ISSN: 0973-7510
E-ISSN: 2581-690X
The formation of extracellular polysaccharide polymers (EPS) is catalyzed by the enzyme Fructosyltransferase (ftf), derived from lactic acid bacteria (LAB). These enzymes produce various homopolymers, including fructans, levans, inulin, and fructooligosaccharides (FOS), which are beneficial as prebiotics, emulsifiers, stabilizers, and gelling or water-binding agents in food products. In the health sector, they also exhibit potential antitumor, antiulcer, and immunomodulatory properties and help lower cholesterol levels. This study aims to identify lactic acid bacteria as probiotic candidates encoding the fructosyltransferase gene (ftf) from Pakoba fruit (Syzygium sp.). The methods used in this study included reculturing six isolates of probiotic candidate LAB-EPS, DNA isolation, amplification of the 16S rRNA gene using universal primers (27F and 1492R), and amplification of the ftf gene using specific primers (5FTF and 6FTF). The amplification of the 16S rRNA gene produced amplicons of approximately 1400 bp, while the amplification of the ftf gene yielded amplicons of approximately 800 bp. Two of the six probiotic LAB-EPS isolates were found to harbor the ftf gene: the PM6.4 and PM5.3 isolates. Identification based on the 16S rRNA gene sequence revealed that the PM6.4 and PM5.3 isolates belong to the species Lactiplantibacillus plantarum. This study concludes that the two LAB-EPS probiotic isolates from Pakoba fruit (Syzygium sp.) contain the FTF gene, identified as Lactiplantibacillus plantarum.
Lactic Acid Bacteria, Pakoba, Fructosyltransferase, Lactiplantibacillus plantarum
Lactic acid bacteria (LAB) are recognized as food-grade bacteria and are widely utilized in the health and food industries as probiotics.1 Many studies have shown that LABs are capable of producing biopolymers, known as exopolysaccharides (EPS),2-6 which are essential for health due to their antitumor, anti-inflammatory, and anti-ulcer activities, as well as their ability to enhance the immune system.7 Several genera of LAB are known to synthesize various EPS, including Fructilactobacillus, Lacticaseibacillus, Lactiplantibacillus, Lactobacillus, Lactococcus, Lactilactobacillus, Lentilactobacillus, Leuconostoc, Limosilactobacillus, Pediococcus, Streptococcus, and Weissella.8,9
Recently, EPS-producing LAB has been explored, with one successful source being the Pakoba fruit (Syzygium sp.), an endemic species from Minahasa, North Sulawesi.10 Pakoba fruit provides an ideal habitat for LAB due to its sour taste. EPS plays a crucial role in enhancing bacterial adhesion to the digestive tract, protecting bacterial cells from toxic compounds, improving resistance to antibiotics and bacteriophages (viruses),11 and increasing the viscosity of food products.
Fructans, homopolymers of fructose, are synthesized by the enzyme fructosyltransferase (ftf), which is classified under the sucrose enzyme family. This enzyme cleaves sucrose and uses the released energy to incorporate fructose units into the growing fructan chain. Fructosyltransferase is found in both plants and bacteria.12 Fructans may contain β-(2-1), β-(2-6), and β-(2-1-6) branching bonds. Fructans with β-(2-6) bonds are called levans, while those with β-(2-1) bonds are called inulins. FTF enzymes producing levan are known as levansucrases, and those producing inulin are known as inulosucrases. Microorganisms such as Streptococcus, Bacillus, and Lactobacillus produce fructans.6,12
Characterizing the ftf gene enables more effective strain selection for probiotic applications. Polymorphisms in the ftf gene may lead to variations in enzymes involved in EPS synthesis, contributing to the diversity of EPS structure and function, which in turn can offer various health benefits.13 Each gene regulates the corresponding enzyme’s formation, function, and activity. The FTF enzyme plays a key role in EPS biosynthesis, making the identification of LAB that produce FTF essential for evaluating their probiotic potential.
