ISSN: 0973-7510
E-ISSN: 2581-690X
Nephropathy is a global health issue that affects more than 20% of the adult population. Nephropathy is expected to be the fifth leading cause of death worldwide over the coming two decades. The introduction of green microalgae in nutrition and therapeutics for their biological activities is increasing. The current study examined the effect of Chlorella sorokiniana on renal health after inducing nephrotoxicity in mice. Preliminary screening of the algal aqueous extract revealed the presence of soluble polyphenols and triterpenoids. Successive intraperitoneal doses of gentamicin were administered to mice to induce nephrotoxicity. Concurrent intraperitoneal doses of the algal extract were administered to the infected mice to evaluate their nephroprotective activity. Two different concentrations of the treatment agent were administered in successive doses to two groups of mice. The tested concentrations were 150 and 300 mg/kg of mouse weight, respectively. The other two groups were either left untreated (normal control) or treated only with antibiotics (negative control). Creatinine, urea, and uric acid levels were analyzed in both serum and urine samples to evaluate the renal health of each animal group. Histochemical examination of the renal tissues was performed to assess the damage and improvement status. In vivo studies revealed a promising and significant nephroprotective activity of C. sorokiniana.
Microalgae, Nephroprotective, Natural Products, Traditional Medicine, Renal Impairment, Chlorella sorokiniana
The human utilization of microalgae in food and health supplements has been well known.1 Food and other supplements incorporated with microalgae extracts can trigger an array of metabolic and immunological processes in the human gut stressed by enteric bacterial pathogens.2 Additionally, microalga extracts have the potential to modulate the human immune response due to the rich-sulfated polysaccharide constituents.3 Several Chlorella spp. have been studied for their health benefits and nutritional values with a focus on Chlorella vulgaris and C. sorokiniana.
The nutrient value of microalgae species, in particular Chlorella, is related to their enrichment content of proteins, minerals, and fatty acids.4 High content of polyunsaturated fatty acids was detected and isolated from stressed Chlorella species. The major detectable fatty acid is eicosapentaenoic acid (EPA).5
Research has focused on the health benefits of consuming various Chlorella species. Biological activities including hypoglycemic activity, blood tonic, increased hemoglobin concentration, and hepato-protective properties of Chlorella have been investigated and reported.6 Additionally, decreased insulin resistance was reported among the health benefits of consuming Chlorella species.7 Improvement of cardiovascular system health after chlorella consumption was demonstrated as well, these include the hypotensive and hypolipidemic effects. In an animal model performed at an earlier time, the cardiovascular function was enhanced, and a hypotensive effect was achieved after consumption of regular oral doses of Chlorella extract.8 Agonists of peroxisome proliferator-activated receptors (PPARs) have been isolated from the green algae C. sorokiniana. PPARs are reportedly involved in lipid metabolism and glucose homeostasis.9
Furthermore, Chlorella species have been proven to have antioxidant and antineoplastic properties.10 A glycoprotein isolated from the C. vulgaris culture supernatant exhibited protective activity against tumor metastasis and chemotherapy-induced immunosuppression. In addition, Chlorella spp. prevent intoxication and reduce the toxicity of several harmful agents. Reports have demonstrated its activity against cadmium-induced toxicity,11 HgCl2-induced oxidative stress and nephrotoxicity,12 digoxin toxicity,13 and ethionine intoxication.14 Toxicity after excessive radiation exposure has been ameliorated and the healing was accelerated after consumption of Chlorella species in experimental animal models.15 C. sorokiniana lipid extract proved to improve short-term memory in rats.16 However, the ecosystem receives benefits from the microalgae organisms as the algae has a fast-growing property and saving of freshwater and arable land. Microalgae can utilize excess salt and waste from water streams and can be incorporated into biofuel synthesis that is less toxic and biodegradable and has a CO2 sequestering activity.17
Folk in developing countries relies heavily on natural products for the treatment and prophylactic purposes of various minor and major health disorders, including diabetes.18 Renal diseases are still among the devastating health disorders that people have tried to treat by self-prescription or following traditional recipes. This study aimed to evaluate the potential nephroprotective activity of a C. sorokiniana isolate from Jordanian water.
