ISSN: 0973-7510
E-ISSN: 2581-690X
Ventilator-associated pneumonia (VAP) is a serious complication in critically ill patients, significantly increasing morbidity and mortality. One concerning organism behind VAP is Acinetobacter baumannii, a multidrug-resistant bacterium has ability to evade treatment, particularly with carbapenems, the last-line antibiotics. This is especially worrisome within the confines of Intensive Care Units (ICUs) of tertiary care hospitals, hubs for high-risk patients and potential reservoirs of antimicrobial resistance. This study focused on identifying carbapenem-resistant Acinetobacter baumannii using both phenotypic and genotypic methods. In 132 isolates of Acinetobacter baumannii, we observed 96% resistance to the cephalosporins while least resistance found to colistin and tigecycline. However, a concerning 51.5% of isolates exhibited carbapenem resistance. Phenotypically confirmation of carbapenem resistance detected in 47% isolates by Combined Disc Test and 51.5% isolates by Modified Hodge Test and E-test. Genotypic analysis with RT-PCR revealed a diverse array of resistance genes: blaIMP (33.82%), blaVIM (25%), blaOXA-Group (20.58%), and blaNDM (8.82%). These findings highlight the alarming prevalence of carbapenem-resistant Acinetobacter baumannii in healthcare settings.
Ventilator-Associated Pneumonia, Acinetobacter baumannii, Multidrug Resistance, Beta Lactamase
VAP is usually a bacterial nosocomial pneumonia that develops in patients with acute respiratory failure after 48 hrs of mechanical ventilation.1 VAP is generally categorized on the basis of time of onset of pneumonia in two categories, early onset and late onset VAP. Early onset VAP is caused by antibiotic sensitive bacteria commonly carries a good prognosis, and occur within 4 days of hospitalization, whereas late onset VAP takes place after 5 or more days of mechanical ventilation, caused by multidrug resistance (MDR) organisms and has high patient mortality and morbidity.2 Patients with early onset VAP, with a history of hospitalization within the past 90 days or administration of prior antibiotics are susceptible for MDR infection, and they should be treated as late onset patients.3
Acinetobacter spp. is one of the prime causes of ventilator-associated pneumonia. It is gram-negative, non-lactose fermenter, non-saccharide fermenter, non-motile, oxidase negative coccobacilli showing intrinsic resistance to multiple antimicrobial drugs. Acinetobacter spp. is associated with opportunistic infections and nosocomial infections because it is widely spread in hospital environment and easily colonized on mucosal surface such as respiratory, urinary and intestinal tract. It has capability to hold on inanimate objects and survive on environment for up to five months even in the absence of nutrients.4 The infections, due to Acinetobacter spp. are mostly treated by cephalosporins, aminoglycosides, carbapenems, and tetracycline but now days, majority of Acinetobacter spp. isolates are susceptible to all currently available antibiotics. MDR Acinetobacter defined as resistance to minimum of three classes of antimicrobial regime such as β-lactamases, aminoglycosides, fluroquinolones and carbapenem.5 The causative factor is the presence of mobile genetic elements such as plasmid and integrons which transfers the antimicrobial resistant gene to other organisms easily.6,7 Different mechanism of multidrug resistance have been identified in Acinetobacter spp. which involves production of β-lactamases, alterations in outer membrane protein, penicillin binding protein and increased activity of efflux pumps.8 One of the chief mechanisms of resistance in Acinetobacter spp. is β-lactamase production which includes extended spectrum β-lactamase, metallo β-lactamase and oxacillinase.9 ESBLs belong to class A β-lactamases, which shows resistance to the third-generation cephalosporin (cefotaxime, ceftriaxone, and ceftazidime) and inhibited by clavulanic acid. Class B β-lactamases, also called as metallo-β-lactamases, act on penicillin’s, cephalosporins and carbapenems but not on monobactam. Class C β-lactamases are ampC β-lactamases which confers resistance to cephalosporins in the oxyimino group, 7α-methoxy cephalosporins which are not affected by β-lactamase inhibitor. Class D β-lactamase or OXA type β-lactamases have high hydrolytic activity against oxacillin and cloxacillin and they confer resistance to ampicillin and cephalothin, which are poorly inhibited by clavulanic acid.10 Carbapenem-resistant organisms are resistant to all those antibiotics to which AmpC β-lactamases producer are resistant. In addition, they are also resistant to carbapenems and resistance cannot overcome by β-lactamases/β-lactamases inhibitors. The most commonly encountered β-lactamases in carbapenem resistance are carbapenem-hydrolysing class D β-lactamases (CHDLs), and class B metallo-β-lactamases (MBLs). So, this study was performed to determine the antibiotic resistance profiles, β-lactamase production by phenotypic method and existence of MBL (IMP, VIM, NDM), OXA (OXA23/40/58), and KPC-encoding gene in Acinetobacter spp. causing VAP.