Probiotic LAB-EPS can be identified through PCR amplification, gene sequencing, or conventional methods. Conventional methods rely on the phenotypic characteristics of bacteria, such as Gram staining, colony morphology, and enzyme activity. However, these traditional methods are often limited. In contrast, PCR and gene sequencing offer more accuracy, objectivity, and speed and do not require optimal bacterial growth or media, making them preferred techniques.14 The target gene for bacterial identification in PCR is the 16S rRNA gene, which encodes the 30S ribosomal subunit and spans 1500 base pairs. The 16S rRNA gene is a reliable parameter for determining phylogenetic relationships at the species level.15
Research on probiotic bacterial candidates from Pakoba fruit (Syzygium sp.) that can produce EPS by detecting the ftf gene encoding the fructosyltransferase enzyme is limited. Therefore, this study was conducted to detect the presence of the fructosyltransferase (FTF) gene and identify LAB-EPS probiotic candidates from Pakoba fruit (Syzygium sp.) that encode this gene.
The sample used in this study was lactic acid bacteria (LAB) from Pakoba fruit (Syzygium sp.), which had previously been tested for its ability to produce EPS and evaluated as a probiotic candidate. Before use, the LAB-EPS probiotic samples were recultured following a dormancy period during which they were stored at freezing temperatures. The bacterial isolates were cultured twice until sufficient bacterial cell biomass was formed.
Method
Reculture of LAB-EPS probiotics
The reculture process of bacterial isolates was conducted using a modified method.16 The process began with preparing MRS liquid medium, which was poured into test tubes containing 5 mL of MRS liquid medium. The composition for 75 mL of MRS broth included 3.93 g of MRS broth and 0.112 g of bile salt. The medium was placed in an Erlenmeyer flask, and 75 mL of aquades was added. The medium was then heated using a hotplate until fully dissolved. Once the medium cooled to room temperature, 5 mL was poured into each test tube. The media was sterilized using an autoclave at 121 °C for 15 minutes. Six bacterial isolates were inoculated into the growth medium and incubated at 37 °C for 24 hours. The reculture process was carried out twice.
Molecular identification with amplification of the ftf (Fructosyltransferase) gene
DNA extraction
Probiotic candidate LAB-EPS were grown on MRS broth medium for 24 hours at 37 °C under anaerobic conditions. 1.5 mL of culture was centrifuged using an Eppendorf Mini Spin Plus (Hamburg, Germany) at 10,000 rpm for 5 minutes to obtain the cell pellets. The pellets were resuspended in 750 μL of lysis buffer, and 20 μL of Proteinase K was added, followed by shaking for 10 minutes. Next, 40 μL of Lysozyme solution was added, and the mixture was incubated for 30 minutes at 55 °C. The sample was centrifuged at 13,000 rpm for 5 minutes, and the supernatant was transferred to a new microcentrifuge tube. 750 μL of phenol was added, and the sample was centrifuged at 13,000 rpm for 10 minutes. The upper layer was separated and transferred to a new microcentrifuge tube, followed by the addition of 1:1 (v/v) cold chloroform. The mixture was gently shaken for 10 minutes, then centrifuged for another 10 minutes. The top layer was separated and transferred to a new microcentrifuge tube, and 1:1 (v/v) cold absolute ethanol was added and incubated at -80 °C for 2 hours. Afterward, the sample was centrifuged at 13,000 rpm for 10 minutes. The supernatant was discarded, and the pellet was washed with 0.5 mL of 70% ethanol and centrifuged for 10 minutes. The pellet was dried, and 50 μL of TE buffer was added. The DNA of the bacterial genome was ready for use.17
Amplification of 16S rRNA gene using universal primers
The primers 27F (5′-AGAGTTT AGTCCTGGCTCAG-3′) and 1492R (5′-GNTACCTTGTTACGACTT-3′) (Integrated DNA Technologies, Singapore) were used to amplify the 16S rRNA gene with a target sequence of approximately 1500 bp. The amplification of the 16S rRNA gene was visualized using a 1% agarose gel and a 1 kb DNA ladder molecular marker. A modified method was used for amplification. The reaction mix included aquabides (NFW) and MyTaq HS Red Mix (Bioline), which contains Taq DNA polymerase, dNTPs, MgCl2, and PCR buffer. The following PCR program was used: initial denaturation at 95 °C for 3 minutes, denaturation at 95 °C for 30 seconds, annealing at 54 °C for 30 seconds, extension at 72 °C for 2 minutes (35 cycles), and final extension at 72 °C for 2 minutes, and hold at 4 °C.16
Amplification of the ftf gene using specific primers
DNA fragments were amplified using a PCR technique with specific primers for the ftf gene, which targets gram-positive bacteria.16,18 The primers used for the amplification of the ftf gene were 5FTF (5′-GAYGTNTGGGAYWSNTGGGCC-‘3) and 6FTF (5’-GATTGAACCTGCATTGCG-‘3) (Integrated DNA Technologies, Singapore). The PCR program was as follows: initial denaturation at 95 °C for 5 minutes, denaturation at 95 °C for 30 seconds, annealing at 52 °C for 30 seconds, extension at 72 °C for 45 seconds (35 cycles), final extension at 72 °C for 3 minutes, and hold at 4 °C. The reaction mix for the ftf gene amplification consisted of aquabides (NFW) and MyTaq HS Red Mix (Bioline), containing Taq DNA polymerase, dNTPs, MgCl2, and PCR buffer. The PCR products were analyzed using 1% agarose gel electrophoresis. The primary attachment temperature variations, ranging from 48 °C to 55 °C, were tested to determine the optimal annealing temperature.