Microalgae species
The algal species were previously isolated from Jordanian water and identified using 18S rRNA and internal transcribed spacer (ITS) DNA markers. The algal specimens were identified and registered in GenBank under the accession numbers MW130898 and MW134039, respectively.19
Propagation of Chlorella sorokiniana biomass
The identified strain of Chlorella was propagated for biomass production in the laboratories of the Biotechnology Department, Faculty of Agricultural Technology, Al-Balqa Applied University, and single colonies were examined for purity using a compound light microscope and grown in 10 mL of Bold`s Basal Medium (BBM) under the following conditions: light: dark (L:D) cycle in 16:8 h, light intensity 120–150 photon·m−2s−1 at 22°C with mixing at 350 rpm. After blooming, the culture was checked for the presence of any contamination and used to seed 100 mL of BBM, after which the culture was upscaled to 500 mL BBM.
Biomass was harvested in the late log phase by centrifugation at 3.99 x103 g for 10 min. The pellet was washed twice with Milli-Q water and centrifuged again to collect cells. The pellet was then freeze-dried using a benchtop manifold freeze dryer (Millrock Technology®, UK).
Phytochemical screening of secondary metabolites
Qualitative preliminary screening tests were performed to investigate the presence of secondary metabolites in algal extracts. The following tests were employed to detect the presence of polyphenols, polysteroids, flavonoids, and triterpenoids:
Qualitative test for polyphenols
Water-soluble phenols were detected by adding drops of ferric chloride solution (1%) to 100 μL of each extract. A change in color to red after gentle shaking indicated a positive result. Insoluble phenols were detected after adding dichloromethane (100 μL) and one drop of pyridine to each extract (100 μL). A change in color indicates a positive result.20
Qualitative test for flavonoids
Qualitative analysis of the flavonoid content was adapted from the Kumar method, and each extract was mixed once with aqueous sodium hydroxide solution, followed by addition of concentrated hydrochloride solution. The appearance of an intense yellow color after alkaline addition and a reversion to the original color after adding the acid indicates the presence of a flavonoid compound.20
Qualitative test for polysteroids
Few drops of acetic anhydride solution (~150 μL) was added to each extracted sample (500 μL), and three drops of concentrated sulfuric acid was then added to this mixture. The mixture was allowed to stand for 5 min and changes in color were observed. Appearance of a blue to green indicates a positive result.20
Qualitative test for triterpenoids
Slow addition of each extract (1 mL) to chloroform (400 μL) followed by the addition of concentrated sulfuric acid (400 μL) was performed in a fume hood cabinet. Changes in color to red, brown, or purple at the interface indicate a positive result.20
Experimental animals
The experimental animals (male BALB/c albino mice) were obtained from the animal house and acclimatized in the research lab at the Faculty of Pharmacy, Mutah University. Mice housing, food, and environment following the “Guide for the care and Use of laboratory animals and “ARRIVE” guidelines. The study protocol was approved by the scientific and ethical committee of the Faculty of Pharmacy, Mutah University (approval number 5/2021/2022).
Induction of nephrotoxicity
Nephropathy was induced in healthy mature male mice after seven successive intraperitoneal doses of gentamicin solution (150 mg/kg mouse body weight). The method was adopted from that described by Edeogu et al.21
Protective treatment with microalgae solutions
Two groups of mice were treated separately with intraperitoneal injections of an aqueous solution of microalgae for seven successive days. Microalgae solutions (150 and 300 mg/kg) were injected eight hours before gentamicin (500 mg/kg) to investigate its protective activity.
The other two animal groups were the normal and negative controls. The first group was only administered distilled water, while the second group was only administered gentamicin. Nephrotoxicity and nephroprotective activity were confirmed using biochemical and histological analyses. The mice were not treated with anesthetics.
