ISSN: 0973-7510
E-ISSN: 2581-690X
Onychomycosis is a fungal infection of the fingernails or toenails caused by fungi. Ciclopirox nail lacquer is generally used for the treatment of patients with onychomycosis, but the duration of treatment is long and indefinite. Hence, Silver nanoparticles were coated with 8% Ciclopirox, and In vitro antifungal susceptibility was done to check its effectiveness over plain 8% Ciclopirox. This Analytical study included a total of 100 nail-clipping/Nail scrapping specimens from patients attending DVL OPD, MGMCRI, with suspected Onychomycosis for a period of one year. The sample was subjected to microscopy and culture identification of the pathogen. In vitro Antifungal susceptibility testing was performed against plain 8% Ciclopirox and 8% Ciclopirox coated on silver Nanoparticles by broth dilution method as per CLSI M38-A. The overall prevalence of Onychomycosis was 39%, caused by 6% of dermatophytes and 33% of non-dermatophytes. The incidence was 65% in toe nail and 35% in fingernail. The female is more predominant than male. Distal and lateral onychomycosis are the commonest clinical types. Aspergillus niger (13%) was the etiologic agent of onychomycosis due to NDOM. The MICs with 8% Ciclopirox coated on silver nanoparticles ranged from 0.4-0.7 μg/ml and the rate of growth inhibition was better when compared to plain 8% Ciclopirox MICs which ranged from 0.5-0.8 μg/ml. The study concludes that A. niger is the agent of NDOM and our findings hold implications of Onychomycosis for both the clinicians and researchers, to improve the clinical outcomes of the patients.
Onychomycosis, Silver Nanoparticles, Non-dermatophytes, Ciclopirox
Onychomycosis, a type of fungal infection of the fingernails or toenails can be caused by yeasts, dermatophytes and non-dermatophytic moulds.1,2 Clinically, it can be seen as onychogryphosis, that is thickening of the nail plate or white to yellow discoloration. It can also be seen as Onycholysis, which is separation of nail from the nail bed. The worldwide prevalence was estimated to be >10% in healthy persons, and 40% in individuals >60 years, and estimates for 50% of nail ailments in clinical practice.3
It is reported that the dermatophytes are the major causative agents whereas, yeasts and Non dermatophytes account for approximately 20% of onychomycosis cases worldwide.4 Non-dermatophytic onychomycosis (NDO) is caused by hyaline5,6 and dematiaceous7,8 filamentous fungi that are commonly found as soil saprophytes or plant pathogens. Unlike dermatophytes, they are generally not keratinolytic.9 They live on the non-keratinized intercellular cement of the host tissue and must take advantage of previous keratin destruction by dermatophytes, trauma, or another nail disease. For this reason, they are sometimes considered secondary invaders of the nail plate.10 Although the list of NDM species that have occasionally been isolated from nails is quite long, only a few are regularly identified as real causes of onychomycosis.
Ciclopirox nail lacquer has demonstrated a broad spectrum of activity with efficacy against Candida species and some non-dermatophytes. Ciclopirox nail lacquer is considered extremely safe regarding causally related treatment emergent adverse-effects.11 Since decades the medical importance of Silver & silver-based compounds has been known and found their applications in many areas of medicine since decades. Nanoparticles (NPs) are of great scientific interest as they differ in physical & chemical parameters from their bulkier counterpart. These size dependent properties of NPs could be attributed to the increased surface area that is exposed for reaction when compared to their bulky counterpart thereby finding application in targeted drug delivery, biosensors, antibacterial agents, cancer treatment, gene therapy and DNA analysis, enhancing reaction rates and magnetic resonance imaging. These NPs have also shown antibacterial, antifungal12 and antiviral activity.13 This study is taken up to identify the non-Dermatophytic causative agents of Onychomycosis and to check the effectiveness of Antifungal drug plain Ciclopirox over Ciclopirox coated on Silver Nanoparticles.
Sampling method
A total of 100 nail-clipping specimens were obtained from patients with suspected onychomycosis attending the Dermatology OPD, at MGMCRI for a period of one year.