Sequencing of the 16S rRNA gene and phylogenetic analysis
An automated DNA sequencer (ABI PRISM 3130 Genetic Analyzer, Applied Biosystems) was used to sequence the amplified DNA with primers 27F and 1492R. The sequencing data were processed using Chromas Pro software. Reference sequences were retrieved from GenBank/DDBJ/EMBL, and the closest identity was determined by searching the EzTaxon website (http://www.ezbiocloud.net/).19 The phylogenetic tree was constructed using the UPGMA method in the MEGA 6.0 program.20
Reculture of LAB-EPS probiotic candidate
The reculture process was based on probiotic LAB-EPS isolates from a previous study. The six probiotic LAB-EPS isolates used in this study were: PM7.2, PM6.4, PM6.10, PM5.1, PM5.3, and PM5.2 (Note: PM refers to Ripe Pakoba). The purpose of the reculture was to revive bacteria that had undergone a dormancy period due to storage at freezing temperatures. The process involved inoculating the isolates in MRS broth growth medium supplemented with 0.15% bile salt to ensure that the cultured isolates were indeed probiotic. The reculture was performed twice to allow the bacteria to regenerate, as indicated by the appearance of white deposits in the test tubes, referred to as bacterial biomass (Figure 1). The isolates were incubated at 37 °C for 24 hours, with the formation of white deposits signaling that the bacteria were still capable of replication.
Figure 1. Reculture results of the second probiotic LAB-EPS isolate; (1) PM7.2 isolate; (2) PM6.4 isolate; (3) PM6.10; (4) PM5.1; (5) PM5.3; (6) PM5.2
On the first day of reculture, no bacterial biomass deposits were formed. This is because the freezing storage had caused a reduction in isolate activity, as the absence of encapsulation led to decreased membrane permeability and potential damage to the bacterial cells.21 Adding bile salts to the MRS broth affects the activity of the dormant cells. Bacterial cells with reduced permeability, which do not yet have regular activity, cannot tolerate the toxic properties of the bile salts. Furthermore,22 bacteria that have undergone a dormancy phase require time to adapt to the new growth medium.
In the second reculture, biomass was observed, indicating that the bacteria had regained the ability to replicate. The increase in biomass demonstrated that the bacterial isolates could adapt to the new growth medium, enabling a more rapid and stable replication process.
Molecular identification
Extraction of genomic DNA
Following the reculture process, the ftf and gtf genes were molecularly detected. The first step in this detection process is DNA isolation, which separates DNA from other cellular components to obtain pure DNA. The principle of DNA isolation is to isolate DNA from other cellular components.23
Based on the reculture results, DNA isolation was successfully performed for the six probiotic LAB-EPS isolates. Subsequently, qualitative analysis was conducted using electrophoresis on a 1% agarose gel, with visualization through a UV transilluminator. During the DNA isolation procedure, the bacteria used were from the second stage of reculture. This is because bacterial biomass had not yet formed during the initial phase of reculture. Following their dormant phase, the bacteria resumed metabolic activity, as evidenced by the emergence of white deposits (Figure 2). A qualitative visualization was performed to assess the effectiveness of DNA isolation from the six LAB isolates. The electrophoregram in Figure 3 shows the bacterial DNA.
