ISSN: 0973-7510
E-ISSN: 2581-690X
In order to determine the association of molecular markers with the pea rust resistance, thirty two diverse pea genotypes were phenotypically screened on the basis of disease reaction followed by molecular screening using four SSR markers – AA446 and AA505 flanking the major QTL Qruf; AD146 and AA416 flanking the minor QTL, Qruf1 associated with pea rust resistance. SSR markers AD146 flanking the minor QTL, Qruf1 were able to identify one moderately resistant (Pant P 42), six moderately susceptible genotypes and four susceptible genotypes (25-30 percent disease severity) with amplified fragment of 430bp. Whereas, SSR markers AA416 flanking the minor QTL, Qruf1amplified a fragment of 280bp in four moderately susceptible genotypes, seven susceptible genotypes (25-30 percent disease severity) and one susceptible genotypes with 49.17 percent severity (HUDP1301). It was observed that most of the germplasm with disease severity of less than 30 percent showed the presence of Qruf and/or Qruf1 governing partial resistance against rust. Therefore, molecular screening of germplasm may conclude that these SSR markers (AA446, AA505, AD146 and AA416) if used together, can be effective in marker assisted selection (MAS) of pea rust resistance.
Molecular Markers, Rust, Disease Severity, Marker Assistant Selection.
Pea is affected by a number of fungal (rust, powdery mildew, downy mildew, root rot, alternaria blight, aschochyta blight, wilt, anthracnose, cercospora leaf spot, damping off, seedling rot etc.), bacterial (bacterial blight and brown spot), nematode (cyst nematode, lesion nematode and root-knot nematode) and viral diseases (cucumber mosaic virus, pea early browning virus, pea enation mosaic, pea mosaic, pea seed borne mosaic, pea streak and pea stunt). These diseases, under the right conditions, can significantly decrease both yield and quality. Among these, the rust of pea caused by Uromyces viciae–fabae (Pers.) J. Schrot (syn. Uromyces fabae (Pers.) de Bary) is considered the most important under warm and humid conditions1. It has been reported from different parts of the country including eastern India2, 3, central India4, southern parts of India5, 6 and from Himalayan region of Uttarakhand and Himachal Pradesh7, 8. In the last few years, disease has been observed in almost epiphytotic form and could cause up to 20-100% losses in yield9, 8. Screening for rust severity indicated wide range of variations for rust resistance in the germplasm lines of pea and none of the genotypes tested were found to be free from infection4, 10, 2, 6, 11, 1. Rust severity is greatly influenced by the environment during initial infection and disease development. This is the major bottleneck in screening and selection for rust resistance. Use of molecular markers would allow indirect selection for rust resistance independent of environmental effects12. Molecular markers associated with pea rust resistance would be useful in marker assisted selection (MAS). For the development of rust resistant varieties there is need for phenotypic as well as molecular screening of existing lines/ germplasms/cultivars, therefore the present research has been carried out.
Screening of thirty two pea germplasm under natural epiphytotic condition was carried out in the field during Rabi season 2013-14 and 2014-15 at N.E. Borlogue Crop Research Centre (NEBCRC), G.B. Pant University of Agriculture and Technology, Pantnagar. The germplasm screening was undertaken following ‘Infector row technique’. Each entry was sown with wider spacing of 30 x 10cm in 3m row with a susceptible check ‘HFP-4’ after every five entries and a susceptible border row for over 2 seasons (Rabi 2013-2014 and 2014-2015). The observation on rust severity was recorded when first symptoms appear and subsequent observations were recorded at ten days interval and final observations was recorded at 20 days before harvesting of entries. Disease severity was determined using 0-9 rating scale13. The genotypes were later grouped into different categories based on 0 to 9 scale of disease severity from immune to highly susceptible according to Mayee and Datar (1986) with slight modifications (Table 1.). Thereafter, molecular screening of thirty two diverse pea genotypes which were phenotypically screened on the basis of disease reaction was evaluated using four SSR markers (Table 2.) (AA446 and AA505 flanking the major QTL Qruf; AD146 and AA416 flanking the minor QTL, Qruf1) associated with pea rust resistance12.
Table (1):
Disease severity scale showing different types of disease reaction .
