The world has been facing a back-to-back hit to life after widespread of viruses since the time of COVID-19. The pandemic had a devastating effect and created history in mankind, but that was not enough for the time. The viruses are been known to be the deadliest microbes by virtue of their ability to reside as inactive for long time and become active again along with new variants when the conditions are favourable. One such noted spread out of virus has been that of Monkeypox Virus in humans. A zoonotic orthopoxvirus that can infect humans, the monkeypox virus (MPV) can cause disease with varied morbidity and death in humans. It has been demonstrated that members of the Orthopoxvirus genus decrease antiviral cell defences, take advantage of host cell machinery, and postpone infection-induced cell death. The name Monkeypox was after its first observation in Macaque monkey but the virus’s origin has been linked to a number of rodents and small mammals. The virus was endemic to Africa and is closely related to notorious variola (smallpox) virus. They both affect people with a febrile rash sickness that is similar to smallpox but has less severity. Monkeypox can spread from person to person and it is frequently related to breathing droplets or direct contact with mucocutaneous lesions of an affected person. There is now no cure available for those who are affected, yet supporting therapies can be used to help people with their symptoms. To better comprehend and prevent human infections, additional study is required on the epidemiology, ecology, mutations and biology of the new virus strains in endemic locations.
Endemic, Monkeypox, Orthopoxvirus, Virus, Zoonotic
Microbes are becoming a topic of concern for animals as well as humankind. Back-to-back hits by pandemics and wide spread of viral infections have blown a heavy hit for the population and other economic aspects as well. One of them being the Monkey Pox Virus or MPX has become of matter of concern since its widespread cases. Monkeypox has about similar clinical appearance as of smallpox’s. Although the disease is indigenous to the DR Congo incidences of monkeypox in humans and wildlife have been reported in other African nations.1,2 The first human case was identified in 1970 in a nine-month-old boy from the DR Congo, there is cause for alarm once more. An uncommon viral zoonosis that is endemic to central and western Africa, human monkeypox connected to the American outbreak from 2003 to 2004 (US).3 Apart from this, the disease has been reported time to time but it was in 2021, it turned out to be a global havoc. A taxonomically diverse spectrum of mammalian species can contract monkeypox, although the actual natural host is unknown. Only two wild animals have been used to isolate the virus: a sooty mangabey in Ivory Coast and a rope squirrel in the DR Congo.4 It is thought that respiratory excretions, saliva, or contact with lesion exudate or crust material are the routes of transmission faeces-based viral shedding could be another form of exposure. The early, frequently at the onset of fever lymph node growth that separates MPX from smallpox is the primary distinction.4 Lesions start to form simultaneously and develop at a comparable rate to those of a rash, which typically develops 1-3 days after the commencement of a fever and lymphadenopathy. Their distribution is primarily peripheral but during a severe sickness, they may cover the entire body. Up to 4 weeks can pass after the infection before the lesion desquamates.5,6 There are two genetic clads of the monkeypox virus i.e, the Congo basin, from Central Africa and another from the West African Clade.7 In human cells, derived from previously infected monkeypox patients, T-cell receptor-mediated T-cell activation was prevented which prevents the production of inflammatory cytokines.8 Based on serologic research in Africa, monkeypox infection is a significant new pathogen that may cause more infections than previously thought. A virulent strain of monkeypox may infect the population where people lack orthopoxvirus immunity, which could result in an epidemic.
Epidemiology
The virus was first found in monkeys in a Danish lab in 1958 when some monkeys from Africa were taken to Denmark for research.9 This is where the name “monkeypox” comes from. A 9-month-old boy, in Zaire, DRC, was first time found infected with monkeypox virus.10 Since then, monkeypox has become common in the DRC and has spread to other African countries, mostly in Central and West Africa. The first cases of monkeypox were found outside of Africa in 2003.11 From 2000 to 2009, monkeypox was found in three African countries: DRC, and South Sudan and Republic of the Congo later in 2010 to 2019, it was spread in seven African countries. Compared to the last three decades of the 20th century, there were more outbreaks and fewer reports of a single case by the year 2000. In the past 50 years most cases of monkeypox were reported in DRC.