Figure 2. Genomic DNA visualization of six probiotic LAB-EPS isolates treated with RNase and non-RNase enzymes; (1) PM7.2 isolate; (2) PM6.4 isolate; (3) PM6.10 isolate; (4) PM5.1 isolate; (5) PM5.3 isolate; (6) PM5.2 isolate
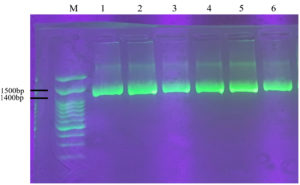
Figure 3. Amplification of 16S rRNA genes from six isolates of LAB-EPS Probiotic; (M) DNA Ladder; (1) PM7.2 isolate; (2) PM6.4 isolate; (3) PM6.10 isolate; (4) PM5.1 isolate; (5) PM5.3 isolate; (6) PM5.2 isolate
Based on the electropherogram from the 1% agarose gel with a 3 Kb marker, it is evident that DNA was successfully extracted from all probiotic LAB-EPS isolates. Electrophoresis enabled the isolation of DNA fragments larger than 1000 base pairs. These results align with previous research,24 which achieved electrophoresis results for DNA fragments exceeding 10,000 bp. The electrophoresis results in Figure 2 also show the presence of RNase and non-RNase impurities. Additionally, there is variation in the thickness of the DNA bands, which is likely due to the insufficient concentration of DNA obtained during the isolation process.25
Amplification of the 16S rRNA gene using specific primers
The success of the genomic DNA isolation process is confirmed by amplifying the 16S rDNA gene. Electrophoresis results showed that the amplification of the 16S rRNA gene achieved a base length of 1400 bp using the universal primers 27F and 1492R (refer to Figure 3). These findings are consistent with the research conducted by Lawalata et al,17 which utilized primers 27F and 1492R to amplify the 16S rRNA gene, resulting in a 1500 bp product.26 Additionally, universal primers, specifically Bact-FI and Uni-B1, have been reported to amplify the 16S rRNA gene, producing a 1400 bp product. The application of universal primers in amplifying the 16S rRNA gene is critical in protein synthesis. The 16S rRNA gene contains a conserved region.
Due to its essential role in cellular function, the 16S rRNA gene contains a conserved region. This significantly reduces mutations, particularly in genes encoding enzymes critical for lactose metabolism. Using the 16S rRNA gene for identification offers several advantages.27 Besides its conserved region, this gene is ubiquitous, with its function identical across all organisms. The gene can evolve according to evolutionary distance, making it an effective evolutionary chronometer. Moreover, the gene has a hypervariable region, which aids in identifying bacterial types.28
The 16S rRNA gene is widely used in various research fields, particularly for identification. Its specificity, stability,29 and universal presence in all living organisms make it a valuable molecular marker for identification.30
Amplification of the Fructosyltransferase (ftf) encoding gene using specific primers
The next stage involves amplifying the ftf gene from six genomic DNA probiotic LAB-EPS isolates using PCR with degenerate primers, specifically 5FTF and 6FTF, under the PCR conditions described by Falih et al.16 The confirmed use of the LAB genus to detect the presence of the ftf gene is supported by Sabbathini et al,14 which states that certain strains of lactic acid bacteria (LAB) possess the ability to synthesize exopolysaccharides (EPS) through the action of prominent extracellular enzymes, notably fructosyltransferase, which is encoded by the ftf gene.
The PCR conditions currently in use are as follows: Pre-denaturation: 95 °C for 2 minutes; denaturation: 95 °C for 30 seconds; primary attachment (annealing): 50 °C for 30 seconds; polymerization: 72 °C for 30 seconds; end elongation: 72 °C for 1 minute; 35 cycles. The results of amplicon analysis through electrophoresis did not show the presence of bands.
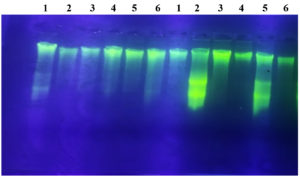
To optimize the results, the annealing temperature was adjusted to 52 °C. Electrophoresis results indicated that the DNA band was faint, and non-specific bands were observed in two isolates: PM6.4 and PM5.3 (Figure 4). Based on the observation of the amplification results using 1% agarose, the size of the ftf gene amplification product was approximately 800 bp, which is smaller than the ftf gene sequence confirmed in GeneBank. This discrepancy is likely due to modifications applied to the primers used.