Rating |
Description |
Disease reaction |
|---|---|---|
0 |
No symptoms on leaf |
Immune (I) |
1 |
Rust pustules small, scattering covering 1% or less of leaf area |
Resistant (R) |
3 |
Rust pustules more in number covering 1-10% of leaf area |
Moderately resistant (MR) |
5 |
Typical rust pustules covering 11-25% of leaf area |
Moderately susceptible (MS) |
7 |
Typical rust pustules covering 26-50% of leaf area. Leaf shedding |
Susceptible (S) |
9 |
Typical rust pustules covering 51% or more of leaf area. Defoliation severe |
Highly susceptible (HS) |
Table (2):
Primers used in molecular screening (Loridon et al., 2005).
Sl. No. |
Primer Name |
Forward sequence |
Reverse sequence |
PIC* |
Tm© (0C) |
|---|---|---|---|---|---|
1. |
AA446 |
5’ TTA GCT TGC AGC CCA CTC 3’ |
3’ ATC CGA CCC ATG GAT TTA 5’ |
0.66 |
55 |
2. |
AA505 |
5’ ATT CAC ACG CGC CCA 3’ |
3’ CAA TTA AGC CCT CAT CCA GA 5’ |
0.69 |
55 |
3. |
AD146 |
5’ TGC TCA AGT CAA TAT ATG AAGA 3’ |
3’ CAA GCA AAT AGT TGT TTT GTT A 5’ |
0.84 |
51 |
4 |
AA416 |
5’ TTA CTG TTA CTT TGC GAC ATC A 3’ |
3’ ATA GTG TCG AAA TTT TCC ATC C 5’ |
0.64 |
61 |
* PlC- Polymorphism information content, ©Tm- Annealing temperature
PCR procedure
DNA from each germplasm was extracted following the CTAB method14. About 100 mg of young leaf tissue was excised from aseptically grown seedlings of each genotype. PCR amplification solution was prepared using 10mM tris-HCl (pH 9.0; 1.5mM MgCl2; 50mM KCl and 0.01% gelatin), 0.5 mM MgCl2, 200 mM dNTPs, 1.25 ìM of primer, 20 to 25ng of DNA and1unit of Taq polymerase per 25Kl reaction volume. SSR markers AA446 and AA505 flanking the major QTL Qruf and AD146 and AA416 flanking the minor QTL, Qruf112 were commercially synthesized and procured from Eurofins Inc., Bengaluru. The amplification reaction was carried out in thermo cycler (MyCycler®, Bio-Rad Laboratories, California). After initial denaturation at 94°C for 5 min, cycle was repeated 40 times; denaturing at 94°C for 1 min, annealing according to primer [15] for 1 min, extension at 72 °C for 2 min, and the final extension segment was hold for 7 min. Thereafter, PCR products were separated electrophoretically in 2% (w/v) agarose gel. Ethidium bromide solution at a final concentration of 0.5 ìg/ml was added to the agarose solution. The gel was visualized and documented using gel documentation system (Transilluminator with filter, GeNei, Benagluru).
Among 32 pea genotypes screened, none of them were found resistant to rust disease during both the seasons. Further, in our search, none of the genotype was found to be completely resistant to the rust disease, which was in agreement with earlier reports2, 16 although these reports were based on the screening of limited genotypes. Only two genotypes showed moderate reaction with 1-10 per cent disease severity (Pant P 244 and Pant P 42). Maximum numbers (18) of genotypes fall under susceptible category followed by moderately susceptible (13) and highly susceptible category (2). Thus, two genotypes showing moderately resistant reaction can be integrated with reduced number of fungicidal spray to obtain maximum yield with minimal rust severity. Screening for rust severity indicated wide range of variations for rust resistance in the germplasms lines of pea and none of the genotypes tested were found to be free from infection4, 10, 2, 6, 11, 1. Pal et al17 screened a total of 292 accessions of pea (Pisum spp.) under field conditions for resistance to rust and he found only three accessions PJ207508, PJ222117, and EC109188 which was resistant to rust. Kumar et al6 used area under disease progress curve (AUDPC) to depict the overall disease stress that the plants were subjected to and described the pea varieties Pant P8, HUP 8063, KPMR 22 to possess good level of partial resistance. Likewise, Chand et.al18 screened 345 accessions, out of which forty-four genotypes were evaluated for disease intensity. Wide range of variation was found for those traits. The genotypes Pant P 11, FC 1, HUDP 16, JPBB 3 and HUP 14 appeared as slow rusting genotypes. Similarly, Mishra et al19 evaluated 107 genotypes of field pea against rust (Uromyces viciae-fabae), out of which genotypes P 9-77, P 2432; P2572 and P 2930 were found resistant, whereas 27 exhibited moderate reaction. Barilli et al20 evaluated 2759 pea accessions for resistance against Uromyces pisi (Pers.) Wint. All accessions in his experiment displayed a compatible interaction (high infection type) both in adult plants under field conditions and in seedlings under growth chamber conditions, but with varying levels of disease reduction. The identified resistance was based on reduction of disease severity with no associated host cell necrosis, which fits the definition of Partial Resistance. No complete resistance or incomplete resistance based on hypersensitivity was observed by them.
