ISSN: 0973-7510
E-ISSN: 2581-690X
Multiple plant growth-promoting attributes with N-acyl homoserine lactone (AHL)-mediated quorum sensing exhibiting bacterial strains can help plants to withstand varying abiotic and biotic stress conditions for improving the plant health and productivity. In total, 306 bacterial isolates were isolated from diverse locations and sites. In our exploration, bacterial isolates were screened based on AHL production, plant growth-promoting attributes, abiotic stress tolerance, and antagonistic activity against phytopathogenic fungi. Among the screened 306 isolates, 4 (11VPKHP4, 7VP51.8, P51.10, NBRI N7) were selected based on their efficiency in AHL production, biofilm formation, enduring different abiotic stress conditions, exhibiting plant growth-promoting attributes, and antagonistic activity. Based on 16S rRNA gene sequencing analyses of the selected 4 isolates belong to Pseudomonas genera. Selected isolates 11VPKHP4, 7VP51.8, P51.10, and NBRI N7 were also proficient in biosurfactant production, emulsification, suggesting that all isolates fabricate emulsifiers. The plant growth promotion potential of selected 4 bacterial isolates showed significant growth enhancement in all the vegetative parameters of Zea mays under control as well as drought stress condition. Biochemical parameters and defense enzymes under drought stress conditions were also modulated in the PGPR treated plants as compared to their uninoculated respective controls. With quorum sensing, multiple PGPR attributes, stress tolerance, biofilm formation, and EPS production the selected isolates have the potential to facilitate enhanced plant growth, rhizosphere colonization, maintenance of soil moisture content under normal and diverse stresses.
Quorum sensing, AHL, PGPR, Biofilm, Drought
All living beings on this planet are affected by varying climatic conditions such as prolonged drought, intense rains, flooding, high and low temperatures that also exert a detrimental impact on plants as well as on soil microbes too. In the coming years, globally it is expected to have double food demand1 and crop productivity loss by 30%, due to abiotic stresses.2 Among all abiotic stresses, drought is a major constrain,1,3 and drought-affected arable land has increased more than twice from the 1970s to early 2000s, and the unaffected land areas are expected to reduce to half by 2050, presenting a great threat to worldwide crop productivity.4 Drought stress affects Zea mays growth during vegetative and reproductive growth5 by altering morphological responses and physiological functions.6 Reactive oxygen species (ROS) produced by plants cause injury to macromolecules and DNA7 facing drought stress. Many approaches are being used to cope up with this critical condition and among them, researchers are focusing on agro-eco-friendly sustainable technology by exploitation of plant growth-promoting rhizobacteria (PGPR) based formulations for improving stress amelioration, soil fertility, and crop productivity.
PGPR possesses multiple plant growth-promoting attributes including phosphate solubilization, phytohormone and siderophore production, nitrogen fixation, nutrients immobilization, exopolysaccharide production, biofilm formation.8-12 PGPR with multiple PGP attributes, enzymatic activities, and abiotic stress tolerance assists host plants in maintaining their health, growth, and development under normal as well as under stressed conditions. Among several reported PGPR, rhizobacteria belonging to Pseudomonas genera are very well worked out and considered as plant probiotics.13 Earlier studies have explored the importance of intercommunication between bacteria in respect to plant growth promotion. Signaling occurs between microbe-microbe: intra- and interspecies via diverse, small molecules like autoinducers, acyl-homoserine lactones (AHL). Quorum sensing via AHL molecules, typically produced by Pseudomonas genera in a cell-density-dependent manner, regulates numerous physiological processes including biofilm formation, virulence, competence, stationary phase, biosurfactant production, antibiotic production, hydrolytic enzymes, catalase activity, motility.14,15 Among genus Pseudomonas, quorum sensing is very well depicted in P. aeruginosa, P. putida, P. aureofaciens, P. fluorescens, P. syringae, P. corrugata and P. fuscovaginae and reported to produce AHLs. Apart from genus Pseudomonas, this sort of signaling has also been recognized in Agrobacterium and Rhizobium, Ochrobactrum, Serratia, and Ensifer.14,16,17 Root colonizing Pseudomonads together with multifarious plant growth-promoting attributes (abiotic stress endurance, biofilm formation, EPS and alginate, phosphate solubilization, IAA production, siderophore production, enzymatic activities, biosurfactant production, and inorganic pollutant degradation) will assist plant growth and development in diverse stress curse conditions. The PGPR endow plant defense by inhibiting the antioxidant biochemical system and enhancing the photosynthetic physiological system under stress conditions. Among all PGPR, the Pseudomonas genus has been well characterized to enhance total plant vegetative parameters in Zea mays, finger millet, sunflower, peas, and ornamental crops facing drought and resource limiting conditions.18-22 Strains from Pseudomonas genera were also explored for stress amelioration in maize, pea, wheat, mung bean, tomato.23,24 Based on earlier researches these bacteria have been isolated from different sources and characterized for the presence of PGP attributes but different geographical locations possessing QS exhibiting Pseudomonas spp. are not yet reported. Moreover, very negligible information on the role of stress-tolerant, quorum sensing exhibiting Pseudomonas spp. in enhancing Zea mays growth under drought stress was reported. Therefore, our main objective of the present study is to exploit the QS exhibiting Pseudomonas spp. from different geographical locations of India to characterize them for enhancing the drought stress tolerance in Zea mays.
Soil samples collection and isolation of bacteria
The soil samples for the isolation of bacteria were collected from various geographic locations from India as depicted in Table S1. These samples were then subjected to sieving with 2 mm mesh to separate plant debris and fauna. After sieving, each was used for the isolation of bacteria. Soil samples were collected in properly labeled sterile polythene sampling bags. For isolation of bacteria from collected soil samples, 1g of each soil sample was serially diluted in sterile saline water (0.85% NaCl) followed by shaking at 150 rpm for 15 min. 100 µL of each dilution was spread on Nutrient Agar (NA; Hi-Media, India) and Petri plates were incubated at 28°C for 24 h. Pure cultures of the bacterial isolates were maintained on NA and simultaneously in 30% glycerol for preservation and stored at -80°C.
Estimation of plant growth-promoting attributes, abiotic stress tolerance, and antagonistic activity of bacterial isolates
Bacterial isolates were subjected to qualitative evaluation for a wide array of PGP traits, abiotic stress tolerance, and antagonistic activity. Among PGR attributes, bacterial isolates were mainly evaluated for phosphate solubilisation25 and indole acetic acid (IAA) production.26 Further, all the isolates were examined for their tolerance against abiotic stresses such as drought/water stress (30%, 45%, and 60% polyethylene glycol 6000; w/v), salt (8% and 12% NaCl; w/v), temperature (37°C and 45°C), and pH (pH3, pH5, pH9, and pH11). All the bacterial isolates were grown in a nutrient broth for 24 h with the continuous shaking of 180 rpm at 28°C (except those tested for temperature stress). Viable cells (CFU/ml) of selected bacterial isolates were counted at different time intervals of 24 h up to 10 days by serial dilution plating on nutrient agar plates. The antagonistic activity was evaluated by comparing the fungal mycelial diameter on control and test plates where all the isolates were streaked on the periphery of Potato Dextrose Agar medium (PDA; HiMedia, India) while the pathogenic fungal mycelium of Rhizoctonia solani and Sclerotium rolfsii at the center. The plates were incubated at 28°C for 7 days. Control plates were prepared with the fungal agar plug without the bacterial streaks.