Figure 4. Gel electrophoresis of PCR amplification results using 5FTF and 6FTF primers. (M) DNA Ladder; (2) PM6.4 isolate; (5) PM5.3 isolate
The DNA bands obtained in the 800 bp region did not show vigorous intensity (Figure 4), likely due to the low concentration of the product. This low concentration may be attributed to factors such as the number of cycles used, the concentration of primers, the DNA template, and the specificity of the degenerate primer. The 35 cycles used were optimized for the reaction. The amplicon size observed in this study (800 bp) differs from the study by Guerin et al,13 where the amplicon was 58 bp. The variation in size may be influenced by environmental factors, differences in origin, strains, and substrates, all of which could result in variations in the genomic structure of the bacteria.
The variability in amplicon sizes, even from a single strain, could also be caused by degenerate primers. Degenerate primers consist of a mixture of oligonucleotides with varying nucleotide sequences. These primers amplify genes with unknown nucleotide base arrangements but belong to the same family. Another contributing factor is the concentration of magnesium chloride (MgCl₂), a cofactor for Taq polymerase, which influences the efficiency and accuracy of the polymerization process. The concentration of the DNA template used can also affect the PCR results. Accurate DNA quantification should be conducted to determine the appropriate amount of DNA required for the PCR reaction.2
The discovery of the ftf gene will contribute to the diversity of enzymes involved in EPS synthesis. EPS’s varied structure and characteristics will enrich the types of polymers produced and enhance their applications in the pharmaceutical, health, and food industries.
DNA sequencing and phylogenetic analysis of LAB-EPS probiotic
Two probiotic EPS-producing LAB isolates were subsequently identified molecularly by analyzing partial sequences of the 16S rRNA gene. The results of BLAST-NCBI analysis show that two isolates contain fructosyltransferase (ftf) encoding genes, namely PM6.4 and PM5.3. Based on the 16S rRNA gene sequence analysis, the PM6.4 isolate shows 100% similarity with Lactobacillus plantarum strain KLB/Lactiplantibacillus plantarum. Meanwhile, the PM5.3 isolate shares 99.93% similarity with Lactiplantibacillus plantarum strain GB386. According to DNA sequencing tests, both the PM6.4 isolate (100%) and PM5.3 isolate (99.93%) are identified as Lactiplantibacillus plantarum (Lb. plantarum), indicating that these isolates belong to the same species. This finding is consistent with the identification made in previous studies based on physiological and biochemical characterization and API tests. Previous research has shown that all six LAB-EPS probiotic candidate isolates belong to the genus Lactobacillus plantarum (93.5%-98.6%).10
Lactiplantibacillus plantarum, previously classified as Lactobacillus plantarum, is a versatile microorganism found in various ecological environments, including the human gastrointestinal (GI) tract and numerous fermented foods.31 In commercial applications, L. plantarum is a key starter culture for food fermentations and is a beneficial probiotic. The L. plantarum strain has been reported to possess various functional properties within the food industry, enhancing nutritional quality, flavor profile, antioxidant capacity, antimicrobial properties, and food preservation while mitigating the presence of undesirable compounds.32 L. plantarum is one of the most significant members of the lactobacilli group and is commonly used as a probiotic due to its exceptional probiotic qualities (e.g., good GI tolerance, adhesion, antioxidant, and antibacterial properties).33
L. plantarum exhibits remarkable antioxidant and antimicrobial properties, notable resilience to acidic pH, gastrointestinal resistance, and a strong ability to adhere to the intestinal mucosa.34 These attributes may confer health benefits to the host. Furthermore, previous research has shown that certain strains of L. plantarum can alter the composition of the gut microbiota.35 Indeed, exploring the interaction between L. plantarum strains and the native gut microbiome has become a prominent area of contemporary research.
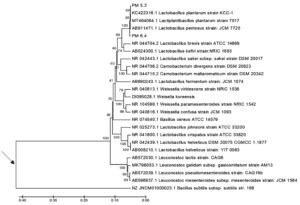
The phylogenetic tree shown in Figure 5 illustrates that the two probiotic LAB-EPS isolates containing the ftf gene from Pakoba fruit (Syzygium sp.), namely PM6.4 and PM5.3, have the closest kinship relationship with Lactobacillus plantarum (Lactiplantibacillus plantarum) compared to other species within the genera Lactobacillus, Leuconostoc, Weissella, and Carnobacterium.