Collection and analysis of biological samples
Blood, urine, and renal tissues were collected from the mice for biochemical and histological analysis. Blood samples were collected from the cardiac ventricles of each experimental mouse, under mild anesthesia using diethyl ether vapors. The blood was then centrifuged and the supernatant was analyzed for biochemical measurements. The kidneys were extracted immediately after sacrificing the animals and stored in formalin solution (10%). Urine samples were collected after spontaneous urination using a clean glass slap. Serum and urine samples were analyzed using liquicolor-HUMAN® kits to determine creatinine (Cr), urea (UA), and uric acid (U) concentrations. The absorbance of each sample was measured at lmax= 490–510, 340 nm, and 520 nm.
Trans-sectional cut tissue samples with a thickness of 5 µm were prepared and stained by an expert in the laboratories of the Specialty Hospital, Amman-Jordan. The sections were then examined by histopathologists, and microscopic images were captured for further examination.
Statistical analysis
Statistical analyses were conducted using SPSS software; Statistical Package for the Social Sciences® software, version 21. Values were considered significantly different if the P-value was ≤ 0.05.
Microalgae species and phytochemical screening of secondary metabolites
According to qualitative screening tests, the aqueous extract of algal biomass showed the presence of water-soluble polyphenols and triterpenoids. Flavonoid and polystyrene qualitative tests revealed negative results for the biomass analyzed.
Experimental animals
Biochemical measurements
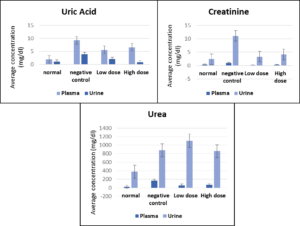
The biochemical measurements of kidney parameters for the four mice groups, namely, control, negative control, low dose (150 mg/kg), and high dose (300 mg/kg), are shown in Table.
Table :
Biochemical measurements of kidney parameters in normal (untreated), negative control (treated with gentamicin only), low-dose (treated with 150 mg/Kg mouse weight of algae extract), and high dose (treated with 300 mg/Kg mouse weight of algae extract).
| Uric acid | ||||
|---|---|---|---|---|
| Sample Group | Normal Control (mg/dl ± SD) |
Negative Control (Gentamicin only) (mg/dl ± SD) |
Low-dose extract (mg/dl ± SD) |
High-dose extract (mg/dl ± SD) |
| Blood samples | 2.02 ± 1.41 | 9.27 ± 3.46 | 5.61 ± 0.56 | 6.62 ± 1.87** |
| Urine samples | 1.12 ± 0.189 | 3.92 ± 0.49 | 2.07 ± 0.75* | 0.89 ± 0.64** |
| Creatinine | ||||
| Blood samples | 0.42 ± 0.085 | 0.95 ± 0.098 | 0.14 ± 0.076* | 0.25 ± 0.099** |
| Urine samples | 2.39 ± 0.82 | 11.09 ± 2.68 | 3.32 ± 0.65* | 4.15 ± 1.67** |
| Urea | ||||
| Blood samples | 23.88 ± 4.96 | 168.67 ± 43.34 | 53.00 ± 7.26* | 67.07 ± 12.72** |
| Urine samples | 378.70 ± 64.99 | 880.93 ± 142.90 | 1103.33±127.95* | 859.67 ± 105.51** |
* indicates a significant decrease in the low-dose treated animals compared to the negative control
** indicates a significant decrease in the high-dose treated animals compared to the negative control.
A significant improvement in kidney function was observed after treating mice for nephropathy. Low-dose treatment (150 mg/kg) with the green microalgae C. sorokiniana showed a significant decrease (P ˂ 0.05) in creatinine and urea serum levels compared to those reported from the negative control. Treatment with a high dose of the preparation significantly decreased serum uric acid levels (P ˂ 0.05). Similar results were obtained after analyzing the urine samples; all the measured kidney parameters responded significantly (P ˂ 0.05) to low-dose treatment compared to the negative control. No significant differences (P ˃ 0.05) were observed between the data obtained after the low-dose treatments and those treated with high doses. Figure 1 shows the comparison between uric acid, creatinine, and urea levels in all the tested groups.