Screening and selection of bacterial isolates demonstrate quorum sensing
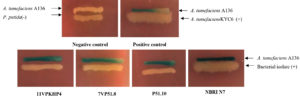
Two biosensors, Agrobacterium tumefaciens (A136; for short-chain AHLs) and Chromobacterium violaceum (CV026; for long-chain AHLs) were used to screen AHL production of all bacterial isolates. Screening of all the bacterial isolates for production AHLs was carried out by agar plate diffusion assay with some modifications.27 The test isolates for induction of AHL of CV026 were streaked on Luria agar (LA; Hi media, India) plates in parallel to the monitor biosensor. Similarly, discovering for AHL production against A136 was done in an analogous mode by supplementing the LA with 45 μg/mL of X-gal (Sigma Aldrich). Agrobacterium tumefaciens KYC6 used as a positive control (AHL producer) and P. putida as a negative control. The Petri plates were incubated for 24 to 48 h at 28°C and the production of purple pigment violacein and blue coloration by β-galactosidase activity was observed as a positive test for AHLs production.27
Quantitative evaluation of PGP attributes of bacterial isolates
Selected bacterial isolates were quantitatively evaluated for biofilm formation,28 exopolysaccharide,29 and alginate30 production. Production of auxin/ indole acetic acid (IAA),26 P- solubilization,25 and siderophore synthesis31 were also determined to understand their potential to be utilized for protecting the host plant under stressed conditions.
Evaluation of other characteristic features of selected bacterial isolates
After estimating bacterial isolates for their PGP traits, they were subjected to drop collapse assay for biosurfactant production described by Jain et al.32 and modified by Bodour and Miller-Maier.33 In addition, the emulsification index (E24) was determined by the method described by Cooper and Goldenberg.34 All the selected bacterial isolates were subjected to evaluation for swimming, swarming, and twitching motility based on zone diameter formation.34 The activity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase among all bacteria was qualitatively estimated by following the protocol of Penrose and Glick.36 Furthermore, selected bacterial isolates were qualitatively evaluated for protease, and cellulose production respectively.37,38 Catalase production was investigated by adding and assorting a drop of 3% hydrogen peroxide to 48h grown bacterial colony on an uncontaminated glass slide. The presence of effervescence is the indicator of catalase activity. Gelatinase activity was determined by preparing and inoculating bacterial isolates in gelatine tubes. Inoculated tubes were incubated at 28°C for 48h. After the incubation period, all tubes were kept on ice. Tubes that get solidified were considered gelatinase positive. Nitrate reduction was scrutinized by using the mannitol motility nitrate medium (MMNM; M1320, HiMedia, India).
16S rRNA gene PCR amplification and phylogenetic analysis of selected isolates
All the selected bacterial isolates were evaluated for 16s rRNA gene sequence amplification. For this bacterial genomic DNA was extracted from 24 h grown culture using a DNA isolation kit (Qiagen) and amplification was carried out for 16s rRNA gene using primers: forward (8F) primer (5’-AGAGTTTGATCCTGGCTCAG-3’) and reverse (1392R) primer (5’-ACGGGCGGTGTGTAC-3’).39 Further, PCR products were purified by QIAquick® PCR purification kit (Qiagen, Germany) followed by sequencing (3730XL DNA analyzer, Applied Biosystems, USA). Further, the sequences were compared with reference type isolate sequences available 16S rRNA database (https://www.eztaxon.org).
Evaluation of bacterial isolates for plant growth promotion under greenhouse condition
Following the screening of bacterial isolates based on their PGP attributes, quorum sensing activity via acyl-homoserine production, and other attributes in vitro, the selected ones were tested for their capability of PGP under plant test using Zea mays (“Maharaja”) as a model plant under control and water stress conditions. Experiments were accomplished in a wholly randomized block design under greenhouse conditions with 4 replicates using non-sterile field soil (2.5 kg soil per pot) from the experimental farm of CSIR-National Botanical Research Institute, Lucknow. Surface sterilized Z. mays seeds were bacterized with four AHL producing isolates i.e., 11VPKHP4; 7VP51.8; P51.10; NBRI N7 as described earlier.40 Simultaneously, two sets with 4 replicates for each treatment and control were grown in a greenhouse for 45 days. After 45 days of seed sowing, drought stress for fifteen days was given by refraining the irrigation in the water stress set. After 60 days of the plantation, plants were harvested from both sets i.e. non-stress as well as drought stress conditions. Vegetative parameters such as shoot and root length, fresh and dry shoot, and root weight were instantly taken and compared with the treated and control plants of both non-stress (NS) and drought stress (DS). For evaluation of dry weights, roots and shoots were kept at 50°C in a hot air oven.
Plant biochemical and antioxidant enzymatic assays
Total chlorophyll and carotenoid content in the leaves were determined accordingly by the protocol of Arnon.41 The proline and soluble sugar assay for leaf tissue were determined accordingly protocols of Bates et al.42 and Dubois et al.43 For determining different antioxidant enzyme activity spectrophotometrically, 500 mg of fresh tissue was homogenized with 100 mM of potassium phosphate buffer (pH 7) containing 0.1 mM EDTA and 1% polyvinylpyrrolidone (w/v) under chilled conditions followed by centrifugation at 15,000 g for 10 min. at 4°C. The collected supernatant was stored at -20°C for further analysis of Superoxide dismutase (EC1.15.1.1), Ascorbate peroxidase (EC1.11.1.11), Guaiacol peroxidize (EC 1.11.1.9) and Catalase (EC1.11.1.6) according to protocols of Beauchamp and Fridovich,44 Nakano and Asada,45 Beyer, and Fridovich,46 Aebi,47 respectively.
Statistical analyses
All data sets in this study were analyzed using SPSS ver 20.0. Initially, means were tested for homogeneity of variance to evaluate the variation among obtained values. Further, these means were compared by analysis of variance (ANOVA), followed by Duncan’s test to determine significance (p≤ 0.05).