More recently, between January and September 2020, the DRC12 reported another 4,594 suspected cases. Nigeria is the second country with the most cases, with 181 confirmed and likely cases since the outbreak began in September 2017.13 The Nigeria CDC report lists 183 cases. However, two cases that started in Nigeria were found in Israel14 and Singapore15 and were linked to travel in those countries. The Republic of the Congo with 97 cases and the Central African Republic with 69 cases of monkeypox were reported. Over the past 50 years, there have been less than 20 confirmed or likely cases of monkeypox in each of the other African countries. Monkeypox wasn’t known to exist outside of Africa until 2003, when 47 likely cases were found in the United States. These cases were caused by exposure to infected pet dogs, which got the virus from infected animals brought in from Ghana.11,16 In the past few years, there have been a number of cases of monkeypox were found due to migration. All of these cases started in Nigeria. One case happened in UK in 2019,12 one in Israel in 2018,14 one in Singapore in 2019,15 and three in the UK in 2018.17 A healthcare worker in the UK got the disease in 2018, making it the fourth case there.18
From 1970 to 1989, most people who got monkeypox were 4 or 5 years old. From 2000 to 2009, the median age was 10 years old, and from 2010 to 2019, it will be 21 years old. In the early years, all monkeypox deaths were in children younger than 10 years old. From 2000 to 2009, 21-year-olds died, and from 2010 to 2019, 21-year-olds will die. In the early years, all monkey pox deaths were in children younger than 10 years old. From 2000 to 2019, only 37.5% of monkeypox deaths were in children younger than 10 years old. The global intensified smallpox eradication programme, which started in 1967,11 and the end of routine smallpox vaccinations in the 1980s,19 seem to fit with these data. In the 2000s, only adults over 20–25 years old had been vaccinated against smallpox. This left people under 20 years old at risk. In the next 10 years, the average age of people who got monkeypox went from 10 to 21. In fact, most of the people who got smallpox were either too young to have been vaccinated or were born after smallpox vaccinations was stopped.
Occurrence
People who live in or near forests may be exposed indirectly or at a low level, which could lead to subclinical infection.20 It was first found in humans in 1970, after smallpox had been wiped out, possibly because the infection was then easier to spot. From 1981 to 1986, surveillance reports in the DRC found 338 cases. During the outbreaks in the DRC in 1996 and 1997, there were 22 cases for every 1000 people. Since 1978, there have been no reports of people getting monkeypox in West Africa. But monkeypox is still spreading strongly in the DRC. In 2003, 11 cases were reported and one person died in DRC. In 2005, 10 cases were reported from Sudan, but no one died.21 Before the outbreak in the Midwestern states in late spring 2003, there were no cases in the United States. In 2003, 71 people who might have had monkeypox were checked out.22
Monkeypox Virus and the Indian Scenario
MPVX is not a native virus of India and there were no cases reported until 27 July 2022 where a fatal case of 22-year-old boy was noted from Kerala,23 and after the same few more cases where being reported from India.24 These were identified as travellers, despite of quarantine checks and all the precautions taken for the travellers, the cases travelled to India.
Risk Factors
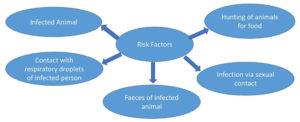
Getting MPX was linked to both direct touching, biting or scratching by an infected animal and indirect by means of contact (Figure 1). In terms of direct exposures, having touched or scratch from an infected animal was a strong predictor of getting MPX when smallpox vaccination was not taken into account. When looking at indirect exposures, being near within 6 feet of an infected animal was not linked to getting MPX. If the cage was cleaned or the used bedding of an infected animal was touched, there was more likely to get MPX, even if the person had been vaccinated against smallpox before.25 Living in the same home with an infected person or using the same objects which has been earlier used by the infected person will increase the risk of viral transmission. Amid In the ongoing monkeypox outbreak, it has also been noted that the person having sexual contact are more likely to get the illness.26
Symptoms
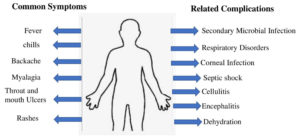
Symptoms as per clinical presentations include chills, fever, rashes etc in which ulcers on skin is the important symptom of the MPXV as listed Below in Figure 2. Apart from this, the complicated symptoms when the disease get severe, includes secondary infections due to decreased immunity, septic shock like similar to COVID-19, respiratory damage, cellulitis etc.
