh different resin extract (ethanol, ethyl acetate, petroleum ether and chloroform) as Conc. (µg / g) and in all extract (ethanol, ethyl acetate and petroleum ether and chloroform) it contained Chlorogenic acid, gallic acid Catechin, Coffeic acid, caffeine, Syringic acid, Coumaric acid, Ferulic acid, Naringenin, 4`.7-Dihydroxyisoflavone, Cinnamic, Propyl Gallate Vanillin, Querectin and Acid Ellagic acid in different concentration percentage and area The effect of Commiphora myrrh (ethanol, ethyl acetate, petroleum ether and chloroform) resin extract against four different pathogenic bacteria Salmonella typhimurium, Pseudomona aeruginosa, Escherichia coli, and Bacillus cereus, were examine by Mueller Hinton Agar and measuring inhibition zone (diameter mm), show that there were significant different among bacteria and different method of extract. All different Commiphora myrrh seed extract (aqueous, ethyl acetate and petroleum ether) have high activity against Candida albicans fungus. The study was conducted to identified the Commiphora myrrh nutritive value, polyphenol Compound and the activity against bacteria and fungi.
Commiphora myrrh, nutritive value, poly phenol constituent, antimicrobial, antifungal
Commiphora myrrh are small tree family Burseraceae. Myrrh native to the northeastern Africa, Somalia, Madagascar, India. Ethiopia, Iran, and Thailand1-7. Myrrh traditionally widely used in folk medicine, which has no toxicity8,9, myrrh resin was used in many medicinal purposes10, Myrrh used for inflammations of the mouth, throat is highly effective antiseptic astringent, has widely used in digestive tract diseases11-14. Its antibacterial14,15. Myrrh have antifungal activity16 anti-parasitic, detoxifying and reduced inflammation17,18. It can be used in remedy for chronic sinusitis. Myrrh is used in skin problems rashes, acne, and inflammatory also used in healing gastric ulcer or skin injury as powder, or essential oil19,20. It play an important role in cosmetics and perfumery.
Commiphora myrrh purchase in the super market and identified in the Department of Biology, Science and Humanity College in Al-kharj, Prince Sattam bin Abdul-Aziz.
Microorganism
Microorganisms used in this work were obtained from laboratories of Microbiology “National Research Centre, Khartoum Sudan. The identification of bacterial by conventional biochemical methods21 according to the standard microbiology techniques. These microbes were, Pseudomonas aeruginosa, Escherichia coli, Bacillus cereus and Salmonella typhimurium.
Moisture content
Oven used for determination of moisture content22.
Ash content
Muffle furnace used for determination of ash%23.
Determination of total fat
A Sox let extractor used for determination of fat23.
Determination of crude fiber
Determination according to the method described by Official methods of analysis of the association of analytical Chemists23.
Determination of protein as total nitrogen
The Kjeldahl method used for determination of protein23.
Determination of minerals
Atomic absorption spectrophotometry used for determination of the minerals23.
Determination of total carbohydrate
According to the method described by Official methods of analysis of the association of analytical Chemists23.
Preparation of plants extracts
100 g, of resin plant were weighed, and subjected to different extraction solvents separately extracted with ethanol 80% at 60°C for 2h in a Sox let apparatus ethyl acetate 80% at 50°C – 60°C for 2 h, petroleum ether 80% at 60°C for 2h Chloroform 80% at 50°C- 60°C for 2 h. The all solvents extract were evaporated by a Buchi Rotary evaporator under reduced pressure, also the resin plant was extracted by distilled water over night at room temperature (25-30°C) filtered and dried24.
HPLC conditions
The Agilent series 1260, Kromasil C18 column (4.6 mm x 250 mm i.d., 5 μm) were used for the separation in HPLC analysis. The mobile phase used consisted of water (A) and 0.1% tri-floro-acetic acid in acetonitrile (B) at a flow rate 1 ml/min. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (82% A); 0–5 min (80% A); 5-8 min (60% A); 8-12 min (60% A); 12-15 min (85% A) and 15-16 min (82% A). The column temperature was maintained at 35°C. The multi-wavelength detector was monitored at 280 nm. The injection volume was 10 μl for each of the sample solutions.