Severity of rust is greatly influenced by the environment during infection initiation and disease development. This is the major bottleneck in screening and selection for rust resistance. Thus, use of molecular markers would allow indirect selection for rust resistance independent of environmental effects.
SSR marker AA446 flanking the major QTL Qruf amplified a fragment of 450 bp in two moderately resistant (Pant P 244 and Pant P 42), seven moderately susceptible and six susceptible genotypes with 25-30 percent disease severity. Whereas, SSR marker AA505 flanking the major QTL Qruf amplified a fragment of 140 bp in two moderately resistant(Pant P 244 and Pant P 42), six moderately susceptible, nine susceptible genotypes (25-30 percent disease severity) and one highly susceptible genotypes(HFP-4) (Table 3 and Plate 1).
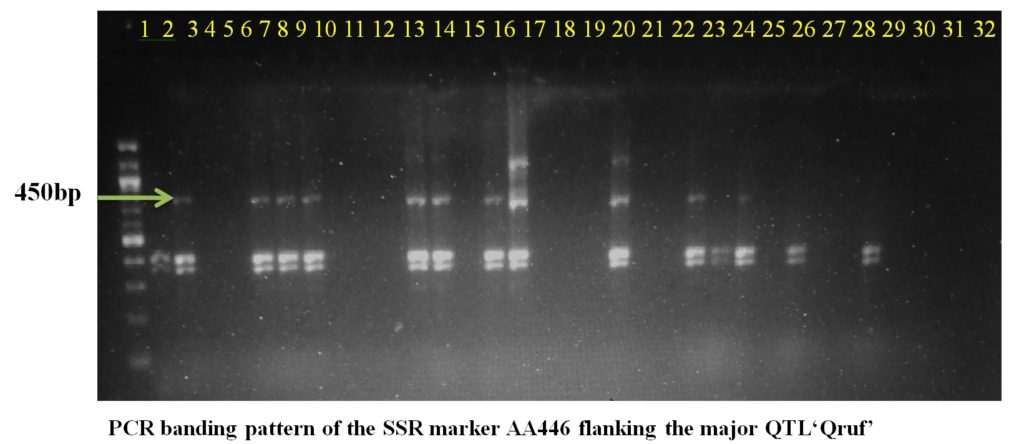
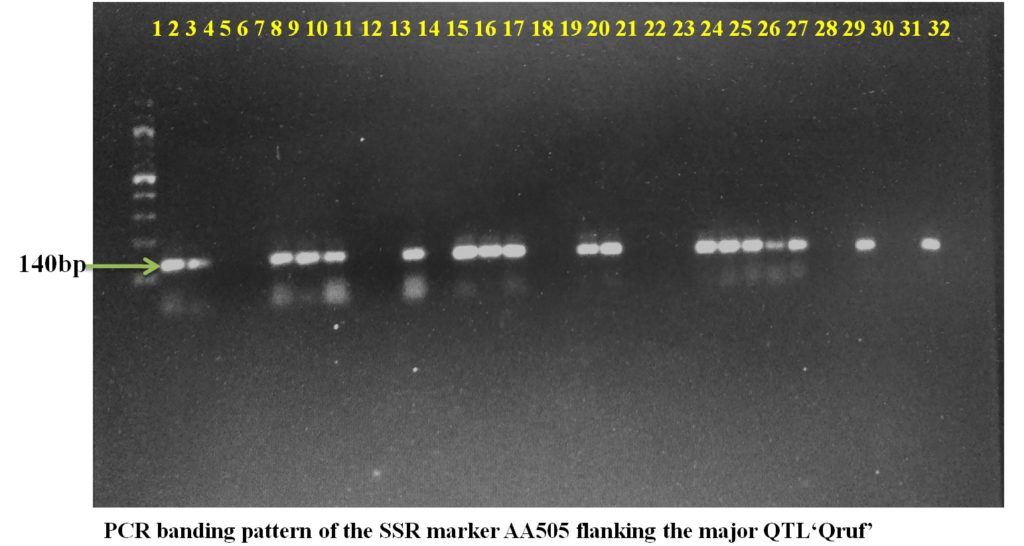 Plate1. PCR banding pattern of the SSR markers AA446 and AA505 flanking the major QTL ‘Qruf’ in selected pea germplasm
Plate1. PCR banding pattern of the SSR markers AA446 and AA505 flanking the major QTL ‘Qruf’ in selected pea germplasmSSR markers AD146 flanking the minor QTL, Qruf1 were able to identify one moderately resistant (Pant P 42), six moderately susceptible genotypes and four susceptible genotypes (25-30 percent disease severity) with amplified fragment of 430bp. Whereas, SSR markers AA416 flanking the minor QTL, Qruf1amplified a fragment of 280bp in four moderately susceptible genotypes, seven susceptible genotypes (25-30 percent disease severity) and one susceptible genotypes with 49.17 percent severity (HUDP1301) (Table 3 and Plate 2).