People who hadn’t been vaccinated had more skin scratches and breaks 27.0% than people who had been vaccinated against smallpox 4.8%.20 Children are more likely than adults to have an infected animal as a pet or been exposed to an infected animal every day or directly. However, neither age group had a higher proportion of exposure overall.21
The number of cases of primary zoonotic transmission is likely to depend on how often and how people interact with infected wildlife. But situations that are often linked to the first introduction of a zoonotic disease may also be linked to more chances of human-to-human transmission. As has been seen with other communicable diseases, this makes the home environment an important sign of disease risk.24
Transmission route
It is recognised that MPV can spread from animals to people. Rodents (rats, squirrels, and dormice) and numerous monkey species are the principal hosts of the virus. However, there has also been evidence of a human-to-human transfer that occurred within and outside of Africa. Direct contact with skin lesions on infected persons or animals, inhalation of human droplets, or consumption of bushmeat are all ways that MPV can be spread. The disease is found to be occurring common in men that have sex with men.27
Pathogenesis
The process of replication and the activation of the host’s immune systems are in a race during infection. The severity of the sickness is measured by the features of the virus and the extent of the host reaction. Orthopoxviruses are vast and complicated, which makes it simple for the host to mount an immune response. While larger viruses like orthopoxviruses need a more elaborate strategy for survival inside the host, smaller viruses can bypass the host’s defenses by slipping through cracks or replicating quickly.28,29
A group of chemicals that are directed against the elements of the host’s immune response and are expressed by virulence genes function as modulators. According to their intracellular and extracellular effects, these modulatory proteins can be separated into two classes. The actions of viral transducer proteins interfere with the cell’s defence mechanisms against infection, such as the oxidative burst and apoptotic pathways. Virostealth proteins are another class of proteins that function within cells. They lower the likelihood of immune system detection by downregulating immune recognition markers including CD4 and major histocompatibility complex class I. Viromimic proteins, another name for viral proteins that operate extracellularly, are responsible for controlling the immune response. They fall under the viroreceptor and virokine categories. Secreted or cell surface glycoproteins called vioreceptors bind host cytokines and chemokines in a competitive manner, inhibiting their function. The formation of virokines, which are viral mimics of host cytokines, chemokines, and growth factors, is efficient in preventing host reactions that are harmful to the survival of the virus and in encouraging reactions that are beneficial for viral reproduction and dissemination.30,31
Although MPVs have been linked to the skin rash, virulent strains that cause a severe immunological reaction can cause mortality in vulnerable persons very quickly. In addition to the Orthopoxviruses’ tissue-damaging cytopathic effects, excessive endogenous mediator and soluble cytokine synthesis might result in sepsis and septic shock, as in the case of COVID-19.32
Diagnosis
Genetic Methods
This is done with PCR or RT-PCR, and the test should be done in a Biosafety Level-3 facility.33 RT-PCR is used to target conserved regions of the extracellular-envelope protein gene (B6R)34 and the DNA polymerase gene (E9L)35 to find DNA of MPXV in clinical and veterinary samples and in MPXV-infected cell cultures. RNA polymerase subunit 18, rpo18,36 and the F3L gene37 depend on DNA. RFLP of PCR-amplified genes or gene fragments is also used to find MPXV DNA,38,39 but it takes a long time and requires virus culture. RFLP of PCR products may not be the right method because in a clinical setting where speed, sensitivity, and specificity of the method are most important and this needs enzyme digestion and gel electrophoresis.40 Whole-genome sequencing with NGS technologies is still the best way to learn about MPXV and other OPVs.41-43 However, the technology is not cost effective, and processing sequencing data after the fact takes a lot of computing power. So, NGS might not be the best way to characterise, especially in the economically backward countries of sub-Sahara Africa that don’t have a lot of resources. Even though RT-PCR is still the best way to diagnose MPXV on a regular basis, it needs to be paired with field genome sequencing technology like the Oxford Nanopore MinION to provide real-time virus genome data, which is essential for epidemiological interventions that are based on facts. MinION field sequencing was used successfully to keep an eye on the Ebola outbreak’s genome in places in West Africa with few resources.44
Phenotypic Methods