Figure 5. The phylogenetic tree, reconstructed using the UPGMA method, shows the kinship relationships between the LAB-EPS probiotic test isolates and reference strains from the genera Lactobacillus, Leuconostoc, Weissella, and Carnobacterium, based on the 16S rRNA gene sequence. The arrow (→) indicates the approximate position of the roots of the phylogenetic tree, established using Bacillus subtilis strain 168 as the outgroup. The scale bar represents one substitution per 100 nucleotides in the 16S rRNA gene sequence
The similarity indices and varying nucleotide differences observed among the 16S rRNA gene sequences compared support the kinship relationship between the two probiotic LAB-EPS isolates and the reference strain of Lactobacillus plantarum (Lactiplantibacillus plantarum). Tables 1 and 2 present the similarity values and nucleotide differences for the 16S rRNA gene sequences of bacterial strains within the genera Lactobacillus plantarum, Lactiplantibacillus plantarum, and Lactobacillus pentosus.
Table (1):
Genetic distance matrix (lower diagonal) and genetic similarity (%) (upper diagonal)
Sequent/Isolate Name |
PM_5.3 |
PM_6.4 |
Lb. plantarum strain KCC-1 |
Lb. pentossus strain JCM 7725 |
Lactiplantibacillus plantarum strain 7017 |
|---|---|---|---|---|---|
PM_5.3 |
– |
99.93 |
99.93 |
99.86 |
99.93 |
PM_6.4 |
0.001 |
– |
100 |
99.93 |
100 |
KC422316.1_Lactobacillus_plantarum_strain_KCC-1 |
0.001 |
0,000 |
– |
99.93 |
100 |
AB911471.1_Lactobacillus_pentosusstrain_JCM_7725 |
0.001 |
0.001 |
0.001 |
– |
99.93 |
MT464064.1_Lactiplantibacillus_plantarum_strain_7017 |
0.001 |
0,000 |
0,000 |
0.001 |
– |
Table (2):
Nucleotide number difference matrix
Sequent/Isolate Name |
PM_5.3 |
PM_6.4 |
Lb. plantarum strain KCC-1 |
Lb. pentossus strain JCM 7725 |
Lactiplantibacillus plantarum strain 7017 |
|---|---|---|---|---|---|
PM_5.3 |
– |
– |
– |
– |
– |
PM_6.4 |
1 |
– |
– |
– |
– |
KC422316.1_Lactobacillus_plantarum_strain_KCC-1 |
1 |
0 |
– |
– |
– |
AB911471.1_Lactobacillus_pentosus strain_JCM_7725 |
2 |
1 |
1 |
– |
– |
MT464064.1_Lactiplantibacillus_plantarum_strain_7017 |
1 |
0 |
0 |
1 |
– |
Tables 1 and 2 present the similarity values and nucleotide differences in the 16S rRNA gene sequences between the two probiotic LAB-EPS isolates and strains of Lb. plantarum strain KCC-1, Lactiplantibacillus plantarum strain 7017, and Lactobacillus pentosus strain JCM 7725. The PM6.4 isolate, which belongs to the genus Lactobacillus or Lactiplantibacillus, shows the highest similarity (100%) with L. plantarum strain 7017, while the PM5.3 isolate shows 99.93% similarity with L. plantarum strain 7017 and Lb. plantarum strain KCC-1, and 99.86% similarity with Lb. pentosus strain JCM 7725. Based on the 16S rRNA gene sequence analysis, both the PM6.4 and PM5.3 isolates, which contain the ftf gene and were isolated from Pakoba fruit (Syzygium sp.), exhibit high nucleotide similarity and a close kinship relationship with Lactiplantibacillus plantarum strain 7017 and Lb. plantarum strain KCC-1.
Examining the 16S rRNA gene sequence is widely used for elucidating the phylogenetic relationships among bacterial strains. 16S rRNA gene sequence analysis provides a more precise and objective method for identifying bacterial strains and differentiating them within a single species.15 Research by 15 demonstrates the advantage of using the 16S rRNA gene, which recognizes bacterial species with over 99% similarity. The similarities obtained can be categorized into two groups: relatively high similarities and more distant (low) similarities.