Isolation and in vitro screening of bacterial isolates for AHL production, PGP attributes, abiotic stress tolerance, and antagonistic activity
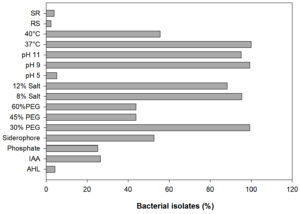
In the present study, a total of 306 bacterial isolates were (Supplementary Table S1) screened for AHL production, plant growth-promoting attributes, abiotic stress tolerance, and antagonistic activity. Results revealed that only 4 out of 306 bacterial isolates were identified as AHL producers with A136; coupling of the traR gene (luxR homolog) with lacZ provides a basis for the detection of acyl-homoserine lactones based on β-galactosidase activity (Fig. 1 and 2) whereas, none of the isolates were found to be positive with CV026. Screening of PGP attributes i.e. IAA production, P solubilization, and siderophore production for all the isolates was carried out. Among 306 isolates, 26.47% exhibited IAA production while 25.16% were able to solubilize phosphate (Fig. 1). Isolates were screened for siderophore production by chrome azurol S (CAS) assay, reported 52.61% out of total 306 isolates positive (Fig. 1). Drought stress tolerance was exhibited by 99.34% isolates at 30% PEG 6000 (w/v) while 43.79% isolates at 45% and 60% PEG 6000 (w/v) (Fig. 1). Salt stress tolerance showed by 95.42% and 88.23% isolates at 8% and 12% NaCl (w/v) and 5.22%, 99.34% and 95.09% isolates tolerant at pH 5, 9 and 11, respectively (Fig. 1). All isolates were able to grow at 37°C and 40°C while 55.55% were able to survive at 45°C. In the case of biotic stress, 2.28% and 3.92% isolates exhibited antagonistic activity against Rhizoctonia solanii and Sclerotium rolfsii, respectively (Fig. 1). Therefore, results of qualitative screening of 306 bacterial isolates lead to the selection of 4 isolates demonstrating AHL production together with PGP attributes and abiotic stress tolerance.
Abiotic stress tolerance of AHL producing selected bacterial isolates
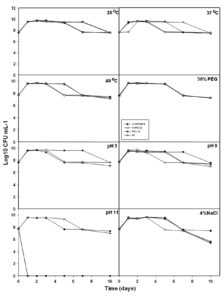
Further, selected 4 isolates were subjected for their abiotic stress tolerance i.e., temperature (37 and 40°C), drought (30%), pH (5, 9, and 11), and salt (NaCl 4%). Under control conditions, all isolates survived with 7 Log10 CFU ml-1 for over 10 days of the experiment (Fig. 3). At temperature 37°Cand 40°C, all isolates demonstrated their survival between 7.09 to 7.42 Log10 CFU ml-1 for over the 10th day (Fig. 3). Under drought stress i.e., 30% PEG, all isolates were competent to survive at the end of the 10th day with 7.22 to 7.27 Log10 CFU ml-1. At pH 5 and pH9, all isolates were able to survive over the 10th day with 7 Log10 CFU ml-1 (Fig. 3) but at pH11, only 11VPKHP4 and NBRI N7 have shown their survival at the end of the 10th day with 7.34 Log10 CFU ml-1 and 6.98 Log10 CFU ml-1 respectively (Fig. 3). Moreover, at 4% salt stress, 11VPKHP4 was competent to survive with 7.41 Log10 CFU ml-1 whereas 7VP51.8, P51.10, and NBRI N7 with 5.37, 5.51, and 5.68 Log10 CFU ml-1 at the end of the 10th day (Fig. 3). Further, four best performing bacterial isolates representing different geographic locations were selected based on their AHL producing ability, PGP, and abiotic stress tolerance ability.
Fig.2. Qualitative evaluation for screening of acyl-homoserine lactone production in all bacterial isolates using Agrobacterium tumefaciens (A136). (-) non-AHL producer, (+) AHL producer.
Identification of bacterial isolates
All four selected potential AHL producers were subjected for identification based on partial 16S rDNA sequences, revealed that all the bacterial isolates belong to Pseudomonas spp. 11VPKHP4, 7VP51.8, P51.10 and NBRI N7 showed the closest homology with P. Sesamii (99.36%), P. hunansis (99.86%), P. asiatica (100%), and P. aeruginosa (99.28%) respectively (Table 1).
Table (1):
Sampling sites along with their geographical coordinates, selected 4 strains with their corresponding 16S rRNA gene Gen Bank nucleotide accession numbers and closest taxonomic relationship.
Sample site |
Location |
Strains |
Accession No. |
Identification |
|---|---|---|---|---|
Kasol, Himachal Pradesh |
32.00N, 77.18E |
11VPKHP4 |
MZ268103 |
P. Sesamii |
Nagpur Pune agriculture field |
18.31N, 73.50E |
7VP51.8 P51.10 |
MZ268104 MZ268105 |
P. hunansis P. asiatica |
Kutch, Gujrat |
23.73N, 69.85E |
NBRI N7 |
MZ268106 |
P. aeruginosa |
Plant growth-promoting attributes of quorum sensing exhibiting Pseudomonas spp.
In the present study, four selected AHL producing isolates with abiotic stress tolerance ability were further evaluated for multiple PGP attributes. All the selected isolates illustrated biofilm formation under in vitro conditions. Significant differences among the isolates were monitored, according to absorbance ranging from 1.0 to 2.2 at 590 nm, depicted in Table 2. Furthermore, out of four bacterial isolates, P51.10 and 11VPKHP4 demonstrated maximum biofilm production i.e., 2.10 and 2.02 absorbance at 590 nm.
Table (2):
PGP attributes of selected AHL producing PGPR strains.
PGP attributes |
11VPKHP4 |
7VP51.8 |
P51.10 |
NBRI N7 |
|---|---|---|---|---|
Biofilm formation (O.D. at 590 nm) |
2.09±0.04b |
1.33±0.008a |
2.09±0.03b |
1.33±0.03a |
Exopolysaccharide production (µg/ml) |
256.62±0.46d |
127.27±0.07b |
120.12±0.32a |
146.16±0.61c |
Alginate (µg/ml) |
110.78±0.09b |
183.71±0.32c |
223.77±0.05d |
39.93±0.11a |
IAA production (µg/ml) |
73.64±1.68c |
65.75±0.34b |
66.15±1.61b |
47.78±0.22a |
Phosphate solubilisation (µg/ml) |
34.12±0.49c |
31.48±0.31b |
52.97±0.74d |
26.78±0.26a |
Siderophore production |
+ |
+ |
+ |
+ |
Values are mean±SE (n=3), compared by analysis of variance (ANOVA), and followed by Duncan’s test. Statistically, differences were determined at p ≤ 0.05 by using SPSS ver 20. “+” siderophore producing.
In the case of exopolysaccharide production after 72 h of growth, significant differences were revealed among all isolates, as varying levels of its production ranges between 120 to 256 μg mL-1 (Table 2). However, among 4 isolates, 11VPKHP4 exhibited maximum EPS (256.62μg mL-1) production. In the case of alginate production, 72 h of growth revealed varying levels of its production among four isolates, ranging between 39 to 223 μg mL-1 (Table 2). Among all four, isolate P51.10 produced maximum alginate 223.77 μg mL-1 followed by 7VP51.8 i.e. 183.71μg mL-1. Notably, varying levels of IAA production were observed among all isolates, ranging between 47 to 73 μg mL-1 as depicted in Table 2. Furthermore, maximum IAA production is reported for 11VPKHP4 at 73.64μg mL-1 followed by 66.15 μg mL-1 and 65.75 μg mL-1 in P51.10 and 7VP51.8, respectively. In the matter of quantitative estimation of P-solubilisation, all four isolates illustrate the varying levels of phosphate solubilization ranging from 26 to 52 μg mL-1. Maximum phosphate solubilization exhibited by P51.10 (52.97 μg mL-1) whereas isolates11VPKHP4 and 7VP51.8illustrated phosphate solubilization 34.12 and 31.48 μg mL-1, respectively (Table 2).