Sample collection
After cleaning the affected area with 70% ethanol, nail scrapings will be collected from the deepest part of the nail and as close as possible to the intact parts of the nail by scraping the nail bed, and the hyponychium. Material will also be obtained from the underside of the nail plate, with emphasis placed on sampling from the advancing infected edge most proximal to the cuticle. This is the area most likely to contain viable hyphae and least likely to contain contaminants. If Candida onycholysis is suspected, the lifted nail bed should be scrapped. The collected specimen will be placed in sterile black paper envelope and folded and sent to Microbiology Laboratory, MGMCRI for further processing.
Microscopy
The specimen should be divided into two portions for direct microscopy and culture. One piece of each nail fragment collected will be subjected to wet mount examination using 20% Potassium Hydroxide and examined under × 40 magnifications to identify the presence of any fungal elements, including hyphae, arthrospores, yeast cells, and pseudohyphae.
Culture
The other part of the nail will be fragmented and inoculated onto three points on two plates of Sabouraud’s dextrose agar (SDA) with 0.05% cycloheximide and the other SDA with 0.005% chloramphenicol, and incubated at 37 °C for 7 days. The cultures will be checked daily for evidence of growth and processed accordingly. No growth after 7 days will be considered a negative culture.
Criteria
The criteria for a diagnosis of NDM onychomycosis will be made based on nail abnormalities consistent with the diagnosis, a positive KOH preparation with the presence of specific hyphae in the nail keratin, and when the culture is done, the failure to isolate a dermatophyte in the culture and growth of identical mould colonies in the inoculation sites of the culture media. Samples with characteristic saprophytic hyphal elements on direct microscopy and significant growth of NDMs on culture will be considered for species identification by colony morphology and a microscopic examination with lactophenol cotton blue preparation according to identification keys.
Antifungal susceptibility testing
Inoculum preparation
The inoculum with conidium sporangiospores was prepared with the help of spectrophotometry procedure. To induce conidium formation, the fungi were grown on potato dextrose Agar for 7 days at 35 °C. After this 1 ml of sterile 0.85% saline was used to cover the sporulating colonies and gentle probing of the colonies was done with the sterile tip of a pipette.
The resulting solution was transferred to a sterile tube and allowed to settle for 3 to 5 minutes. The result the resulting supernatant homogenised suspension was transferred to sterile screw capped container. This mixture was then vortexed for 15 seconds.
From this mixture 1:50 dilution of inoculum was prepared in adequate volume to inoculate 1 ml into each well.
Drug preparation
Plain 8% Ciclopirox was commercially obtained. 8% Ciclopirox coated on Silver Nanoparticles was prepared by stirring and sonication method.
Antifungal susceptibility
The antifungal efficacy of plain 8% Ciclopirox and 8% Ciclopirox coated on Silver Nanoparticles was determined by microbroth dilutions method as per CLSI M38-A2 document. The MIC ranges tested were 0.06-2 μg/ml for both the drug formulations. After a 7-day incubation period at 28 °C, the tubes were analysed. Since the end points of ciclopirox is less well defined as per CLSI M38 A2, allowing some Turbidity above the mic was considered as the end point. For ciclopirox the turbidity allowed corresponded to approximately 80% or more reduction in the growth compared to the growth in the control well.
Onychomycosis
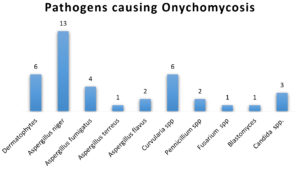
Of the 100 patients screened, the prevalence of Onychomycosis accounted for 39%, with 6% due to dermatophytes and 33% due to Non dermatophytes. The incidence was 65% in toenail onychomycosis and 35% in fingernail onychomycosis. The prevalence ratio of male to female was found to be 1:1.7, with a female preponderance. The most common clinical types seen were Distal & lateral subungual onychomycosis. Aspergillus niger (13%), was the most isolated etiological agent of NDOM. Others included various Aspergillus species, Curvularia, Fusarium, Candida, etc., as shown in Figure 1.