Figure 1. Changes in uric acid, creatinine and urea serum and urine levels (± SE) in the four tested groups (normal, negative control, low dose, and high dose)
Histological analysis
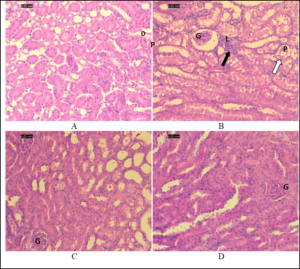
The kidney sections of the control group exhibited normal glomeruli and capsular space; proximal tubules appeared filled because of the long microvilli of the brush border, while the lumens of distal tubules appeared empty (Figure 2A). Kidney sections of the gentamicin-treated mice showed shrunken glomeruli with a wide capsular space. The outer layer of the cortex showed more damage than the medulla did. Tubular alterations in the gentamicin-treated group included necrosis, degeneration, vacuolization, and tubular dilatation in most of the proximal convoluted tubules and, to a lesser extent, in the distal tubules, in addition to the loss of the proximal tubular brush border (Figure 2B). High-dose gentamicin/algae-treated mice showed less damage than low-dose gentamicin/algae-treated mice (Figure 2C), and gentamicin-treated groups (Figure 2D).
Figure 2. Photomicrographs of the renal cortex sections stained by hematoxylin and eosin. (A) Normal kidney (control group). (B) Kidney of a mouse treated with gentamicin. (C) Kidney of a mouse treated with a low dose of algae with gentamicin. (D) Kidney of a mouse treated with high dose of algae with gentamicin.
Note shrunken glomerulus with wide capsular space, leukocytic infiltration (black arrows), and tubular degeneration (white arrows) in B. Also, note that tubular damage is less in D than that in gentamicin-treated group (B). D: distal tubules; G: glomerulus; L: leukocyte infiltrate; P: proximal tubules
Nephropathy is considered a global health issue affecting more than 20% of the adult population.22 Nephropathy is expected to be the fifth cause of death worldwide in the coming two decades.23 Gentamicin is a well-known broad-spectrum antibiotic with a common side effect of nephrotoxicity.24 To the best of our knowledge, the use of green microalgae C. sorokiniana isolated from Jordanian water as a prophylactic remedy to preserve renal health is yet to be investigated.
Preliminary screening of the aqueous extract revealed the presence of some secondary metabolites that are well-known for their antioxidant activity, consistent with previous reports published recently.25 Reversing or ameliorating the negative effect of antibiotics after microalgae consumption could be related to the antioxidant activity of microalgal extracts.
The results obtained in the current study are consistent with previously published data and support our hypothesis. Nephroprotective activity has been linked to the polyphenol content in numerous microalgae species, in addition to other biological properties. Maadane et al.26 have correlated the presence of polyphenolic compounds with their potential biological activities in numerous microalgae species.26 The tested species isolated from Moroccan water include Dunalliela, Tetraselmis, and Nannochloropsis gaditana species. In addition, compounds such as gallic acid, protocatechuic acid, catechin, chlorogenic acid, and epicatechin have been identified.27
Phenolic compounds were identified among other active phytoconstituents in plant extracts and these compounds were linked to the antioxidant and nephroprotective activity of the plants.28 These phytochemical constituents include succinic, benzoic, hydroxybenzoic, coumaric, caffeic acids, tetrahydroflavone, spiraeoside, and rutin.29,30 Moreover, variable phytoconstituents have been identified and isolated from C. sorokiniana. These compounds include carotenoids, lutein, and a-, β-, and g-tocopherols.31
The obtained results from the biochemical studies showed a significant improvement in kidney functions. All the tested parameters, with respect to creatinine, uric acid, and urea levels, responded well to the algal doses and minimized the harmful effects of aminoglycosides. At the cellular level, consumption of regular prophylactic doses of the algal extract reduced the damage precipitated by gentamicin compared with those left untreated. Biochemical and histochemical data were concomitant and supported our hypothesis. Microalgae have the potential to preserve the damage caused by gentamicin by optimizing its function and enhancing the signs of inflammation and necrosis.