Biosurfactant production, emulsification index, motility, and enzymatic activities of selected AHL producing Pseudomonas spp.
In an evaluation for drop collapse assay for biosurfactant production, out of four AHL producers, only P51.10 and NBRI N7 were proficient in biosurfactant production. In addition, all the isolates were tested positive for emulsification and produces in the range of 18 to 54%, suggesting that all isolates fabricate emulsifiers (Table 3). It is conceivable that twitching motility, swarming as well as swimming motility was observed based on zone diameter formation. Twitching motility of isolates P51.10 and NBRI N7 assessed in between 2.0 to 2.5 cm and 1.5 to 2.0 cm diameter. Swarm diameter of P51.10 and 7VP51.8 assessed in between 1.5 to 2.0 cm and 1.0 to 1.5 cm respectively (Table 3). Swimming motility of isolates P51.10 and 11VPKHP4 was assessed more than 2.5 cm whereas that of 7VP51.8 and NBRI N21 in between 2.0 to 2.5 cm of diameter respectively (Table 3). Furthermore, all four isolates were proficient in ACC deaminase and catalase activity. However, among all the four isolates, only NBRI N7 demonstrated protease activity. In addition, 7VP51.8 and P51.10 were tested positive for gelatinase activity whereas only P51.10 tested positive for nitrate reduction. However, amylolytic and cellulolytic activity was not exhibited by any of these isolates under study (Table 3).
Table (3):
Biosurfactant production, emulsification index and enzymatic activities of selected AHL producing PGPR strains.
Characteristics |
11VPKHP4 |
7VP51.8 |
P51.10 |
NBRI N7 |
|---|---|---|---|---|
Biosurfactant production |
– |
– |
+ |
+ |
Emulsification index (E24%) |
18.53±1.14a |
19.44±1.38a |
25.75±1.51a |
54.66±2.66b |
Twitching motility |
– |
– |
+++ |
++ |
Swarming motility |
– |
+ |
++ |
– |
Swimming motility |
+++ |
++++ |
++++ |
++ |
ACC deaminase |
+ |
+ |
+ |
+ |
Proteolytic |
– |
– |
– |
+ |
Amylotic |
– |
– |
– |
– |
Cellulolytic |
– |
– |
– |
– |
Nitrate reduction |
– |
– |
+ |
– |
Catalase |
+ |
+ |
+ |
+ |
Gelatinase |
– |
+ |
+ |
– |
+ positive, – negative; ++++ more than 2.5 cm, +++ 2 to 2.5 cm of bacterial colony diameter; ++ 1.5 to 2.0 cm of bacterial colony diameter; + 1.0 to 1.5 cm of bacterial colony diameter; – 0.5 cm of bacterial colony diameter
Evaluation of plant growth promotion under control and drought condition in Zea mays
In the present study, greenhouse results demonstrated the enhanced plant growth parameters in PGPR treatments as compared to uninoculated controls under non-stress as well as drought stress curse conditions (Table 4). Significant differences in plant root and shoot length along with the fresh and dry mass of root have been recorded, in which 11VPKHP4 illustrated maximum enhanced shoot length followed by P51.10 (Table 4). However, P51.10 has been verified for maximum enhanced root length followed by 7VP51.8 and 11VPKHP4 under drought stress conditions (Table 4). Moreover, under drought stress conditions, 11VPKHP4 and P51.10 enhanced shoot and root length by 96.61%; 27.93%, and 76.24%; 66.32% respectively. In addition, P51.10 enhanced fresh root weight and dry root weight by 249.21% and 225.52% while fresh shoot weight and dry shoot weight enhanced by 230.65% and 227.17%. However, 11VPKHP4 enhanced fresh root weight and dry root weight by 116.24% and 134.26% while fresh shoot weight and dry shoot weight enhanced by 230.09% and 237.17% respectively (Table 4).
Table (4):
Plant growth promotion by AHL producing selected PGPR strains using Zea mays under control and drought stress.
| Bacterial isolates | Root Length (cm) |
Shoot Length (cm) |
Fresh Weight (g) | Dry Weight (g) | Ratio RDW/SDW |
||
|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | ||||
| Control | 36.0±0.40a | 56.7±0.61b | 3.88±0.37b | 7.44±0.32b | 1.11±0.02b,c | 1.83±0.36b | 0.60±0.01d |
| Control+D | 37.05±0.40a | 48.72±1.76a | 2.07±0.23a | 4.51±0.18a | 0.51±0.00a | 1.15±0.06a | 0.44±0.01b |
| 11vPKHP4 | 42.2±2.36b | 99.85±0.41h | 5.09±0.63c | 17.16±0.65f | 1.8±0.06d | 4.27±0.01g,h | 0.42±0.01b |
| 11vPKHP4+D | 47.4±0.42c | 95.8±0.39g | 4.49±0.11b,c | 14.88±0.24de | 1.2±0.16b,c | 3.87±0.22e,f | 0.31±0.03a |
| 7VP51.8 | 56.65±0.63d | 75.97±0.76d | 4.69±0.29b,c | 12.70±0.90d | 1.35±0.08c | 3.21±0.02d | 0.42±0.02b |
| 7VP51.8+D | 57.95±0.55d | 66.37±1.30 c | 3.61±0.10b | 9.52±0.21c | 0.97±0.08b | 2.46±0.12c | 0.30±0.02a |
| P51.10 | 55.25±0.75d | 85.95±1.44f | 7.4±0.54d | 17.42±0.17f | 2.29±0.17c | 4.35±0.02h | 0.52±0.03c |
| P51.10+D | 61.62±0.40e | 85.87±0.69f | 7.25±0.27d | 14.91±0.11e | 1.66±0.15d | 3.76±0.16e,f | 0.44±0.03b |
| N7 | 43.3±0.31b | 80.3±0.50e | 4.62±0.27b,c | 17.03±0.41f | 1.34±0.07c | 3.97±0.04f,g | 0.33±0.01a |
| N7+D | 44.52±0.65b | 77.15±0.45d | 3.72±0.27b | 14.98±0.35e | 0.90±0.03b | 3.55±0.17e | 0.25±0.01a |
Values are mean±SE (n=3), compared by analysis of variance (ANOVA), and followed by Duncan’s test. Statistically, differences were determined at p ≤ 0.05 by using SPSS ver 20.
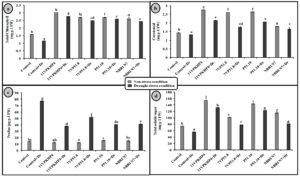
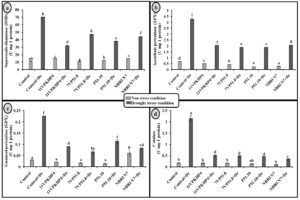
Fig. 4. Effect of PGPR on (a) Total chlorophyll (b) carotenoid content (c) proline and (d) Total soluble sugar in Zea mays plants grown under normal and drought stress conditions.