On the basis of clinical results and diagnosis, the incubation period for MPXV is determined to be between 4 and 21 days which is usually followed by symptoms like swollen lymph nodes, pharyngitis, fever, back pain, myalgia, severe asthenia, severe headache, severe headache, and malaise. It is marked by vesiculopustular rashes that start on the face and spread all over the body in 1–10 days. Lesions caused by MPXV are uniform, about the size of a pea, and hard, which is similar to smallpox. The MPXV lesion looks like a crop, and it doesn’t spread in a strong centrifugal pattern like smallpox. The main clinical difference between MPXV and smallpox is the fact that MPXV causes lymphadenopathy.45,46 For monitoring to discover suspected instances, it is crucial to presume MPX based on clinical signs, but not without laboratory confirmation. It has been shown in a group of 645 people to have high sensitivity (93–98%) and low specificity (9%–26%).47,48
Immunological Methods
This includes using ELISA to detect IgG and IgM antibodies and immunohistochemistry to find viral antigens. Using polyclonal or monoclonal antibodies against all OPVs, immunochemistry can be used to tell the difference between a poxvirus infection and one caused by the herpes virus. It has been shown that both T-cell responses and antiviral antibodies rise around the time a disease starts. IgM and IgG, on the other hand, are found in serum about after five days and when rash appear, it is found more than eight days. If IgM and IgG antibodies are found in a person who has never been vaccinated but has a history of rash and severe illness, this could be a sign that they have MPXV. But none of these tests are specific for MPX,49,50 and can also show that other OPV species are present. IgM can be used to tell if someone with a history of smallpox vaccination51 is infected with MPX. Positive IgM capture ELISA shows that the person has recently been exposed to OPV (likely MPXV in endemic areas), while positive IgG capture ELISA shows that the person has been exposed to OPV before, by through vaccination or by natural infection.52 So, the recent exposure to OPV can be determined by the presence of both IgM and IgG in a sample of the person, even if they have been vaccinated or have had the disease before. So, the presence of IgM in people who have been vaccinated against smallpox in areas where MPX is common indicates the fact that they have recently been exposed to MPXV.
Electron Microscopy
Under an electron microscope, MPXV looks like a brick with two sides and a central core that is about 200–300 nm long. Even though this method isn’t a surefire way to tell what kind of virus it is because OPV species can’t be told apart by their appearance, it does show that the virus is in the Poxviridae family.48,53
Prevention
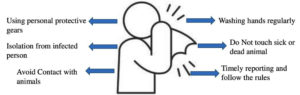
Data suggest that immunization against smallpox virus may decrease the infection of monkeypox or the severity of infection may be less severe.54 There are three licenced smallpox vaccines in the US Strategic National Stockpile (SNS): JYNNEOSTM (also known as IMVAMUNE, IMVANEX, MVA-BN) and ACAM2000®. The Aventis Pasteur Smallpox Vaccine (APSV) could be used for smallpox under an investigational new drug (IND) protocol.46 The US Centres for Disease Control and Prevention (CDC) say that JYNNEOSTM is a live viral vaccine made from the modified vaccinia Ankara-Bavarian Nordic (MVA-BN strain) and is an attenuated orthopoxvirus that does not replicate. It can now be used to prevent smallpox and monkeypox disease in individuals 18 years of age and older who are at a high risk of contracting smallpox or monkeypox thanks to FDA approval in September 2019. Other common preventive measures advised by government agencies are depicted in Figure 3. Historical data show that the smallpox vaccine with vaccinia virus was about 85% effective against monkeypox.55 Europe has approved the vaccine for smallpox as IMVANEX®, but the UK has been using it off-label to treat monkeypox cases.56 ACAM2000® is used to actively protect against smallpox disease in people who are at high risk for getting it.
Pre exposure Prophylaxis
The Advisory Committee on Immunization Practices (ACIP) says that some people who might be exposed to orthopoxviruses57 at work should get vaccinated. It is recommended that people who work in research laboratories, clinical laboratories that do diagnostic testing for orthopoxviruses, and designated response teams who could be exposed to orthopoxviruses at work get vaccinated.
Post exposure Prophylaxis
To get monkeypox, you have to be in close contact with someone who has it for a long time. Brief interactions and those done with the right personal protective equipment (PPE) and standard precautions do not pose a high risk and do not usually require post-exposure prophylaxis (PEP). The CDC has made guidelines to help people assess the risk of exposures and make smart choices about PEP. For disease prevention, the CDC says that the first dose of a vaccine should be given within 4 days of exposure.58 If the vaccination is given 4-14 days after the date of exposure, it may lessen the symptoms of the disease, but it may not stop the disease from happening.59
Unprotected skin-to-skin contact with mucous membranes, lesions, bodily fluids such as sexual contact, getting saliva splashed in one’s eyes or mouth, or touching a patient without wearing gloves, or infected objects is prohibited (e.g., linen, clothing). Not wearing a N95 or comparable respirator (or higher) and eye protection while doing any procedure in a patient’s room or within 6 feet of a patient that could produce aerosols from oral secretions.