Mueller Hinton Agar
MHA (Mueller Hinton Agar) (Becton Dicknson M. D USA), media was prepared according to the manufacturer’s instruction. Sterile Mueller Hinton agar plates were inoculated with the test culture by surface spreading using sterile wire loops and each bacterium evenly spread on the entire surface of the plate to obtain uniformity of the inoculum. Concentrations of 12.5, 25, 50 and 100mg/ml prepared from the seed different extract (aqueous, ethyl acetate and petroleum ether) agar were used for antibacterial analysis. Petri dishes prepared by agar and allow to solidify. Each plate was then seeded with a test bacterium. Four holes were made in each of the plate with a sterile 2.0 mm diameter cork borers. Each of the four holes was filled with a given concentration of the extract mixed with plane sterile agar. The plates were then incubated at 37°C for 24 hours. The diameters of zones of inhibition were measured using a meter rule and the mean value for each organism was recorded25.
Preparation of fungal organism
The fungal is culture as fallow at temperature 25°C for 4 days put in Peptone water, sterile normal saline used for the harvested growth mat of fungal, washed and suspended stored in refrigerator till used26.
Statistical analysis
It was done according to Duncan, Multiple Range Test127.
Table (1):
Nutritional value per 100 g (3.5 oz) of Commiphora myrrh. resin extract.
Moisture |
0.10 |
Ash |
15.40 |
Fiber |
0.0 |
Protein |
9.97 |
Carbohydrate |
56.01 |
Fat |
17.96 |
Table (2):
Mineral constituents (ppm) of Commiphora myrrh. Resin extract.
Ca |
20.80 |
Fe |
139.540 |
Mg |
359.203 |
Na |
39.1 |
Cu |
0.654 |
Zn |
0.6561 |
Table (3):
Commiphora myrrh resin ethanol extract (20 mg/ml).
Area |
Conc.(µg / ml = µg / 20 mg) |
Conc. (µg / g) |
|
|---|---|---|---|
Gallic acid |
67.35 |
4.34 |
216.84 |
Chlorogenic acid |
28.36 |
1.75 |
87.56 |
Catechin |
61.17 |
10.15 |
507.51 |
Caffeine |
83.83 |
2.43 |
121.71 |
Coffeic acid |
99.76 |
3.29 |
164.33 |
Syringic acid |
101.70 |
3.82 |
190.96 |
Rutin |
171.24 |
25.79 |
1289.50 |
Pyro catechol |
0.00 |
0.00 |
0.00 |
Ellagic acid |
0.00 |
0.00 |
0.00 |
Coumaric acid |
41.05 |
0.92 |
45.79 |
Vanillin |
46.57 |
1.63 |
81.74 |
Ferulic acid |
176.56 |
3.76 |
188.11 |
Naringenin |
207.26 |
8.42 |
420.76 |
Propyl Gallate |
824.35 |
20.03 |
1001.61 |
4`.7DihydroxyisoFlavone |
600.45 |
15.10 |
754.82 |
Querectin |
674.27 |
54.31 |
2715.62 |
Cinnamic acid |
461.17 |
3.76 |
187.84 |
Table (4):
Commiphora myrrh resin ethyl acetate extract (Conc. 15 mg/ml).
| Commiphora myrrh Ethyl acetate (Conc. 15 mg/ml) | |||
|---|---|---|---|
| Area | Conc. (µg / ml = µg / 15 mg) |
Conc. (µg / g) |
|
| Gallic acid | 273.98 | 20.37 | 1357.68 |
| Chlorogenic acid | 198.63 | 12.82 | 854.34 |
| Catechin | 234.47 | 37.76 | 2517.42 |
| Coffeic acid | 193.22 | 6.67 | 444.43 |
| Syringic acid | 311.13 | 11.37 | 758.04 |
| Rutin | 1037.40 | 134.54 | 8969.27 |
| Ellagic acid | 1066.67 | 67.05 | 4470.27 |
| Coumaric acid | 278.07 | 11.67 | 778.15 |
| Vanillin | 304.74 | 7.15 | 476.75 |
| Ferulic acid | 395.72 | 13.04 | 869.28 |
| Naringenin | 1797.77 | 100.81 | 6720.83 |
| Querectin | 530.56 | 211.14 | 14076.01 |
| Cinnamic acid | 375.73 | 3.41 | 227.29 |
Table (5):
Commiphora myrrh resin petroleum ether extract (Conc. 15 mg/ml).