Values are mean±SE (n=3), compared by analysis of variance (ANOVA), and followed by Duncan’s test. Statistically, differences were determined at p ≤ 0.05 by using SPSS ver 20.
Effect of Pseudomonas spp. on biochemical and antioxidant enzymes of Zea mays
Inoculation with all four Pseudomonas spp. has significantly increased the chlorophyll (total), carotenoid, proline, and soluble sugar as compared to uninoculated plants under drought stress conditions (Fig. 4). However, 11VPKHP4 revealed a maximum increase in total chlorophyll content by 137.18%, while 56.64%, 49.72%, and 48.31% by P51.10, NBRI N7, 7VP51.8, respectively under drought stress condition whereas carotenoid content was enhanced by 62.29%, 56.50%, 32.98% and 25.17% in 11VPKHP4, P51.10, NBRI N7, 7VP51.8, respectively (Fig. 4). Plants inoculated with 11VPKHP4 and P51.10 revealed maximum enhanced total soluble sugar by 136.23% and 85.87% while 20.4% and 16.95% by NBRI N7 and 7VP51.8 under drought stress conditions (Fig. 4). Plants under drought stress revealed increased levels of proline but modulated by 50.76% and 47.47%, 46.47%, and 32.85% inoculation of 11VPKHP4, P51.10, NBRI N7, and 7VP51.8, respectively (Fig. 4). Our results revealed that plants growing under drought stress conditions have demonstrated a significant increase in all antioxidant enzyme activities. Bacterial isolates, 11VPKHP4, P51.10, NBRI N7 and 7VP51.8 modulated SOD activity by 54.12%, 45.72%, 37.04%, and 32.40% respectively (Fig. 5). In the case of APX, inoculation of P51.10, 7VP51.18, 11VPKHP4, and NBRI N7 modulated plants by 56.30%, 55.89%, 52.34%, and 51.50%, respectively. GPX activity was modulated by 16.02%, 14.44%, 13.61%, and 11.30% by inoculation of 7VP51.18, NBRI N7, 11VPKHP4, and P51.10, respectively. Similar to other soil enzymes, catalase activity was also modulated 83.62%, 78.61%, 77.45%, and 76.211% by inoculation of NBRI N7, P51.10, 7VP51.18, and 11VPKHP4, respectively (Fig. 5).
Fig. 5. Effect of PGPR on (a) superoxide dismutase (SOD), (b) ascorbate peroxidize (APX) (c) guaiacol peroxidase (GPX) and (c) catalase (CAT) in Zea mays plants are grown under normal and drought stress conditions.
Values are mean±SE (n=3), compared by analysis of variance (ANOVA), and followed by Duncan’s test. Statistically, differences were determined at p ≤ 0.05 by using SPSS ver 20.
Abiotic stresses affect our agro-ecosystem every year by generating a large area of uncultivable land and therefore impact food security, significantly. To overcome this problem, soil-inhabiting microbes with multifarious PGP and abiotic stress mitigating attributes have emerged as a solution.48,49 However, intensive research has been done towards characterizing the bacterial isolates from different locations for the presence of PGP attributes and their efficiency for enhancing plant growth under abiotic stresses.48,49 Our findings demonstrated that the Pseudomonas spp. with quorum sensing potential isolated from different geographic locations demonstrated multiple PGP attributes and stress ameliorating abilities. The information available on the characterization of QS exhibiting stress-tolerant Pseudomonas spp. along with other attributes for drought stress amelioration in Zea mays is very limited. However, few studies have exposed the presence of QS in bacteria.50,51 In our study, out of 306 bacterial isolates, 4 potent bacterial isolates were screened and selected based on exhibiting quorum sensing activity via AHL induction. These isolates belong to different geographical locations having different climatic conditions and soil types. In the present study, all 4 quorum sensing exhibiting bacterial isolates i.e., 11VPKHP4, 7VP51.8, P51.10, and NBRI N7, with multiple PGP attributes, and abiotic stress tolerance are reported from different geographical locations (Table 1). Notwithstanding, the novel attribute of quorum sensing activity via AHL production has been earlier reported in other members of Pseudomonas but for the first time reported in P. sesamii, P. hunansis, and P. asiatica.51 Moreover, quorum sensing signaling interaction between rhizospheric isolates was reported to produce AHLs.50,51 AHLs also act as inter-kingdom interacting signal molecules affecting plant growth, gene expression, and induction of systemic resistance in plants.53 On recognition of AHL molecules by plants, these AHL molecules modify root, shoot gene expression, amending defense, and cell growth responses, and also lead to lateral root development.54
In the present study, all isolates possess multifarious plant growth-promoting attributes like biofilm formation, exopolysaccharide production, alginate production, auxin production, phosphate solubilization, siderophore production, ACC deaminase activity, and abiotic stress endurance. Although, previous studies have also well documented that PGPR belonging to Pseudomonas genus with the PGP attributes demonstrated plant growth promotion under diverse abiotic stresses.55,56 In the context of AHL production, all four isolates were found to be the best biofilm formers (Table 2) as well as able to produce exopolysaccharides (EPS). Biofilm-forming PGPR confers resistance against various abiotic stresses, produces antibacterial compounds, enhances protection from desiccation.15 As compared to planktonic cells, biofilms formed from Pseudomonas, Bacillus, Trichoderma, Bradyrhizobium, Brevibacterium, Pantoea, and Acinetobacteria showed superior production of auxin, phosphate solubilization, siderophore production, nitrogenase activity.49,57 These were also found potent in plant growth promotion under diverse environmental stress conditions.57 Also, in the context of biofilm formation, all the isolates were proficient against drought (30% PEG), salt (4% salt), pH (5, 9 and 11), and temperature (37°C and 40°C) abiotic stresses as compared to control conditions till 10th day. Our findings are in corroboration with earlier studies,58,49 therefore, it is expected that all promising isolates will be able to withstand abiotic stress and abiotic stress amelioration. EPS secretion maintains soil structure and fertility by sustaining the water holding capacity and moisture content of the soil. Among all reported PGPR, the Pseudomonas genus has been well characterized to enhance plant vegetative parameters in maize, finger millet, sunflower, peas, and ornamental crops facing drought and resource limiting conditions.55,22,20 Among all the selected four isolates, 11VPKHP4 exhibited maximum EPS production (Table 2). However, Konnova et al.59 reported the role of EPS in the defense of A. brasilense Sp245 cells against desiccation. A considerable correlation was observed between the EPS amount produced by cowpea Bradyrhizobium strains and desiccation tolerance. P. putida KT2440 strain was able to synthesize four types of EPS, performing various functions in plant growth promotion.60 Alginate, an extracellular polysaccharide produced by a variety of Gram-negative bacteria including Azotobacter vinelandii, P. fluorescens, and P. aeruginosa. Earlier, Nivens et al.61 demonstrated that alginate production is not crucial for biofilm development but it plays role in biofilm 3-dimensional structure formation. In our findings, among all four isolates, P51.10 produced maximum alginate followed by 7VP51.8 (Table 2). Furthermore, in the present study, maximum IAA production was observed in 11VPKHP4 followed by P51.10 and 7VP51.8 (Table 2). Since, IAA secreted by bacteria may promote plant growth directly, by stimulating tissue differentiation, cell elongation, or cell division and tactic responses,62 the observed positive effect of our isolates on the biomass of Zea mays plants could be due to the mentioned mechanism. A higher level of IAA production by Pseudomonas was reported by other workers also.63 Numerous forms of phosphate including inorganic and organic phosphate persist in soil. PGPR, potent in the mineralization of most organic