Exposure that is changed to this risk level at the discretion of public health officials (i.e., exposure that ordinarily would be considered a lower-risk exposure, raised to this risk level because of unique circumstances). With an intermediate level of exposure, it would be recommended to keep an eye on the person and make an informed clinical decision on an individual basis to see if the benefits of PEP are worth the risks.
Exposure characteristics for intermediate degree of exposure include
Being within 6 feet of a patient without a mask for at least 3 hours without at least a surgical mask. Activities (like turning, bathing, or helping with a transfer) in which a person’s sleeves or other parts of their clothing come into contact with a patient’s skin lesions, bodily fluids, or soiled linens or dressings, but they are not wearing a gown. Exposure that is moved to this risk level because of special circumstances, which are up to the public health officials to decide for e.g., Public health authorities may decide to lower the risk rating from high to intermediate if there is a chance of an aerosol exposure.
Treatment
Supportive Care
Most people who get monkeypox don’t need medical care to get better. People who have stomach problems (like vomiting or diarrhoea) will need oral or intravenous rehydration to stop them from losing too much fluid.60
Antivirals
Several antivirals might be able to treat monkeypox, even though they were approved for treating smallpox based on studies with animals. Humans have been used to test the doses of these drugs, but the effectiveness of these drugs has not been fully defined.60
Tecovirimat
Tecovirimat (also called TPOXX) is the first antiviral approved to treat smallpox in adults and children weighing at least 3 kg, and it is considered the best treatment. When the disease is very bad, it may be used with brincidofovir. Tecovirimat block the viral envelope protein VP37. This stops the last steps of the virus maturing and getting out of an infected cell, which stops the virus from spreading in an infected host.61,62 Even though this agent hasn’t been tested on humans for monkeypox, studies have shown that animals treated with tecovirimat have a better chance of surviving lethal monkeypox virus infections than animals treated with a placebo. In a larger safety study of 359 people who took tecovirimat, the side effects of the placebo were mostly the same as those of tecovirimat.63 Tecovirimat was used with vaccinia immune globulin (VIG) to treat smallpox vaccine side effects like eczema vaccinatum,64,65 and progressive vaccinia.66,67
The CDC’s Emergency Access Investigational New Protocol lets doctors use tecovirimat to treat monkeypox and other infections caused by orthopoxviruses that are not caused by the variola virus. The protocol also says that for kids who weigh less than 13 kg, they can open an oral capsule and mix its contents with liquid or soft food. The Strategic National Stockpile has Tecovirimat in the form of oral capsules or an intravenous vial [TPOXX] (tecovirimat).
Brincidofovir and Cidofovir
Since June 2012, Brincidofovir has been approved by FDA to treat smallpox.68 Brincidofovir (orally) is similar to the drug cidofovir, which is given through an IV. It may be safer than cidofovir69 because it is less toxic to the kidneys. The viral DNA polymerase is stopped from doing its job by these drugs.70 Even though there aren’t many studies on brincidofovir being used to treat monkeypox in animal models but it was found effective against orthopoxvirus infections.61 There isn’t any clinical data on how well cidofovir works against monkeypox in humans, but it has been found to be effective in vitro against lethal monkeypox virus infections in animals.62 When treating with cidofovir through an IV route, normal saline and probenicid must be given simultaneously. Before and during treatment with brincidofovir, liver function tests must be done because brincidofovir may raise serum transaminases and serum bilirubin.