| Commiphora myrrh Petroleum ether (Conc. 15 mg/ml) | |||
|---|---|---|---|
| Area | Conc. (µg / ml = µg / 15 mg) |
Conc. (µg / g) | |
| Gallic acid | 0.00 | 0.00 | 0.00 |
| Chlorogenic acid | 6.89 | 0.44 | 29.66 |
| Catechin | 9.35 | 1.51 | 100.40 |
| Coffeic acid | 0.00 | 0.00 | 0.00 |
| Syringic acid | 32.22 | 1.18 | 78.50 |
| Rutin | 38.84 | 5.04 | 335.78 |
| Ellagic acid | 58.12 | 3.65 | 243.57 |
| Coumaric acid | 22.16 | 0.93 | 62.02 |
| Vanillin | 82.42 | 1.93 | 128.94 |
| Ferulic acid | 86.74 | 2.86 | 190.55 |
| Naringenin | 90.78 | 5.09 | 339.38 |
| Querectin | 197.10 | 78.44 | 5229.17 |
| Cinnamic acid | 41.65 | 0.38 | 25.20 |
Table (6):
Commiphora myrrh resin chloroform extract (Conc. 15 mg /ml).
| Commiphora myrrh Chloroform (Conc. 15 mg/ml) | |||
|---|---|---|---|
| Area | Conc. (µg/ml = µg/15 mg) | Conc. (µg / g) | |
| Gallic acid | 0.00 | 0.00 | 0.00 |
| Chlorogenic acid | 14.62 | 0.94 | 62.87 |
| Catechin | 16.81 | 2.71 | 180.51 |
| Coffeic acid | 19.79 | 0.68 | 45.51 |
| Syringic acid | 34.57 | 1.26 | 84.24 |
| Rutin | 49.20 | 6.38 | 425.35 |
| Ellagic acid | 237.37 | 14.92 | 994.79 |
| Coumaric acid | 56.46 | 2.37 | 157.99 |
| Vanillin | 258.43 | 6.06 | 404.29 |
| Ferulic acid | 210.19 | 6.93 | 461.71 |
| Naringenin | 55.69 | 3.12 | 208.20 |
| Querectin | 458.47 | 182.45 | 12163.41 |
| Cinnamic acid | 114.01 | 1.03 | 68.97 |
Table (7):
Inhibition zone (in mm) for different concentrations of Commiphora myrrh ethanol resin extract.
| Microorganism | Concentration of the of Commiphora myrrh ethanol resin extract (μg/disc) | Mean Microorganism | |||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 12 | 13 | 14 | 15 | 13.5 |
| Pseudomonas aeruginosa | 10 | 12 | 12 | 15 | 12.25 |
| Escherichia coli | 12 | 13 | 14 | 15 | 13.5 |
| Bacillus cereus | 10 | 14 | 15 | 16 | 13.75 |
| Candida albicans | 10 | 13 | 14 | 15 | 13 |
| Mean Commiphora myrrh ethanol resin extract | 10.8 | 13 | 13.8 | 15.2 | |
Table (8):
Inhibition zone (in mm) for different concentrations of Commiphora myrrh ethyl acetate resin extract.
| Microorganism | Concentration of the Commiphora myrrh ethyl acetate resin extract (μg/disc) | Mean Microorganism |
|||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 12 | 13 | 15 | 16 | 14 |
| Pseudomonas aeruginosa | 12 | 12 | 13 | 15 | 13 |
| Escherichia coli | 14 | 15 | 16 | 16 | 15.25 |
| Bacilluscereus | 10 | 12 | 12 | 13 | 11.75 |
| Candida albicans | 12 | 13 | 14 | 15 | 13.5 |
| Mean Commiphora myrrh ethyl acetate resin extract | 12 | 13 | 14 | 15 | |
Table (9):
Inhibition zone (in mm) for different concentrations of Commiphora myrrh petroleum ether resin extract.