The use of green microalgae in nutrition and therapeutics is still limited despite their valuable content and plentiful resources. C. sorokiniana isolated from Jordanian water has promising nephroprotective activity owing to its enriched polyphenol and antioxidant contents. The hypothesis regarding the nephroprotective property of the algae is related to the secondary metabolite content, particularly polyphenols, was supported by the obtained results. These findings should vitalize the use of microalgae extracts in health supplements owing to their enriched content, low cost, and eco-friendly nature. Further studies analyzing the microalgae content under optimal and stressed conditions are warranted.
ACKNOWLEDGMENTS
The authors would like to thank Technical and Support Staff in the laboratories of the Faculty of Pharmacy, Mutah University, the Pharmacological and Diagnostic Research Centre, Faculty of Pharmacy, Al-Ahliyya Amman University, and the Department of Biotechnology, Faculty of Agricultural Technology, Al-Balqa Applied University for their help and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Faculty of Pharmacy, Mutah University, Jordan with approval number 5/2021/2022.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Nazih H, Bard JM. Microalgae in human health: interest as a functional food. Microalgae in Health and Disease Prevention. 2018:211-226.
Crossref - Hulst M, Van der Weide R, Hoekman A, Van Krimpen M. Transcriptional response of cultured porcine intestinal epithelial cells to micro algae extracts in the presence and absence of enterotoxigenic Escherichia coli. Genes Nutr. 2019;14:8.
Crossref - Park GT, Go RE, Lee HM, et al. Potential anti-proliferative and immunomodulatory effects of marine microalgal exopolysaccharide on various human cancer cells and lymphocytes in vitro. Marine Biotechnol. 2017;19(2):136-146.
Crossref - Rani K, Sandal N, Sahoo PK. A comprehensive review on chlorella-its composition, health benefits, market and regulatory scenario. The Pharma Innovation Journal. 2018;7(7):584-589.
- Harwood JL. Algae: critical sources of very long-chain polyunsaturated fatty acids. Biomolecules. 2019;6;9(11):708.
Crossref - Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allah EF. Microalgae metabolites: A rich source for food and medicine. Saudi J Biol Sci. 2019;26(4):709-722.
Crossref - Jeong H, Kwon HJ, Kim MK. Hypoglycemic effect of Chlorella vulgaris intake in type 2 diabetic Goto-Kakizaki and normal Wistar rats. Nutr Res Pract. 2009;3(1):23-30.
Crossref - Shimada M, Hasegawa T, Nishimura C, et al. Anti-hypertensive effect of g-aminobutyric acid (GABA)-rich Chlorella on high-normal blood pressure and borderline hypertension in placebo-controlled double blind study. Clin Exp Hypertens. 2009;31(4):342-354.
Crossref - Chou YC, Prakash E, Huang CF, et al. Bioassay-guided purification and identification of PPARa/g agonists from Chlorella sorokiniana. Phytother Res. 2008;22(5):605-613.
Crossref - Raghuwanshi N, Arora N, Varshney R, Roy P, Pruthi V. Antineoplastic and antioxidant potential of phycofabricated silver nanoparticles using microalgae Chlorella minutissima. IET Nanobiotechnol. 2017;11(7):827-834.
Crossref - Rehman A, Shakoori AR. Tolerance and uptake of cadmium and nickle by Chlorella sp., isolated from tannery effluents. Pak J Zool. 2004;36(4):327-331.
- Blas-Valdivia V, Ortiz-Butron R, Pineda-Reynoso M, Hernandez-Garcia A, Cano-Europa E. Chlorella vulgaris administration prevents HgCl2-caused oxidative stress and cellular damage in the kidney. J Appl Phycol. 2011;23(1):53-58.
Crossref - Lee I, Tran M, Evans-Nguyen T, et al. Detoxification of chlorella supplement on heterocyclic amines in Korean young adults. Environ Toxicol Pharmacol. 2015;39(1):441-446.
Crossref - Sulaiman S, Shamaan NA, Ngah WZ, Yusof YA. Chemopreventive effect of Chlorella vulgaris in choline deficient diet and ethionine induced liver carcinogenesis in rats. Int. J. Cancer Res. 2006; 2(3):234-241.