Vaccinia Immune Globulin (VIG)
The FDA has given permission for VIG, which is a hyperimmune globulin, to be used to treat some side effects of the polio vaccine. These include eczema vaccinatum, severe generalised vaccinia, progressive vaccinia, vaccinia infections in people with skin conditions, and unusual infections caused by the vaccinia virus (except in cases of isolated keratitis, like eye infections).71 Even though VIG could be used to treat monkeypox and smallpox, there isn’t a lot of information about how well it works, and it hasn’t been tested on humans. Since vaccinia virus vaccine shouldn’t be given to people with severe T-cell immunodeficiency, VIG may be given instead to those who have been exposed to the virus in the past.72
Vaccination
Studies have shown that getting vaccinated against smallpox also protects you from other OPVs, like MPXV. Based on the information we have, about 90% of the cases have never been exposed to OPV. A lot of these people were born after the programme to get rid of smallpox ended in 1965. People who had been vaccinated against smallpox were found to be 85% less likely to get sick from MPXV.73 During the 2003 MPXV endemic in the United States, the Centre for Disease Control and Prevention (CDC) recommended a smallpox vaccine called ACAM2000TM. This vaccine did not prevent disease, but it did lessen the symptoms.74 As a result, this vaccination is not available to the public and is not used in areas where MPXV is common. This is because there are some problems, such as not knowing how the vaccine will affect people with weak immune systems and not knowing if the live vaccinia virus in the vaccine is safe. The Food and Drug Administration (FDA) and the European Medicine Agency (EMA) have also authorised a replication-deficient, attenuated third-generation modified vaccinia Ankara (MVA) vaccine to protect against smallpox and monkeypox in adults 18 years of age and older who have a high risk of contracting VARV named IMVAMUNE. At the moment, neither ACAM2000 nor IMVAMUNE can be used by the general public. So, it’s still not clear whether or not these licenced smallpox vaccines will protect against MPX in areas where MPXV is common.75,76
The monkeypox epidemic after the COVID-19 pandemic, have demonstrated that zoonotic diseases pose a severe threat to humanity. Any infection that emerges from any place could pose a threat to all nations and to all of humanity in the rapidly globalising planet. The favourable conditions for the revival and comeback of monkeypox has been created by the declining population immunity brought on by the vaccination of the smallpox. This is quite understood by the increase in the number of cases of those who contact monkeypox. Apart from this, although, MPXV is not spreading rapidly and diagnostic tests and vaccines being available even before the current outbreak, long-standing flaws in the public health system are providing MPXV a chance to spread. Similar to SARS-CoV-2, MPVX’s transmissibility has probably been boosted by mutations and natural selection. Policymakers, researchers, and healthcare professionals can all work together to effectively address the MPXV outbreak using what we have learnt from the COVID-19 pandemic. On the other part, policy makers should come forward and undertake programs to spread awareness related to the infectious and zoonotic diseases for the common man along with building of healthcare units and hospitals, appointing more health care workers and rapid action force in such situations. Whereas, the researchers should come forward with new inventions and interventions in the field of infectious disease and its spread in the community. Awareness amongst the communities, regarding need of good hygiene and identification of symptoms, getting proper treatment and isolation should be followed by the common people.
ACKNOWLEDGMENTS
The authors would like to thank GLA University, Mathura, India for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Sklenovska N, Ranst MV. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front Public Health. 2018;4(6):241.
Crossref - Quiner CA, Moses C, Monroe BP, et al. Presumptive risk factors for monkeypox in rural communities in the Democratic Republic of the Congo. PLoS One. 2017;12(2):e0168664.
Crossref - Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342-350.
Crossref - MacNeil A, Abel J, Reynolds MG, et al. Serologic evidence of human orthopoxvirus infections in Sierra Leone. BMC Res. Notes. 2011;4:1–5.
- Radoni’c A, Metzger S, Dabrowski PW, et al. Fatal monkeypox in wild-living sooty mangabey, Cote d’Ivoire, 2012. Emerg Infect Dis. 2014;20(6):1009-1011.
Crossref - Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465-470.
- Hutson CL, Olson VA, Carroll DS, et al. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J Gen Virol. 2009;90(Pt 2):323-33.
Crossref - McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260-267.
Crossref - von Magnus P, Andersen EA, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Path Microbiol Scand. 1959;46(2):159.
Crossref - Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58(2):165-182. PMID: 6249508
- Centers for Disease Control and Prevention. Monkeypox. Available from: https://www.cdc.gov/ poxvirus/monkeypox/index.html
- Public Health England. Monkeypox case confirmed in England 2019. https://www.gov. uk/government/news/monkeypox-case-confirmed-in-england
- World Health Organization. Regional Office for Africa. 2020. Weekly Bulletin on Outbreak and other Emergencies: Week 2020;41:05-11. https://apps.who.int/iris/handle/10665/ 336026
- Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980-983.