| Microorganism | Concentration of the Commiphora myrrh petroleum ether resin extract (μg/disc) | Mean Microorganism |
|||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 10 | 10 | 11 | 12 | 10.75 |
| Pseudomonas aeruginosa | 11 | 11 | 12 | 13 | 11.75 |
| Escherichia coli | 12 | 12 | 13 | 14 | 12.75 |
| Bacilluscereus | 10 | 11 | 11 | 13 | 11.25 |
| Candida albicans | 11 | 12 | 13 | 15 | 12.75 |
| Mean Commiphora myrrh resin extract | 10.8 | 11.2 | 12 | 13.4 | |
Table (10):
Inhibition zone (in mm) for different concentrations of Commiphora myrrh chloroform extract.
| Microorganism | Concentration of the Commiphora myrrh chloroform resin extract (μg/disc) | Mean Microorganism | |||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| Salmonella typhimurium | 9 | 10 | 10 | 11 | 10 |
| Pseudomonas aeruginosa | 10 | 10 | 11 | 12 | 10.75 |
| Escherichia coli | 11 | 12 | 13 | 14 | 12.5 |
| Bacilluscereus | 10 | 10 | 11 | 12 | 10.75 |
| Candida albicans | 12 | 13 | 14 | 15 | 13.5 |
| Mean Commiphora myrrh | 10.4 | 11 | 11.8 | 12.8 | |
| chloroform resin extract | |||||
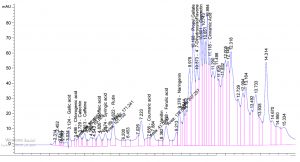
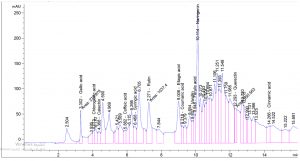
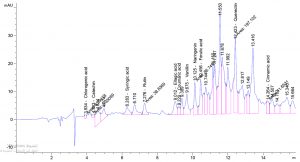
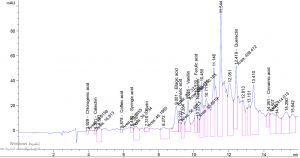
Table 1. show the approximate nutritive constituent of Commiphora myrrh resin contain Moisture % (0.10) , ash% (15.40), fiber% (0.0), protein% (9.97), carbohydrates% (56.01), fat% (17.96). Table 2. show the minerals content of the myrrh (ppm) such as Ca (20.80), Fe (139.540), Mg (359.203), Na (39.1), Cu (0.654) and Zn (0.6561), (26,28). Fig. 1 and Table 3. show the 17 Polyphenol constituents of Commiphora myrrh ethanol resin extract according to concentration and area, Querectin gave the highest concentration (54.31Conc. (µg / ml = µg / 20 mg) while Pyro catechol and Ellagic acid gave (0.00%) Table 4. and figure 2 show the Commiphora myrrh resin extracted by ethyl acetate contained 13 polyphenol the highest concentration is Querectin (211.14 Conc. (µg / ml = µg / 15 mg) while the lower one is Cinnamic acid (3.41 Conc. (µg / ml = µg / 15 mg). Table 5. and figure3 show the Commiphora myrrh resin extracted by petroleum ether contained 13 Polyphenol constituents the highest one is Querectin (78.44 Conc. (µg / ml = µg / 15 mg) while the lower one is Gallic acid and Coffeic acid (0.00 Conc. (µg / ml = µg / 15 mg) Table 6. and figure 4 show the Commiphora myrrh resin extracted by Chloroform contained 13 Polyphenol constituents the higher one is Querectin (182.45 Conc. (µg / ml = µg / 15 mg) while the lower one is Gallic acid (0.00 Conc. (µg / ml = µg / 15 mg) In all extract show that the Querectin have high concentration, many studies reported the presence of phenolic compounds prevent body against oxidation, cancer and inflammation28-30. The antibacterial activity of the Commiphora myrrh by different method (ethanol, ethyl acetate, petroleum ether) seed extract against four different pathogenic organisms Escherichia coli, Pseudomona aeruginosa, Salmonella typhimurium and Bacillus cereus and one fungus Candida albicans (the lowest concentration of the Commiphora myrrh seed extract is (12.5 mg/ml) and the highest one is (100 mg/ml) there were differences effect among bacteria, Table 7 shows the inhibition zone (in mm) for different concentrations of Commiphora myrrh ethanol resin extract, the highest inhibition zone was detected against Bacillus cereus (13.75) and