phosphorous compounds carried out employing phosphatase enzymes. Many solubilization reactions including acidification, chelation, and secretion of organic acids as gluconic acid for conversion of an insoluble form of P to a soluble form, carried out by diverse groups of microorganisms belonging to the genera Pseudomonas, Serratia, Acinetobacter, Bacillus, Burkholderia, Paenibacillus, Pantoea, Acinetobacter, Enterobacter.64 In the present study, maximum phosphate solubilization was exhibited by P51.10 followed by 11VPKHP4 and 7VP51.8 (Table 2). Recently, it was explored that phosphate solubilizing strain P34-L of Pseudomonas was able to elevate wheat growth by developing root system and phosphorous content.64 Phosphate solubilizing Pseudomonas libanensis EU-LWNA-33 was evaluated for wheat growth promotion under drought stress.24 Few PGPR enhance plant growth by siderophore production. Siderophore-producing bacteria chelate iron from a nearby medium, making it accessible to plants. In the present study, we found that all four Pseudomonas sp. were efficient in siderophore production. Earlier, Shah et al.66 explored that the siderophore producing RSP5 strain of Pseudomonas aeruginosa was able to enhance iron content of all vegetative parameters in Zea mays, thereby enhancing overall plant growth and development. Therefore, it is expected that in our exploration, all four bacterial isolates promising for siderophore production shall also execute iron content in crop plants. Moreover, all four isolates were tested positive for ACC deaminase and catalase enzymatic activity whereas only NBRI N7 was positive for proteolytic activity. Based on recent past year studies, Pseudomonas spp. have also been characterized for enzymatic activities including catalase, nitrate reduction, and gelatinase.67 Isolates 7VP51.8 and P51.10 illustrated gelatinase activity while only P51.10 illustrate nitrate reduction. Numerous studies have reported that with their ACC deaminase assistance, PGPR is capable of suppressing the ethylene levels in plants by cleaving ACC (the precursor of ethylene) and converting it into a-ketobutyrate and ammonia which re-establishes the plant’s normal growth and function.68 Earlier, researchers have proved that consortium or all alone ACC deaminase producing PGPR (Pseudomonas, Bacillus, Ochrobactrum) with other PGP attributes elevate plant health under water deficit conditions which can be further employed for sustainable agriculture.58,48,49 Synergism of P. aeruginosa (LSE-2) and Bradyrhizobium (LSBR-3) reveals Soybean growth promotion via improved nutrient acquisition and soil health.69
One of the most interesting characteristic features of the four isolates is motility and motility helps in colonization. Microbial factors such as the release of quorum sensing signaling molecule, ability to form biofilm on the root surface, and motility are required to establish a relationship with the plants. Another characteristic feature of bacteria is biosurfactant production and emulsification index, isolate P51.10 and NBRI N7 illustrate biosurfactant production while all isolates illustrated emulsification (Table 3). All isolates are thus considered emulsifiers. Biosurfactants are low molecular weight surface-active compounds widely produced by bacteria, yeast, and fungi. Biosurfactant produced by PGPR, effectively employed for oil recovery, improving soil quality by removing hydrocarbons as well as heavy metals,70,71 plant-microbe interaction, pharmaceutical, cosmetics, petroleum, and food industries.72
In the present study, Zea mays growth promotion was evaluated by considering the vegetative plant growth parameters as root and shoot length, fresh, dry root and shoot weight, and dry root and shoot weight ratio. Results indicated that all the four PGPR isolates showed significantly enhanced growth in all parameters under drought stress as compared to uninoculated control. Mishra et al.48 have reported the role of biofilm-producing abiotic stress-tolerant PGPR for ameliorating drought stress and enhancing maize productivity. Several other studies are indicating that inoculation with biofilm-forming PGPR has better plant growth promotion as compared to non-biofilm former inoculants.73 Earlier, extensive studies have revealed the profound effects of EPS producing PGPR in ameliorating the drought stress and enhancing plant growth. It is speculated that the EPS producing PGPR assist plants to cope up with drought stress. Increased Zea mays vegetative parameters including root and shoot length, leaf area, leaf protein, and sugar contents under drought stress conditions were observed on bacterization of Zea mays seeds with EPS producing PGPR. Similar results were also observed by Khan et al.74 for wheat plants under the sandy soil. Our results could be corroborated with these findings as our PGPR also produces different levels of EPS.
In our present study, we elucidated the treatment of stress-tolerant Pseudomonas spp. exhibiting quorum sensing activity has significantly increased plant enzymatic and non-enzymatic biochemical systems (total chlorophyll, carotenoid, proline, soluble sugar, and antioxidant enzymes) as well as vegetative parameters which found to be in covenant with earlier studies findings.49,48,21 Accretion of osmolytes as proline and total soluble sugar induced by PGPR lead plants to cope up with drought stress by preventing electrolyte leakage through stabilizing membrane integrity thereby bringing ROS levels back to normal range via sustaining osmotic turgor.75,49,48 As a result, this eradicates oxidative damage in plants. Our results of Pseudomonas inducing a substantial increase in soluble sugar levels in plants under drought stress were covenant with previous findings.49,48 Under drought stress, our results also revealed a substantial increase in proline accretion levels in plants but our PGPR treatment eliminates those induced substantial increase levels of proline accretion as compared to untreated ones. Thus, our findings of PGPR treatment in eliminating the substantial increase of proline levels in plants to cope up with drought stress are in corroboration with earlier findings.29,48,21
It is speculated that a substantial increase in hydrogen peroxide (H2O2) levels in plants growing under stress conditions leads to cellular metabolites damage and worsening stress resistance power.76 In our present study, we observed an increase in H2O2 levels in untreated plants facing drought stress but reduced in all four Pseudomonas PGPR treatments with slight variation in reduction levels. Earlier researchers have speculated for the reduced levels of H2O2 in plants on PGPR treatment but the exact mechanisms for this are still not wholly known. Among all four Pseudomonas isolates, maximum SOD levels were reduced by 11VPKHP4 and P51.10 followed by NBRI N7 and 7VP51.8 while in APX by 7VP51.8 and 11VPKHP4 followed by NBRI N7 and P51.10. Plants facing drought stress, GPX activity was reduced maximum by 7VP51.8, NBRI N7, 11VPKHP4 followed by P51.10. Catalase activity in plants was modulated by all four isolates under stressed conditions, thus, these findings are in agreement with the earlier findings.49,48
Bacterial strains efficient in quorum sensing and biofilm formation together with multifarious plant growth-promoting attributes contributed to enhanced maize biomass under drought stress. It can be hypothesized that strains with their quorum sensing activity (AHL production) facilitate the plant-microbe interaction and nutrient acquisition. Their proficiency in biofilm formation and EPS production will further help in rhizosphere colonization, maintenance of soil moisture content for a longer duration under normal and diverse stresses. Detailed research work is required to emphasize the effects of quorum sensing and its genes engage in multifarious activity for enhancing crop productivity through signaling under diverse stresses.