Crossref - Yong SEF, Ng OT, Ho ZJM, et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826-1830.
Crossref - Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742-1751.
Crossref - Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38).
Crossref - Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26(4):782-785.
Crossref - Jezek Z, Khodakevich LN, Wickett JF. Smallpox and its post-eradication surveillance. Bull World Health Organ. 1987;65(4):425-434. PMID: 3319266
- Durski KN, McCollum AM, Nakazawa Y, Petersen BW, Reynolds MG, Briand S, Harouna DM, Olson V, Damon IK, Khalakdina A. Emergence of Monkeypox—West and Central Africa, 1970–2017. Morb. Mortal. Wkly. Rep. 2018; 67:306
- Reynolds, MG, Damon, IK. Outbreaks of human monkeypox after cessation of smallpox vaccination. Trends Microbiol. 2012;20(2):80-87.
Crossref - Centers for Disease Control and Prevention. Update: multistate outbreak of monkeypox – Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin. Morb Mortal Wkly Rep. 2003;52:642-646.
- Yadav PD, Reghukumar A, Sahay RR, et al. First two cases of Monkeypox virus infection in a traveller returned from UAE to India, July 2022. J Infect. 2022;85(5):e145-e148.
Crossref - Sah R, Mohanty A, Siddiq A, et al. Monkeypox reported in India South East Asia Region: Health and economic challenges. Lancet Reg Health – Southeast Asia. 2022;4:100063.
Crossref - Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus. 2022;14(7):e26531.
Crossref - Okyay RA, Bayrak E, Kaya E, et al. Another epidemic in the shadow of Covid 19 pandemic: a review of monkeypox. EJMO. 2022;6:95-99.
Crossref - Angelo KM, Petersen BW, Hamer DH, Schwartz E, Brunette G. Monkeypox transmission among international travellers-serious monkey business? J Travel Med. 2019;26:taz002.
- Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence EJMO 99 of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41:1765-1771.
Crossref - Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(Pt 10):2661-2672.
Crossref - Stanford MM, McFadden G, Karupiah G, Chaudhri G. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol Cell Biol. 2007;85(2):93-102.
Crossref - Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46-63.
Crossref - Liszewski MK, Leung MK, Hauhart R, et al. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J Immunol. 2006;176(6):3725-3734.
Crossref - Fang L-Q, Yang Y, Jiang J-F, et al. Transmission dynamics of Ebola virus disease and intervention effectiveness in Sierra Leone. Proc Natl Acad Sci U S A. 2016;113(16):4488-4493.
Crossref - Fowotade A, Fasuyi TO, Bakare RA. Re-emergence of monkeypox in Nigeria: A cause for concern and public enlightenment. Afr J Clin Exp Microbiol. 2018;19, 307.
- Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36(3):194-203.
Crossref - Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: A clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872-879.
Crossref - Reynolds MG, Carroll DS, Olson VA, et al. A Silent Enzootic of an Orthopoxvirus in Ghana, West Africa: Evidence for Multi-Species Involvement in the Absence of Widespread Human Disease. Am J Trop Med Hyg. 2010;82(4):746-754.
Crossref - Orba Y, Sasaki M, Yamaguchi H, et al. Orthopoxvirus infection among wildlife in Zambia. Gen Virol. 2015;96(Pt 2):390-394.
Crossref - Kulesh DA, Loveless BM, Norwood D, et al. Monkeypox virus detection in rodents using real-time 30 -minor groove binder TaqMan®® assays on the Roche Light Cycler. Lab Investig. 2004;84(9):1200-1208.
Crossref - Meyer H, Pfeffer M, Rziha H-J. Sequence alterations within and downstream of the A-type inclusion protein genes allow differentiation of Orthopoxvirus species by polymerase chain reaction. Gen Virol. 1994;75(Pt 8):1975-1981.
Crossref - Ropp SL, Jin QI, Knight JC, Massung RF, Esposito JJ. PCR Strategy for Identification and Differentiation of Smallpox and Other Orthopoxviruses. Clin Microbiol. 1995;33(8):2069-2076.
Crossref - Bourquain D, Dabrowski PW, Nitsche A. Comparison of host cell gene expression in cowpox, monkeypox or vaccinia virus-infected cells reveals virus-specific regulation of immune response genes. Virol. 2013;10:1–13
- Farlow J, Ichou MA, Huggins J, Ibrahim S. Comparative whole genome sequence analysis of wild-type and cidofovir-resistant Monkeypoxvirus. Virol J. 2010;7:1-15.