Antifungal Susceptibility Testing
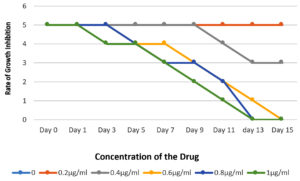
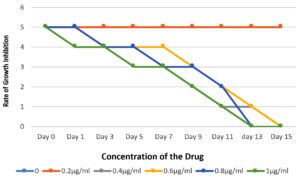
In vitro antifungal susceptibility by micro dilution method for Ciclopirox, the MIC was the lowest concentration showing 80-100% growth inhibition. The 33 non-dermatophytic strains were subjected to antifungal susceptibility testing against Plain 8% Ciclopirox and 8% ciclopirox coated on silver Nanoparticles. The MIC with plain 8% Ciclopirox for Aspergillus sp., Curvularia, Fusarium, Blastomyces and Penicillium ranged between 0.5-0.8 μg/ml and with 8% ciclopirox coated on silver nanoparticles the MICs ranged from 0.4-0.7 μg/ml. The rate of growth inhibition of the two formulations also revealed that in the tubes with the plain drug, the reduction in turbidity was observed from day 5 onwards, whereas, in the tubes with 8% ciclopirox coated on silver nanoparticles, the reduction in turbidity was observed from day 3 onwards as shown in Figures 2 and 3.
Onychomycosis, a fungal infection caused mainly in the toe or finger nails. It is more common in adults as well as elderly individuals, and its prevalence is higher in populations with certain risk factors such as diabetes, immunosuppression, poor foot hygiene, and those who frequently wear closed-toe shoes in hot and humid climates.4 In the present study, 100 patients were screened for fungal infections and they found that 39% of Onychomycosis infection, with 6% of dermatophytes and remaining 33% of Non dermatophytes. The incidence was more in toenail (65%) compared to fingernail (35.1%). The results of this study found that females are more predominant than the male population for this fungal infection. The study results are highly similar with Rafat et al., and also shows the higher incidence of infection was at the age of >50 years.2 Aspergillus niger were the predominant etiological agent of NDOM followed by other agents like Curvularia, Aspergillus species, Fusarium, Candida etc., In our study, in vitro antifungal susceptibility testing was performed using the microdilution method for Ciclopirox, which demonstrated 80-100% growth inhibition at the lowest concentration. Studies and surveys on the prevalence of onychomycosis in India have reported varying rates. Some studies have suggested prevalence rates ranging from 0.5% to 5.0% in different populations across the country.14 However, these rates may not fully capture the extent of the problem due to under-reporting and under-diagnosis, as many cases of onychomycosis may go unnoticed or untreated.
Non-Dermatophytic Onychomycosis
The main intention of the study is to investigate non-dermatophytic onychomycosis (NDO) with critical insights of aetiology and its management. This research is mainly to estimate the prevalence of Onychomycosis and test the efficacy of Silver Nanoparticles coated with 8% Ciclopirox as well as plain Ciclopirox.
Prevalence of onychomycosis
The present study has identified a spectrum of non-dermatophytic fungi responsible for onychomycosis, emphasizing the diversity within this category. Kaur et al., recorded that Trichophyton rubrum (46.67%) was the predominant fungus followed by Trichophyton mentagrophytes (20.0%) and Candida albicans (15.6%) from 60 patients.1 Another large multinational cross-sectional study using validated questionnaires from 532 patients which includes 284 toe nail, 248 finger nail and this questionnaire became a vital tool for the improvement of patient’s lives with onychomycosis.15
Prevalence of Non dermatophytic onychomycosis
Contrary to the prevailing notion that dermatophytes are the primary causative agents, but the study results indicate a significant number of non-dermatophytic fungi that can also cause a serious disease. The prevalence of NDOM accounts to 30.1% and 39%16 and were 29.3% and 32.7% by moulds and by yeasts, respectively,17 is quite similar ranges (33%) to the present study. Based on the above statement, moulds are considered as a nail invader which ranges from 1.45% to 17.60%. The incidence may vary due to geographic differences in mould distribution or diagnostic methods. This current study highlights the epidemiological features of Onychomycosis and shows that A. niger is the predominant pathogenic isolates causing NDOM. Various studies from different countries like Sri Lanka, Cameroon, India, Turkey, Italy and UK reported that A. fumigatus and A. terreus are the predominant pathogens.18-24