have the lowest one is Pseudomona aeruginosa (12.25). Table 8 show that the Commiphora myrrh resin extracted by ethyl acetate, the high inhibited zone against Escherichia coli (15.25), while the lower one against Bacillus cereus (11.75).Table 9 show that the Commiphora myrrh resin extracted by petroleum ether , the high inhibited zone against Escherichia coli (12.75) and the lower inhibition zone against Salmonella typhimurium (10.75). Table 10 show the inhibition zone (in mm) for different concentrations of Commiphora myrrh chloroform extract the high inhibited zone against Escherichia coli (12.50) and the lower inhibition zone against Salmonella typhimurium (31,32,33,34). All different Commiphora myrrh resin extract (ethanol, ethyl acetate petroleum ether and chloroform) have the high activity against Candida albicans fungus these results agree with those who obtained that their in extract of Commiphora myrrh seed extract have potent effect against bacteria and fungi35.
The Commiphora myrrh contain polyphenol compound had potent antioxidant, antibacterial and antifungal activity in all different leaf extract.
ACKNOWLEDGMENTS
We would like to thanks the all staff of department of Biology, Science and Humanity College in Al-kharj, Prince Sattam bin Abdul-Aziz University Al-Khaarj Saudi Arabia and department of Chemistry, Faculty of Science and Arts in Mukhwa, University of Albaha, Saudi Arabia for providing assistance on bioinformatics analysis. We would like to thank all the staff of the laboratories of Chemistry and Microbiology “National Research Centre, Khartoum Sudan.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Kebu B, Ensermu K, Zemede A. Indigenous medicinal utilization, managemetn and threats in Fentale area, Eastern Shewa, Ethiopia. Ethi J Biol Sci. 2004;3:1-7.
- Evans WC, Evans T. Pharmacognosy. 14th Ed, London: WB Saunders, 1996. 14. Langenheim JH. Plants resins: Chemistry, Evolution, Ecology, and Ethnobotany. Timber Press, Portland, Oregon, USA. 2003.
- Nikam P, Kareparamban J, Jadhav A, Kadam V: Future Trends in Standardization of Herbal Drugs. J of Applied Pharmaceutical Science. 2012:38-44
- Chevallier A. Ed.: “The Encyclopedia of Medicinal Plants”, 4th ed., 84 (1996) 2 Wallis, T. E. Ed.: “Text book of Pharmacognosy”, 5th ed., 497 (1967)
- Trease GE, Evans WC, Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685.
Crossref - Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10:210-229.
Crossref - Shen T, Li GH, Wang XN, Lou HX. The genus Commiphora: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2012;142:319-330.
Crossref - Mekonen Y, Dekebo A, Dagne E. Toxcity study in mice of resins of three Commiphora species. SINET: Ethiop. J. Sci. 2003;26:151-153.
- Rao RM, Khan ZA, Shah AH. Toxicity studies in mice of Commiphora molmol oleo-gum-resin. J Ethnopharmacol. 2001;76(2):151-154. Frankincense, Myrrh. Economic Botany. 40:425-433.
Crossref - Greene DA Gold. frankincense, myrrh and, medicine. NC Med. J. 1993;54:620-622.
- Dolara P, Luceri C, Ghelardini C, et al. Analgesic effects of myrrh. Nature. 1996;379:29.
Crossref - Shulan S, Tuanjie W, Jino D, et al. Anti-inflammatory and analgesic activity of different extracts of Commiphora myrrha. J Ethnopharmacol. 2011;134(2):251-258.
Crossref - Shalaby MA, Hammouda A-E. Analgesic, anti-infl ammatory and anti-hyperlipidemic activities of Commiphora molmol extract(Myrrh). J Intercult Ethnopharmacol. 2014;3(2):56-62.