Additional file: Additional Table S1.
ACKNOWLEDGMENTS
The authors are thankful to the Director, CSIR-NBRI, Lucknow for providing crucial facilities to accomplish this research work. Thank to Department of Science and Technology, New Delhi, India, for INSPIRE fellowship. The authors are also thankful to Prof. RJC. Bob Mclean of University of Texas, USA for providing Agrobacterium tumefaciens (A136) for discovering AHL production of all isolates.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PSC and AS conceived the idea and designed the experiments. AS performed the experiments. AS and PSC inscribed the manuscript. AS and PSC edited the manuscript. Both the authors read and approved the final manuscript for publication.
FUNDING
This study was supported by the financial assistance from the CSIR Network Project Biostimulants for stress amelioration, enhanced plant productivity and soil health (MLP049) and In-house Project Endophytes and biostimulants for enhancing Crop Production (OLP109).
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Leng G, Hall J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci Total Environ. 2019;654:811-821.
Crossref - Singh RB. Climate change and abiotic stress management in India. In: Tuteja N, Gill SS (eds). Climate Change and Plant Abiotic Stress Tolerance. Wiley, Weinheim. 2013;1:57-77.
Crossref - Lesk C, Rowhani P, Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016;529(7584):84-87.
Crossref - Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J. Control of drought stress in wheat using plant-growth-promoting bacteria. J Plant Growth Regul. 2013;32(1):122-130.
Crossref - Wang B, Liu C, Zhang D, He C, Zhang J, Li Z. Effects of maize organ-specific drought stress response on yields from transcriptome analysis. BMC Plant Biology. 2019;19(1): 335.
Crossref - Zhang X, Mi Y, Mao H, Liu S, Chen L, Qin F. Genetic variation in ZmTIP1 contributes to root hair elongation and drought tolerance in maize. Plant Biotechnol J. 2020;18(5):1271-1283.
Crossref - Ghosh D, Sen S, Mohapatra S. Drought-mitigating Pseudomonas putida GAP-P45 modulates proline turnover and oxidative status in Arabidopsis thaliana under water stress. Ann Microbiol. 2018;68(9):579-594.
Crossref - Qurashi AW, Sabri AN. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz J Microbiol. 2012;43(3):1183-1191.
Crossref - Abiala MA, Odebode AC, Hsu SF, Blackwood CB. Phytobeneficial properties of bacteria isolated from the rhizosphere of maize in southwestern Nigerian soils. Appl Environ Microbiol. 2015;81(14):4736-4743.
Crossref - Oteino N, Lally RD, Kiwanuka S, et al. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015;6:745.
Crossref - Gadhave KR, Hourston JE, Gange AC. Developing soil microbial inoculants for pest management: can one have too much of a good thing? J Chem Ecol. 2016;42(4):348-356.
Crossref - Dutta J, Thakur D. Evaluation of multifarious plant growth promoting traits, antagonistic potential and phylogenetic affiliation of rhizobacteria associated with commercial tea plants grown in Darjeeling, India. PLoS ONE. 2017;12(8):e0182302.
Crossref - Kim YC, Andrson AJ. Rhizosphere pseudomonads as probiotics improving plant health. Mol Plant Pathol. 2018;19(10):2349-2359.
Crossref - Antunes LCM, Ferreira RBR. Intercellular communication in bacteria. Crit Rev Microbiol. 2009;35(2):69-80.
Crossref - Singh A, Chauhan PS. Ecological significance of soil associated plant growth promoting biofilm forming microbes for stress management. In: Iqbal Ahmad (ed) Biofilms in plant and soil health. Wiley and Sons UK. 2017.
Crossref - Jung BW, Khan AR, Hong SJ, et al. Quorum sensing activity of the plant growth-promoting rhizobacterium Serratia glossinae GS2 isolated from the sesame (Sesamumindicum L.) rhizosphere. Ann Microbiol. 2017;67(9):623-632.
Crossref - Gosai J, Anandhan S, Bhattacharjee A, Archana G. Elucidation of quorum sensing components and their role in regulation of symbiotically important traits in Ensifer nodulating pigeon pea. Microbiol Res. 2020;231:126354.
Crossref - Zahir ZA, Munir A, Asghar HN, Shaharoona B, Arshad M. Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisumsativum) under drought conditions. J Microbiol Biotechnol. 2008;18(5):958-963.
- Sandhya V, Ali SKZ, Grover M, Reddy G, Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-p45. Biol Fertil Soils. 2009;46(1):17-26.
Crossref - Chandra D, Srivastava R, Glick BR, Sharma AK. Drought tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusinecoracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere. 2018;28(2):227-240.
Crossref - Ansari FA, Jabeen M, Ahmad I. Pseudomonas azotoformans FAP5, a novel biofilm forming PGPR strain, alleviates drought stress in wheat plant. Int J Environ Sci Tech. 2021;18(8):3855-3870.
Crossref - Nordstedt NP, Chapin LJ, Taylor CG, Jones ML. Identification of Pseudomonas Spp. that increase ornamental crop quality during abiotic stress. Front Plant Sci. 2020;10:1754.
Crossref - Kaushal M, Wani SP. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol. 2016;66(1):35-42.
Crossref - Kour D, Rana KL, Sheikh I, et al. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc Natl Acad Sci India, Sect B Biol Sci. 2020;90(18):785-795.
Crossref - Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170(1):265-270.
Crossref - Brick JM, Bostock RM, Silverstone SE. Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. App Environ Microbiol. 1991;57(2):535-538.
Crossref - Lade H, Paul D, Kweon JH. Isolation and molecular characterization of biofouling bacteria and profiling of quorum sensing signal molecules from membrane bioreactor activated sludge. Int J Mol Sci. 2014;15(2):2255-2273.
Crossref - OToole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30(2):295-304.
Crossref - Titus S, Gasnkar N, Srivastava KB, Karande AA. Exopolymer production by a fouling marine bacterium Pseudomonas alcaligenes. Indian J Mar Sci. 1995;24(2):45-48.
- Wozniak DJ, Wyckoff TJO, Starkey M, et al. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa Biofilms. Proc Natl Acad Sci U S A. 2003;100(13):7907-7912.
Crossref - Meyer JM, Abdallah MA. The florescent pigment of Pseudomonas fluorescens biosynthesis, purification and physical chemical properties. J Gen Microbiol. 1978;107(2):319-328.
Crossref - Jain D, Collins-Thompson D, Lee H, Trevors JT. A drop-collapsing test for screening surfactant-producing microorganisms. J Microbiol Methods. 1991;13(4):271-279.
Crossref - Bodour AA, Miller Maier RM. Application of a modified drop collapse technique for surfactant quantification and screening of biosurfactant-producing microorganisms. J Microbiol Methods. 1998;32(3):273-280.