Antifungal susceptibility profile
Ciclopirox is an antifungal medication which is commonly used to treat the fungal infections of the skin as well as nails, including onychomycosis (fungal nail infection). It targets a variety of metabolic processes in the fungal cell.11 Ciclopirox (Penlac 8%) chelates with the polyvalent cations (Fe3+ and Al3+) that are involved in fungal enzymatic activity, by interrupting intracellular energy production, toxic peroxide degradation, depletion of amino acids and nucleotides which results in reduction of protein synthesis.11 Our study findings also contribute to the understanding of treatment strategies for NDOM. In Turkey Erdogan et al., reported a new technique, a mild keratolytic to be invaded under nail bed for Onychomycosis unresponsive to antifungal treatment.25 Notably, the demonstration of 8% Ciclopirox coated on silver nanoparticles yielded a higher in vitro antifungal efficacy against the non-dermatophytes isolates when compared to plain 8% Ciclopirox. This is on par with the clinical findings of Foley et al., who stated that only low-quality evidence supported the efficacy of ciclopirox 8% lacquer.26 Testing of Ciclopirox have compiled with broad antibiotic susceptibility profile to dermatophytes as well as non-dermatophytes.26-29 The present study also indicated a higher rate of fungal growth inhibition with the silver nano-coated drug compared to the plain drug. Further studies are warranted to establish its optimal use and long-term efficacy of the clinical settings.
Future Directions and Recommendations
The study recommends that the incorporation of silver nanoparticles is highly helpful to enhance the efficacy of antifungal agents. In vitro animal model testing of 8% Ciclopirox coated on silver-nanoparticles is highly potential than plain 8% Ciclopirox.
The study concludes that present research contributes valuable insights into the multifaceted aspects of non-dermatophytic onychomycosis. To the extent of our knowledge we elaborated our research on the fungal causative agents, clinical presentations, and their advanced treatment strategies for the benefit of the patients as well as clinicians. Our findings hold implications for both clinicians and researchers to improve the clinical management and their outcomes for the patients affected by NDO.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Sri Balaji Vidyapeeth (Deemed to be University) and the Department of Microbiology, MGMCRI, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by Sri Balaji Vidyapeeth deemed-to-be university, India, reference number SBV/IRC/SEEDMONEY/125/2022.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Mahatma Gandhi Medical College and Research Institute, Puducherry, India, vide reference number MGMCRI/2023/IRC/04/IHEC/30
- Kaur R, Kashyap B, Makkar R. Evaluation of clinicomycological aspects of onychomycosis. Indian J Dermatol. 2008;53(4):174-178.
Crossref - Rafat Z, Hashemi SJ, Saboor-Yaraghi AA, et al. A systematic review and meta-analysis on the epidemiology, casual agents and demographic characteristics of onychomycosis in Iran. J Mycol Med. 2019;29(3):265-272.
Crossref - Drake LA, Patrick DL, Fleckman P. The impact of onychomycosis on quality of life: Development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol. 1999;41(2)1999:189-196.
Crossref - Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28(2):151-159.
Crossref - Hattori N, Shirai A, Sugiura Y, et al. Onychomycosis caused by Fusarium proliferatum. Br J Dermatol. 2005;153(3):647-649.
Crossref - Ramani R, Ramani A, Shivananda PG. Penicillium species causing onychomycosis. J Postgrad Med. 1994;40(2):87-88.
- Romano C, Paccagnini E, Difonzo EM. Onychomycosis caused by Alternaria spp. in Tuscany, Italy from 1985 to 1999. Mycoses. 2001;44(3-4):73-76.
Crossref - Barde AK, Singh SM. A Case of Onychomycosis caused by Curvularia lunata (Wakker) Boedijn/Ein Fall einer Onychomykose durch Curvularia lunata (Wakker) Boedijn. Mycoses. 1983;26(6):311-316.
Crossref - Farwa U, Abbasi SA, Mirza IA, et al. Non-dermatophyte moulds as pathogens of onychomycosis. J Coll Physicians Surg Pak. 2011;21(10):597-600.
Crossref - Rippon J. Philadelphia: W.B. Saunders Co.; Medical Mycology: The pathogenic fungi and the pathogenic actinomycetes. 1988:276-296.
- Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43(4 Suppl):S70-S80.