Thus, molecular phylogenetic analysis based on the 16S rRNA gene sequence can confirm the novelty status of the two probiotic LAB-EPS isolates containing the ftf gene. However, further research is necessary to conclusively determine that these two isolates in the Pakoba fruit (Syzygium sp.) represent novel species and strains.
The ftf gene was amplified in two probiotic LAB-EPS isolates, PM6.4 and PM5.3. Both isolates possess the ftf gene, as indicated by a DNA band at approximately 800 bp, although the intensity was not vigorous. Molecular identification through 16S rRNA gene analysis confirmed that the PM6.4 and PM5.3 isolates belong to Lactiplantibacillus plantarum (formerly Lactobacillus plantarum).
ACKNOWLEDGMENTS
The authors thank the Indonesian Ministry of Education, Culture, Research, and Technology for funding this study through the Fundamental Research Grant (Regular).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
HJL conceptualized the study. HJL, S, JK and IK designed the experiments, performed isolation and identification of lactid acid bacteria as probiotic candidates encoding FTF Gene and data analysis. NWS, SMT, CT performed result interpretation. HJL wrote the manuscript. S and JK revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
The Indonesian Ministry of Education, Culture, Research, and Technology funded this study through the Fundamental Research Grant (Regular).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Moradi M, Guimaraes JT, Sahin S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr Opin Food Sci. 2021;40:33-39.
Crossref - Van Hijum SAFT, Kees Bonting, van der Maarel MJEC, Dijkhuizen L. Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol Lett. 2001;205(2):323-328.
Crossref - Rawat HK, Nath S, Sharma I, Kango N. Recent developments in the production of prebiotic fructooligosaccharides using fungal fructosyltransferases. Mycology. 2024;15(4):564-584.
Crossref - Alfaruqi HQD, Anindita NS, Bimantara A. Molecular Studies on Probiotic of Human Breast Milk in the Synthesis of Exopolysaccharide (EPS). Indonesian journal of biotechnology and bioscience. 2021;8(1):114-122.
Crossref - Wang Y, Peng Q, Liu Y, et al. Genomic and transcriptomic analysis of genes involved in exopolysaccharide biosynthesis by Streptococcus thermophilus IMAU20561 grown on different sources of nitrogen. Front Microbiol. 2024;14:1328824.
Crossref - Zhang J, Li L, Gu S, et al. Characterization of a Novel Fructosyltransferase from Lactobacillus crispatus, InuCA, That Attaches to the Cell Surface by Electrostatic Interaction. Appl Enviromen Microbiol. 2022;88(4):aem.02399-21.
Crossref - Korcz E, Varga L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci Technol. 2021;110: 375-384.
Crossref - Angelin J, Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol. 2020;162:853-865.
Crossref - Zheng JS, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782-2858.
Crossref - Lawalata HJ, Kumajas J, Tengker SM, Runtuwene KM, Hasani RS, Weken MM. Lactic Acid Bacteria as an Exopolysaccharides (EPS) Producing Starter from Pakoba Fruit (Syzygium sp.), Endemic Species at Minahasa, North Sulawesi. J Pure Appl Microbiol. 2023;17(4):2536-2546.
Crossref - Korcz E, Kerenyi Z, Varga L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018;6:3057-3068.
Crossref - van Hijum SAFT, van Geel-Schuten GH, Rahaoui H, van der Maarel MJEC, Dijkhuizen L. Characterization of a novel fructosiltransferase from Lactobacillus reuteri that synthesizes high-molecular weight inulin and inulin oligosaccharides. Appl Env Microbiol. 2002;68(9):4390-4398.
Crossref - Guerin M, Da Silva CR, Garcia C, Remize F. Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits. Fermentation. 2020;6(4):115.
Crossref - Sabbathini GC, Pujiyanto S, Wijanarka P, Lisdiyanti P. Isolation and Identification of Bacteria of the Genus Sphingomonas from Rice Leaves (Oryza sativa) in the Cibinong Rice Fields. Jurnal Biologi. 2017;6(1):1-6.
- Yang M-Q, Wang Z-J, Zhai C-B, Chen L-Q. Research progress on the application of 16S rRNA gene sequencing and machine learning in forensic microbiome individual identification. Front Microbiol. 2024;15:1360457.