Crossref - Cooper DG, Goldenberg BG. Surface-active agents from two Bacillus species. Appl Environ Microbiol. 1987;53(2):224-229.
Crossref - Adler J. Chemotaxis in Bacteria. Science. 1966;153(3737):708-716.
Crossref - Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Plant Physiol. 2003;118(1):10-15.
Crossref - Chu WH. Optimization of extracellular alkaline protease production from species of Bacillus. J Ind Microbiol Biotechnol. 2007;34(3):241-245.
Crossref - Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr Microbiol. 2008;57(5):503-507.
Crossref - Dastager SG, Lee JC, Ju YJ, Park DJ, Kim CJ. Leifsonia kribbensis sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2009;59(1):18-21.
Crossref - Nautiyal CS. A method for selection and characterization of rhizosphere-competent bacteria of chickpea. Curr Microbiol. 1997;34(1):12-17.
Crossref - Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1-15.
Crossref - Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205-207.
Crossref - DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350-356.
Crossref - Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276-287.
Crossref - Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867-880.
Crossref - Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987;161(2):559-566.
Crossref - Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-126.
Crossref - Mishra SK, Khan MH, Misra S, et al.Drought tolerant Ochrobactrum sp. inoculation performs multiple roles in maintaining the homeostasis in Zea mays L. subjected to deficit water stress. Plant Physiol Biochem. 2020;150:1-14.
Crossref - Misra S, Chauhan PS. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech. 2020;10:119.
Crossref - Zuniga A, Poupin MJ, Donoso R, et al. Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN. Mol Plant Microbe Interact. 2013;26(5):546-553.
Crossref - Venturi V, Keel C. Signaling in the rhizosphere. Trends Plant Sci. 2016;21(3):187-198.
Crossref - Wei HL, Zhang LQ. Quorum-sensing system influences root colonization and biological control ability in Pseudomonas Xuorescens 2P24. Antonie Van Leeuwenhoek. 2006;89(2):267-280.
Crossref - Venturi V, Fuqua C. Chemical signaling between plants and plant pathogenic bacteria. Annu Rev Phytopathol. 2013;51:17-37.
Crossref - Ortiz-Castro R, Contreras-Cornejo HA, Macias-Rodriguez L, Lopez-Bucio J. The role of microbial signals in plant growth and development. Plant Signal Behav. 2009;4(8):701-712.
Crossref - Dahaji PA, Atajan FA, Omidvari M, Tahan V, Kariman K. Mitigation of Copper Stress in Maize (Zea mays) and Sunflower (Helianthus annuus) Plants by Copper resistant Pseudomonas Strains. Cur Microbio. 2021;78(4):1335-1343.
Crossref - Haque MM, Mosharaf MK, Khatun M, et al. Biofilm producing rhizobacteria with multiple plant growth-promoting traits promote growth of Tomato under water-deficit stress. Front. Microbiol. 2020;11:542053.
Crossref - Ansari FA, Ahmad I. Isolation, functional characterization and efficacy of biofilm forming rhizobacteria under abiotic stress conditions. Antonie van Leeuwenhoek. 2019;112(12):1827-1839.
Crossref - Misra S, Dixit VK, Khan MH, et al. Exploitation of agro-climatic environment for selection of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt tolerant indigenous plant growth promoting rhizobacteria. Microbiol Res. 2017;205:25-34.
Crossref - Konnova SA, Brykova OS, Sachkova OA, Egorenkova IV, Ignatov VV. Protective role of the polysaccharide containing capsular components of Azospirillum brasilense. Microbiol. 2001;70(4):436-440.
Crossref - Costa-Gutierrez SB, Lami MJ, Santo MCC, et al. Plant growth promotion by Pseudomonas putida KT2440 under saline stress: role of eptA. Appl Microbiol Biotechnol. 2020;104(10):4577-4592.
Crossref - Nivens DE, Ohman DE, Williams J, Franklin MJ. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J Bacteriol. 2001;183(3):1047-1057.
Crossref - Etesami H, Beattie GA. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front Microbiol. 2018;9:148.
Crossref - Rupal KS, Raval VH, Saraf M. Biosynthesis and purification of indole-3-acetic acid by halotolerant rhizobacteria isolated from Little Runn of Kachchh. Biocatal Agric Biotechnol. 2020;23:101435.
Crossref - Chawngthu L, Hnamte R, Lalfakzuala R. Isolation and characterization of rhizospheric phosphate solubilizing bacteria from wetland paddy field of Mizoram, India. Geomicrobiol J. 2020;37(4):366-375.
Crossref - Liu X, Jiang X, He X, et al. Phosphate-solubilizing Pseudomonas sp. strain P34-L promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J Plant Growth Regul. 2019;38(4):1314-1324.
Crossref - Sah S, Singh N, Singh R. Iron acquisition in maize (Zea mays L.) using Pseudomonas siderophore. 3 Biotech. 2017;7:121.
Crossref - Biswas JK,Mondal M, Rinklebe J, et al. Multi-metal resistance and plant growth promotion potential of a wastewater bacterium Pseudomonas aeruginosa and its synergistic benefits. Environ Geochem Health. 2017;39(6):1583-1593.
Crossref - Siddikee MA, Sundaram S, Chandrasekaran M, Kim K, Selvakumar G, Sa T. Halotolerant bacteria with ACC deaminase activity alleviate salt stress effect in canola seed germination. J Korean SocAppl Biol Chem. 2015;58(2):237-241.
Crossref - Kumawat KC, Sharma P, Sirari A, et al. Synergism of Pseudomonas aeruginosa(LSE-2) nodule endophyte with Bradyrhizobium sp. (LSBR-3) for improving plant growth, nutrient acquisition and soil health in soybean. World J Microbiol Biotechnol. 2019;35(3):47.
Crossref - Haloi S, Sarmah S, Gogoi SB, Medhi T. Characterization of Pseudomonas sp. TMB2 produced rhamnolipids for ex situ microbial enhanced oil recovery. 3 Biotech. 2020;10(3):120.
Crossref - He S, Ni Y, Lu L, et al. Simultaneous degradation of n-hexane and production of biosurfactants by Pseudomonas sp. strain NEE2 isolated from oil-contaminated soils. Chemosphere. 2020;242:125237.
Crossref - Sachdev DP, Cameotra SS. Biosurfactants in agriculture.Appl Microbiol Biotechnol. 2013,97(3):1005-1016.
Crossref - Singh A, Jain A, Sarma BK, Upadhyay RS, Singh HB. Rhizosphere competent microbial consortium mediates rapid changes in phenolic profiles in chickpea during Sclerotium rolfsii Infection. Microbiol Res. 2014;169(5-6):353-360.
Crossref - Khan N, Bano A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE. 2019;14(9):e0222302.
Crossref - Grover M, Madhubala R, Ali SZ, Yadav SK, Venkateswarlu B. Influence of Bacillus spp. strains on seedling growth and physiological parameters of sorghum under moisture stress conditions. J Basic Microbiol. 2014;54(9):951-961.
Crossref - Hossain MA, Bhattacharjee S, Armin SM, et al. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci. 2015;6:420.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.