Crossref - Gaikwad S, Ingle A, Gade A, et al. Antiviral activity of mycosynthesized silver nanoparticles against Herpes Simplex virus and Human Parainfluenza Virus Type 3. Int J Nanomed. 2013;8(1):4303-4314.
Crossref - Iravani S. Green synthesis of metal nanoparticles using plants. Green Chemistry. 2011;13(10):2638-2650.
Crossref - Adekhandi S, Pal S, Sharma N. Incidence and Epidemiology of Onychomycosis in Patients Visiting a Tertiary Care Hospital in India. Cutis. 2015;95(1):E20-E25.
- Drake LA, Patrick DL, Fleckman P, Andr J, Baran R, Haneke E, Sapède C, Tosti A. The impact of onychomycosis on quality of life: development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol. 1999;41(2 Pt 1):189-96.
- Halvaee S, Daie-Ghazvini R, Hashemi SJ, Khodavaisy S, Rahimi-Foroushani A, Bakhshi H, Rafat Z, Ardi P, Abastabar M, Zareei M, Borjian-Boroujeni Z and Kamali Sarvestani H (2021) A Mycological and Molecular Epidemiologic Study on Onychomycosis and Determination In Vitro Susceptibilities of Isolated Fungal Strains to Conventional and New Antifungals. Front. Cell. Infect. Microbiol. 11:693522.
Crossref - Motamedi M, Ghasemi Z, Shidfar MR, Hosseinpour L, Khodadadi H, Zomorodian K, Mirhendi H. Growing Incidence of Non-Dermatophyte Onychomycosis in Tehran, Iran. Jundishapur J Microbiol. 2016; 9(8):e40543.
- Ranawaka RR, de Silva N, Ragunathan RW. Non-dermatophyte mold onychomycosis in Sri Lanka. Dermatol. Online J. 2012;18:7.
- Nkondjo MS, Fabrizi V, Papini M. Onychomycosis in Cameroon: a clinical and epidemiological study among dermatological patients. Int. J. Dermatol. 2012;51:1474–1477.
Crossref - Adhikari L, Das GA, Pal R. Singh TS. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J. Pathol. Microbiol. 2009;52: 194-197.
Crossref - Das, N. K. et al. A study on the etiological agent and clinico-mycological correlation of fngernail onychomycosis in eastern India. Indian J. Dermatol. 2008;53:75-79.
Crossref - Hilmioglu-Polat, S. et al. Non-dermatophytic molds as agents of onychomycosis in Izmir, Turkey: a prospective study. Mycopathologia. 2005 160;125-128.
Crossref - Gianni C, Romano C. Clinical and histological aspects of toenail onychomycosis caused by Aspergillus spp.: 34 cases treated with weekly intermittent terbinafne. Dermatology. 2004;209:104-110.
Crossref - English MP, Atkinson R. Onychomycosis in elderly chiropody patients. Br. J. Dermatol. 1974;91:67-72.
Crossref - Erdogan FG, Yıldırım D, Çakır Akay GA. Onychomycosis Unresponsive to Antifungals: Etiology and Treatment with a New Direct Technique. Indian J Dermatol. 2019;64(6):476-481.
- Foley K, Gupta AK, Versteeg S, Mays R, Villanueva E, John D. Topical and device-based treatments for fungal infections of the toenails. Cochrane Database Syst Rev. 2020;1(1):CD012093.
Crossref - Rana R, Gupta P, Khurana M. Mechanistic insights into the antifungal action of ciclopirox and silver nanoparticle-ciclopirox formulations on non-dermatophytic fungi. 2023. Nanomedicine and Mycology, 2023;8(4):301-314.
- Rodríguez-Tudela JL., Alastruey-Izquierdo A, Cuenca-Estrella M. Non-dermatophytic onychomycosis: Emerging etiological agents and treatment challenges. Journal of Fungal Infections, 2023;14(2):112-130.
- Kim SH, Park JW, Choi SH, Lee JY. Evaluation of antifungal activity of ciclopirox and silver nanoparticle-ciclopirox conjugate against non-dermatophytic fungi isolated from onychomycosis. Antifungal Research Journal, 2023;26(5): 307-315
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.