Crossref - Beheshtipour H, Mortazavian AM, Mohammadi R, Sohrabvandi S, Khosravi-Darani K. Supplementation of Spirulina platensis and Chlorella vulgaris algae into probiotic fermented milks. Comprehensive Reviews in Food Science and Food Safety. 2013;12(2):144-154.
Crossref - Morgese MG, Mhillaj E, Francavilla M, et al. Chlorella sorokiniana extract improves short-term memory in rats. Molecules. 2016;21(10):1311.
Crossref - Zhang Y, Kendall A. Consequential analysis of algal biofuels: Benefits to ocean resources. J Clean Prod. 2019;231:35-42.
Crossref - Al-Halaseh LK, Al-Jawabri NA, Al-Btoush H, et al. In vivo investigation of the potential hypoglycemic activity of Pennisetum setaceum: Justification of the traditional use among Jordanians. Res J Pharm Technol. 2022;15(7):3185-3189.
Crossref - Sweiss MA. Microalgae for Wastewater Treatment and Biomass Production from Bioprospecting to Biotechnology. (Bath,UK). 2017.
- Cock IE, Kukkonen L. An examination of the medicinal potential of Scaevola spinescens: Toxicity, antibacterial, and antiviral activities. Pharmacognosy Res. 2011;3(2):85-94.
Crossref - Edeogu CO, Kalu ME, Famurewa AC, Asogwa NT, Onyeji GN, Ikpemo KO. Nephroprotective effect of Moringa oleifera seed oil on gentamicin-induced nephrotoxicity in rats: biochemical evaluation of antioxidant, anti-inflammatory, and antiapoptotic pathways. J Am Coll Nutr. 2020;39(4):307-315.
Crossref - Faria J, Ahmed S, Gerritsen KG, Mihaila SM, Masereeuw R. Kidney-based in vitro models for drug-induced toxicity testing. Arch Toxicoly. 2019;93(12):3397-3418.
Crossref - Luyckx VA, Al-Aly Z, Bello AK, et al. Sustainable development goals relevant to kidney health: an update on progress. Nat Rev Nephrol. 2021;17(1):15-32.
Crossref - Ali BH. Gentamicin nephrotoxicity in humans and animals: some recent research. Gen Pharmacol. 1995;26(7):1477-1487.
Crossref - Safitri NM, Hsu JL, Violando WA. Antioxidant Activity from the Enzymatic Hydrolysates of Chlorella sorokiniana and Its Potential Peptides Identification in Combination with Molecular Docking Analysis. Turkish Journal of Fisheries and Aquatic Sciences. 2021;11;22(4).
Crossref - Maadane A, Merghoub N, Ainane T, et al. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J Biotechnol. 2015;215:13-19.
Crossref - Jerez-Martel I, Garcia-Poza S, Rodriguez-Martel G, Rico M, Afonso-Olivares C, Gomez-Pinchetti JL. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J Food Qual. 2017;2017.
Crossref - Hasan A, Issa R, Al-Halaseh LK, Abbas M, Al-Jawabri N, Al-Suhaimat R. Investigation of the nephroprotective activity of Moringa peregrina leaves aqueous extract in mice. Pharmacia. 2022 (in press).
- Cienfuegos-Pecina E, Ibarra-Rivera TR, Saucedo AL, et al. Effect of sodium (S)-2-hydroxyglutarate in male, and succinic acid in female Wistar rats against renal ischemia-reperfusion injury, suggesting a role of the HIF-1 pathway. Peer J. 2020;8:e9438.
Crossref - Radwan RR, Fattah SM. Mechanisms involved in the possible nephroprotective effect of rutin and low dose g irradiation against cisplatin-induced nephropathy in rats. J Photochem Photobiol B. 2017;169:56-62.
Crossref - Matsukawa R, Hotta M, Masuda Y, Chihara M, Karube I. Antioxidants from carbon dioxide fixing Chlorella sorokiniana. J Appl Phycol. 2000;12(3):263-267.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.