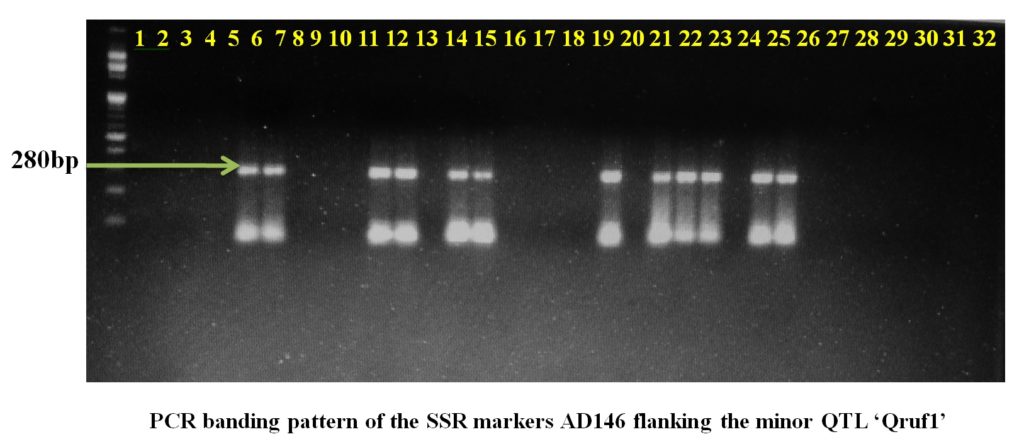
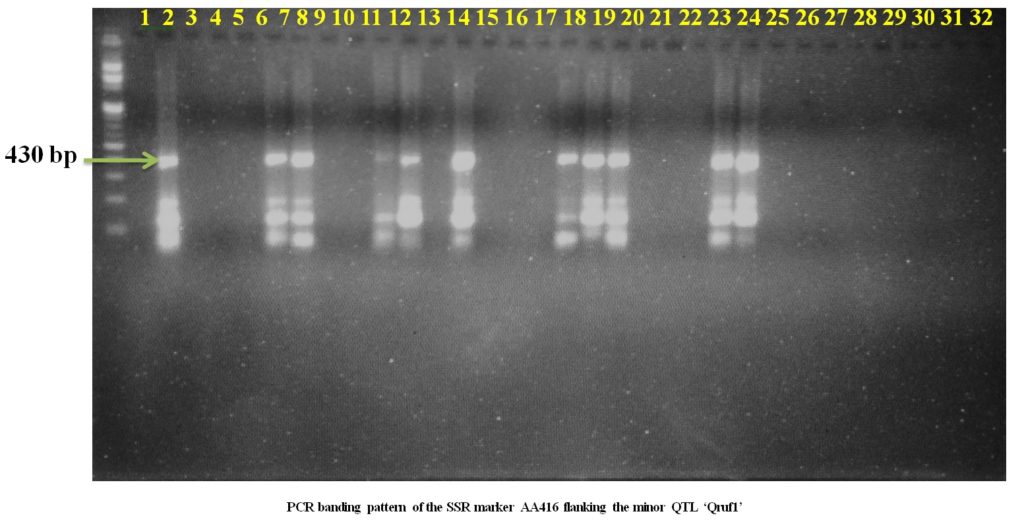
Plate 2. PCR banding pattern of the SSR markers AD146 and AA416 flanking the major QTL ‘Qruf1’ in selected pea germplasm.