Crossref - Falih AN, Anindita NS, Bimantara A, Khumaira A. The Detection of Genes Encoding Enzyme Fructosyltransferase the Exoplysaccharide EPS) Produce Potential Probiotic Candidates for Breast Milk (ASI). Int J Health Sci Technol. 2022;4(1):40-50.
Crossref - Lawalata HJ, Rungkat JA. Characterization of Lactic Acid Bacteria That Have Ability to Improved Number of Carnosine on Bakasang Based on I6S rRNA Gene. Eur J Eng Res Sci. 2019;4(1):106-109.
Crossref - Malik A, Radji M, Kralj S, Dijkhuizen L. Screening of Lactic Acid Bacteria From Indonesia Reveals Glucansucrase And Fructansucrase Genes In Two Different Weissella Confusa Strains From Soya. FEMS Microbiol Lett. 2009;300(1):131-138.
Crossref - Yoon S, Ha S, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67(5):1613-1617.
Crossref - Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725-2729.
Crossref - Bilang M, Tahir M, Haedar D. Study Viability Encapsulation of Probiotic Cells (Lactobacillus plantarum and Streptococcus thermophilus) on Ice Cream. Canrea Journal: Food Technology, Nutritions, and Culinary Journal. 2018;1(1):41-52.
Crossref - Sionek B, Szydlowska A, Trzaskowska M, Kolozyn-Krajewska D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation. 2024;10(6):298.
Crossref - Giyatno DC, Retnaningrum E. Isolation And Characterization Of Exopolysaccharide Producer Lactic Acid Bacteria From Kersen Fruit (Muntingia calabura L.). Basic Sains Journal. 2020;9(2):42-49.
Crossref - Reller LB, Weinstein MP, Petti CA. Detection and identification of microorganisms by gene Amplification and Sequencing. Clin Infect Disc. 2007;44(8):1108-1114.
Crossref - Noer S. Molecular Identification of Bacteria Using 16S rRNA. Biological and Education Journal (EduBiologia). 2021;1(1):1-6.
Crossref - Karamoy E, Kepel B. Isolation and Identification of Mercury Resistant Bacteria Through Analysis of the 16S RNA Gene in the Urine of Patients with Amalgam Fillings. eBiomedicine Journal. 2017;5(2):1-8.
Crossref - Akihary CV, Kolondam BJ. Utilization of the 16S rRNA Gene as a Bacterial Identification Tool for Research in Indonesian. Pharmacon Pharmaceutical Scientific Journal. 2020;9(1):2302-2493.
Crossref - Patten DA, Laws AP. Lactobacillus produced exopolysaccharides and their potential health benefits: a riview. Benef Microbes. 2015;6(4):457-471.
Crossref - Noor S, Pramono H, Aziz S. Detection of Diversity of Cattle Rumen Methanogenic Bacterial Species Using 16S rRNA Gene Cloning and Sequencing. Scripta Biologica. 2014;1(4):1-8.
- Singh V, Chaudhary D, Mani I. Molecular Characterization and Modeling of Secondary Structure of 16S rRNA from Aeromonas veronii. Int J Appl Biol Pharm Technol. 2012;3(1):253-260.
- Ariestanti DM, Nurfachtiyani A, Arry Y, Malik A. Screening for glucosyltransferase gene (gtf) from exopolysaccahride producing lactic acid bacteria. Makara Sains, 2008;12(1):1-6.
Crossref - Yilmaz B, Bangar SP, Echegaray N, et al. The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: a review of current knowledge. Microorganisms. 2022;10(4):826.
Crossref - Wang Y, Qin Y, Xie Q, Zhang Y, Hu J, Li P. Purification and characterization of plantaricin LPL-1, a novel class IIa bacteriocin produced by Lactobacillus plantarum LPL-1 isolated from fermented fish. Front Microbiol. 2018;9:2276.
Crossref - Liu Y, Zheng S, Cui J, Guo T, Zhang J. Lactiplantibacillus plantarum Y15 alleviate type 2 diabetes in mice via modulating gut microbiota and regulating NF-κB and insulin signaling pathway. Braz J Microbiol. 2022;53(2):935-945.
Crossref - Hang S, Zeng L, Han J, et al. Lactobacillus plantarum ZJ316 improves the quality of Stachys sieboldii Miq. pickle by inhibiting harmful bacteria growth, degrading nitrite and promoting the gut microbiota health In vitro. Food Funct. 2022;3:1551-1562.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.