Crossref - Cohen-gihon I, Israeli O, Shifman O, et al. Identification and Whole-Genome Sequencing of a Monkeypox. Microbiol Resour Announc. 2020;9(10):e01524-19.
Crossref - Quick J, Loman NJ, Duraffour S, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016;530(7589):228-232.
Crossref - Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010;140(3-4):229-236.
Crossref - Kabuga AI, El Zowalaty ME. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J Med Virol. 2019;91(4):533-540.
Crossref - Nasir IA, Dangana A, Ojeamiren I, Emeribe AU. Reminiscing the recent incidence of monkeypox in Nigeria: Its ecologic-epidemiology and literature review. Port Harcourt Med. J. 2018; 11:1–9.
- Kaysser P, Von Bomhard W, Dobrzykowski L, Meyer H. Genetic diversity of feline cowpox virus, Germany 2000–2008. Vet. Microbiol. 2011; 141:282–288
- Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13(10):1-20.
Crossref - Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Revolut. 2008;225:96-113.
Crossref - MacNeil A, Abel J, Reynolds MG, et al. Serologic evidence of human orthopoxvirus infections in Sierra Leone. BMC Res Notes. 2011;4:1-5.
Crossref - Risi GF. Orthopoxviruses. In Nebraska Isolation and Quarantine Manual; Theodore, J., Mark, C., Kortepeter, G., Christopher, J., Kratochvil, J.V.L., Eds.; University of Nebraska Medical Center: Omaha, NE, USA, 2019; pp. 125–138.
- Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693-702.
Crossref - Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11(9):1005-11.
Crossref - Fine PEM, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643-650.
Crossref - Kupferschmidt K. As monkeypox threat grows, scientists debate best vaccine strategy. Science. 2022;376(6598):1142-1143.
Crossref - US Centers for Disease Control and Prevention (CDC). Monkeypox and smallpox vaccine guidance. 2019. https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpoxvaccine.html. Accessed 25 May 2022.
- Reynolds MG, McCollum AM, Nguete B, Lushima RS, Petersen BW. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9(12):380.
Crossref - Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8P):1153-1162.
Crossref - Russo AT, Grosenbach DW, Chinsangaram J, et al. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev Anti Infect Ther. 2021;198(3):331-344.
Crossref - Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379(1):44-53.
Crossref - Quenelle DC, Buller RML, Parker S, et al. Efcacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob Agents Chemother. 2007;51(2):689-695.
Crossref - Marcinak J, Vora S, Weber S, et al. Household transmission of vaccinia virus from contact with a military smallpox vaccine- Illinois and Indiana, 2007. Morb Mortal Wkly Rep. 2007.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46(10):1555-1561.
Crossref - Hahne S, Macey J, Binnendijk RV, et al. Progressive vaccinia in a military smallpox vaccine-United States, 2009. Pediatr Infect Dis J. 2009;39(12):e388-e392.
Crossref - US Food and Drug Administration: FDA approves drug to treat smallpox. https://www.fda.gov/drugs/drug-safety-and-availabili ty/fda-approves-drug-treat-smallpox. Accessed 25 May 2022.
- Chittick G, Morrison M, Brundage T, Nichols WG. Short-term clinical safety profle of brincidofovir: a favorable beneft-risk proposition in the treatment of smallpox. Antiviral Res. 2017;143:269-277.
Crossref - Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses. 2010;2(12):2740-2762.
Crossref - Rice AD, Adams MM, Wallace G, et al. Efcacy of CMX001 as a post exposure antiviral in New Zealand white rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses. 2011;3(1):47-62.
Crossref - Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 200357(1-2):13-23.
Crossref - Wittek R. Vaccinia immune globulin: current policies, preparedness, and product safety and efcacy. Int J Infect Dis. 2006100(3):193-201.
Crossref - Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 200541(12):1765-1771.
Crossref - Brown K, Leggat PA. Human monkeypox: Current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1(1):8.
Crossref - Formenty P, Muntasir MO, Damon I, et al. Human Monkeypox Outbreak Caused by Novel Virus Belonging to Congo Basin clade, Sudan, 2005. Emerg. Infect. Dis. 2010;16:1539–1545
- Petersen E, Abubakar I, Ihekweazu C, et al. Monkeypox-Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78-84.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.