It was observed that most of the germplasm with disease severity of less than 30 percent showed the presence of Qruf and/or Qruf1 governing partial resistance against rust. Therefore, molecular screening of germplasm may conclude that these SSR markers (AA446, AA505, AD146 and AA416) if used together, then it can be more effective in marker assisted selection (MAS) for pea rust resistance. Similarly, Singh et al21 utilize molecular markers associated with the pea rust resistance for evaluation of 30 diverse pea genotypes using these four SSR markers. On the basis of marker allele analysis they concluded that these SSR markers can be used in MAS of pea rust resistance. Vijayalakshmi et al22 suggested two RAPD makers, viz., SC10-82360, and SCRI-711000, flanking the rust resistance gene (Ruf) if used together, the effectiveness of marker assistant selection for rust resistance would be improved considerably. Avila et al23 also identified random amplified polymorphic DNA (RAPD) markers linked to resistance gene (Uvf-1). This result conclude that for the development of rust resistant varieties there is need for phenotypic followed by molecular screening of existing Table.3
Table (3):
Molecular screening of selected germplasm for rust resistance in pea.
| S. No. | Germplasm | Disease severity (%) | Disease reaction | 1Qruf | 2Qruf1 | ||
|---|---|---|---|---|---|---|---|
| AA446(450bp) | AA505(140bp) | AD146(430bp) | AA416(280bp) | ||||
| 1. | Pant P 244 | 8.17 | MR | + | + | _ | _ |
| 2. | Pant P 42 | 8.50 | MR | + | + | + | _ |
| 3. | KPMR 522 | 42.50 | S | _ | _ | _ | _ |
| 4. | HUVP 1 | 55.83 | HS | _ | _ | _ | _ |
| 5. | HFP 530 | 30.00 | S | + | + | _ | + |
| 6. | HFP 1016 | 25.83 | S | + | + | + | + |
| 7. | HFP 9907 | 21.67 | MS | + | + | + | _ |
| 8. | KPMR 925 | 45.00 | S | _ | _ | _ | _ |
| 9. | VL 202 | 43.33 | S | _ | _ | _ | _ |
| 10. | Pant P223 | 27.50 | S | _ | + | + | + |
| 11. | VL 59 | 21.67 | MS | + | _ | + | + |
| 12. | Pant P 222 | 19.17 | MS | + | + | _ | _ |
| 13. | Pant P 217 | 10.33 | MS | _ | + | + | + |
| 14. | Pant P 213 | 12.17 | MS | + | + | _ | + |
| 15. | VL 58 | 13.17 | MS | + | _ | _ | _ |
| 16. | KPMR 853 | 47.50 | S | _ | _ | _ | _ |
| 17. | HUDP 1302 | 22.50 | MS | _ | + | + | _ |
| 18. | HUDP 1209 | 25.83 | S | _ | + | + | _ |
| 19. | RFP 2009-2 | 19.17 | MS | + | _ | + | + |
| 20. | RFP 2009-3 | 39.17 | S | _ | _ | _ | _ |
| 21. | HUDP 1301 | 49.17 | S | _ | _ | _ | + |
| 22. | KPMR 851 | 25.83 | S | + | + | _ | + |
| 23. | KPM 928 | 25.83 | S | + | + | + | + |
| 24. | HUDP 15 | 20.83 | MS | + | + | + | _ |
| 25. | IPFD 13-14 | 25.83 | S | _ | + | _ | + |
| 26. | IPF 10 | 30.83 | S | + | + | _ | + |
| 27. | IPFD 5-19 | 23.33 | MS | _ | _ | _ | _ |
| 28. | IPFD 99-13 | 44.17 | S | _ | _ | _ | _ |
| 29. | IPFD 11-5 | 25.83 | S | + | + | _ | _ |
| 30. | IPFD 12-2 | 45.83 | S | _ | _ | _ | _ |
| 31. | IPFD 13-4 | 39.17 | S | _ | _ | _ | _ |
| 32. | HFP-4 (check) | 65.00 | HS | _ | + | _ | _ |
1Qruf flanked by SSR markers AA446 and AA505 (Rai et al., 2011), 2Qruf1 flanked by SSR markers AD146 and AA416 (Rai et al., 2011), + indicates presence of a band and “ indicates absence of the specific band
- Chand, R., Srivastava, C.P. and Kushwaha, C. Screening technique for pea (Pisum sativum L.) genotypes against rust disease (Uromyces fabae Pers. de Bary). Indian J. Agric. Sci., 2004; 74: 166-7.
- Gupta, R.P. Evaluation of pea germplasm for their reaction to powdery mildew and rust. Indian J. Pul. Res., 1990; 3: 186-8.
- Chand, R., Srivastava, C.P., Singh, R.M. and Singh, R.B. Pea specific strains in Uromyces fabae. Indian J. Pul. Res., 1997; 10: 127-8.