Crossref - Mohammed AA, Ali SI, EL-Baz FK, Hegazy AK, Kord MA. Chemical composition if essential oil and in vitro antioxidant and antimicrobial activities of crude extracts of Commiphora myrrha resin. Industrial Crops and Products. 2014;57:10-16.
- Omar SA, Adam SEI, Mohammed OB. Antimicrobial activity of commiphora myrrha against some bacteria and Candida albicans Isolated from Gazelles at King Khalid Wildlife Research Centre. Research Journal of Medicinal Plants. 2011;5(1):65-71.
Crossref - Duwiejua M, Zeitlin IJ, Waterman PG, Chapman J, Mhango GJ, Provan GJ. Anti-inflammatory activity of resins from some species of the plant family Burseraceae. Planta Med.1993;59(1):12-16.
Crossref - Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS, Parmar NS. Anti-inflammatory activity of Commiphora molmol. Agents Actions. 1986;17(3-4):381-219.
Crossref - Haffor A-SS. Effect of myrrh (Commiphora molmol) on leukocyte levels before and during healing from gastric ulcer or skin injury. J Immunotoxicol. 2010;7:68-75.
Crossref - al-Harbi MM, Qureshi S, Raza M, Ahmed MM, Afzal M, Shah AH. Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J Ethnopharmacol. 1997;55(2):141-50.
- Cheesebrough M. District laboratory practice in tropical countries Part 2. Cambridge University Press; Cambridge: 2000: 63-70.
- Sadzawka A, Carrasco MA, Demanet R, et al. Method of analysis for vegetables. 2nd Ed.Santiago, Chile. Instituto de Investigaciones Agropecuarias INIA, 2007:140.
- AOAC. Official methods of analysis of the association of analytical Chemists, Washington D.C. 1990:12-13.
- Nobosse P, Fombang EN, Mbofung CMF. The effect of steam blanching and drying method on nutrients, phytochemicals and antioxidant activity of Moringa (Moringa oleifera L.) leaves. American Journal of Food Science and Technology. 2017:5(2):53-60.
Crossref - Omenka CA, Osuoha JO. Antimicrobial potency of grapefruit seed extract on five selected pathogens. Nig J Microbiol. 2000:14(2):39-40.
- Albuquerque TR, Camara RDR, Marian L, Willadino Marcelin C. Antimicrobial action of the essential oil of Lippia gracilisSchauer. Braz Arch Bio Tech. 2006:49(4):527-535.
Crossref - Othman R, Hameed A-SRR, Naji NA. Studying of Phytochemical, Nutritive values and Antioxidant ability of Commiphora myrrha. Al-Mustansiriyh Journal of Pharmaceutical Scienses. 2016:17(1):21-33.
- Gomez KA, Gomez AA. Statistical procedures for Agriculture Research. seconded . John Wiley and Sons, Inc. New York, 1983.
- Duyff RL. American Dietetic Association Complete Food and Nutrition Guide. 4th ed. Hoboken, N.J.: John Wiley & Sons. 2012:55.
- Su S, Wang T, Duan J, et al. “Anti-inflammatory and analgesic activity of different extracts of Commiphoramyrrha,” J Ethnopharmacol. 2011;134(2):251-258.
Crossref - Su S, Hua Y, Wang Y, et al. “Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha, and Boswellia carterii,” J Ethnopharmacol. 2012;139(2):649-656.
Crossref - Kumar VP, Chauhan NS, Padh H, Rajani M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharmacol.2006;107:182.
Crossref - Joshi B, Lekhak S, Sharma A. Antibacterial property of different medicinal plants: Ocimum sanctum, Cinnamomum zeylanicum, Xanthoxylum armatum and Origanum majorana. Kathmandu Univ J Sci Eng Technol. 2009;5(1):143-50.
Crossref - Das K, Tiwari RK, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J Med Plants. 2010;4(2):104-111.
Crossref - Savluchinske-Feio S, Curto MJM, Gigante B, Roseiro JC. Antimicrobial activity of resin acid derivatives. Appl Microbiol Biotechnol.2006;72(3):430-436. Res. 2010;4:104-111.
Crossref - Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem.2003;10(10):813-829.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.