- Narsinghani, V.G., Singh. S.P. and Pal, B.S. Note on rust resistance pea varieties. Indian. J. Agric. Sci., 1980; 50: 453.
- Sokhi, H.S., Sokhi. S.S. and Rawal, R.D.Vertical reaction of pea to powdery mildew (Erysiphe polygoni) and rust (Uromyces vicia fabae). Mysore J. Agril. Sci., 1974; 8: 529-532.
- Kumar, T.B.A., Rangaswamy, K.T. and Ravi, K. Assessment of tall field pea genotypes for slow rusting resistance. Legume Res., 1994; 17: 79-82.
- Chauhan, R.S., Sugha, S.K. and Singh, B.M. A note on the prevalence and distribution of pea rust in Himachal Pradesh. Him. J. Agric. Res., 1991; 17: 105-107.
- Sharma, A.K. Epidemiology and management of rust disease of French bean. Veg. Sci., 1998; 25: 85-88.
- Stavely, J.R. Compendium of Bean Diseases. APS Press, St Paul MN., 1991; pp. 24-25.
- Singh, R.M. and Srivastava, C.P. Evaluation, classification and usefulness of pea germplasm lines for quantitative characters. Legume Res., 1985; 8: 68-73.
- Xue, A.G. and Warkentin, T.D. Reaction of field pea varieties to three isolates of Uromyces fabae. Cana. J. Pl. Sci., 2002; 82: 253-255.
- Rai, R., Singh, A.K., Singh, B.D., Joshi, A.K., Chand, R. and Srivastava, C.P. Molecular mapping for resistance to pea rust caused by Uromyces fabae (Pers.) de-Bary. Theor. Appl. Genet., 2011; 123: 803-813.
- Mayee, C.D. and Datar, V.V. Phytopathometry. Technical Bulletin-1 (Special Bulletin 3), Marathwada Agric. Univ. Parbhani., 1986; p 218.
- Doyle, J.J. and Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull., 1987; 19: 11-15.
- Loridon, K., McPhee, K., Morin, J., Dubreuil, P., Pilet-Nayel, M.L., Aubert, G., Rameau, C., Baranger, A., Coyne, C., Lejeune-Henaut, I. and Burstin, J. Microsatellite marker polymorphism and mapping in pea (Pisum sativum L.). Theor. Appl. Genet., 2005; 111: 1022-1031.
- Anil Kumar, T.B., Rangaswamy, K.T. and Ravi, K. Assessment of tall field pea genotypes for slow rusting resistance. Legume Res., 1994; 17: 79–82.
- Pal, A.B., Brahmappa, R., Rawal, D. and Ullasa, B. A. Field resistance of pea germplasm to powdery mildew (Erysiphe polygoni) and rust (Uromyces fabae). Plant Dis., 1980; 64: 1085-1086.
- Chand, R., Srivastava, C.P., Singh, B.D. and Sarode, S.B. Identification and characterization of slow rusting components in pea (Pisum sativum L.). Genet. Resour. Crop. Ev., 2006; 53: 219-224.
- Mishra, R.K., Pandey, K.K. and Pandey, P.K. Screening of pea (Pisum sativum) genotypes against rust caused by Uromyces fabae. Indian J. Agr. Sci., 2009; 79: 402-403.
- Barilli, E., Sillero, J.C., Aparicio M.F. and Rubiales, D. Identification of resistance to Uromyces pisi (Pers.) Wint. in Pisum spp. Germplasm. Field Crop Res., 2009; 114: 198-203.
- Singh, A.K., Rai, R., Singh, B.D., Chand, R., Srivastava, C.P. Validation of SSR markers associated with rust (Uromyces fabae) resistance in pea (Pisum sativum L.). Physiol. Mol. Biol. Plant., 2015; 21: 243-247.
- Vijayalakshmi, S., Yadav, K., Kushwaha, C., Sarode, S.B., Srivastava, C.P., Chand, R. and Singh, B.D. Identification of RAPD markers linked to the rust (Uromyces fabae) resistance gene in pea (Pisum sativum.). Euphytica, 2005; 144: 265–274.
- Avila, C.M., Sillero, J.C., Rubiales, D., Moreno, M. T. and Torres, A. M. Identification of RAPD markers linked to the Uvf-1 gene conferring hypersensitive resistance against rust (Uromyces viciae-fabae) in Vicia faba L. Theor. Appl. Genet., 2003; 107: 353–358.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