Bacterial Isolates
In the present study, we collected a total of 340 endotracheal aspirates using a mucous extractor under aseptic conditions.11 Among these, patients who met the inclusion criteria, including patients on mechanical ventilation in ICU for >48 hours, Fever greater than 38.5°C, Leucocytosis (≥ 10,000 cells/mm3) or Leukocytopenia (≤4,000 cells/mm3), Radiograph infiltrates and all endotracheal aspirate samples were growing Acinetobacter baumannii isolates in significant >105cfu/ml in number were included in this study. Quantitative cultures were performed on ET aspirates and threshold was considered 105cfu/ml for diagnosis of VAP. Primary inoculation of the samples was done on blood agar (BA), and MacConkey agar (MA) and all plates were incubated overnight at 37°C and observed for growth after 24 hr. Identification of bacteria was done by vitek-2 compact automated system.
Antibiotic susceptibility testing (Kirby-Bauer’s Disk Diffusion Method)12
Kirby-Bauer’s disk diffusion method was used for antimicrobial susceptibility test. AST was done on Muller Hinton Agar (MHA) media according to clinical and laboratory standard institute (CLSI) guidelines.13 MDR strains were screened as per criteria illustrated by European Centre for Disease Control and Prevention and Centre for Disease Control and Prevention. Colistin susceptibility was determined by colistin broth disk elution method as per CLSI guidelines.13
Metallo Beta Lactamase (MBL) Detection by Phenotypic Methods: MBL production was screened by:-
Combined Disc iTest (CDT)14
The test culture was made on the Muller Hinton Agar (MHA) plate, as recommended by CLSI guidelines. Imipenem (10µg) and imipenem /iEDTA (10+750µg) discs were put on MHA at an interval of 20mm, distance from centre to centre. Plates were incubated for 16-18 hrs at 37°C. If the inhibition zone was ≥7mm around the IMP/EDTA disc, then IMP disc alone, indicated as MBL producers.
Modified Hodge Test14
The modified Hodge test was carried out to identify the activity of carbapenemase. E. Coli ATCC 25922 strain was inoculated on iMuller Hinton Agar (MHA) plate. Imipenem discs (10µg) were placed on the plate centre and test culture was streaked from the disc edge to the plate periphery. Plate was incubated for 16-18 hours at 37°C. Interpretation was made by presence of clover leaf zone or distortion of inhibition around the imipenem disc, indicated as presumptive carbapenems producer.
E-Test14
All imipenem resistance isolates were subjected to E-Test (Ezy MICTM Strip, HI media) to detect minimum inhibitory concentration ratio and to confirm MBL production. The Imipenem (4-256 g/ml) and Imp-EDTA (1-64 g/ml) dilution ranges are double-sided on the E-Test MBL strip. On MHA agar, a lawn culture of 0.5 McFarland opacity test isolation was done. E-test strip was inoculated and incubated at 37°C overnight on agar surface. The plates were examined for imipenem and imipenem-EDTA minimum inhibitory concentration (MIC) values where the inhibition ellipses intersected the strip. When the MIC ratio of imipenem/imipenem plus EDTA was greater than eight, the test was considered MBL positive. The appearance of a phantom zone or a distortion of the imipenem ellipse was also considered positively.
Metallo Beta Lactamase (MBL) detection by Genotypic Method
PCR for Carbapenem resistance: Dynex laboratories manufactured kit, Praha 1, Prauge was used for detection of carbapenemase activity of VIM, IMP, KPC, NDM & OXA-23/40/58 genes on Real Time PCR. ABI i7500 Fast Dx Real Time PCR (Peltier Block technology) & Type specific primer & probe were used to detect target genes. Bacterial DNA was isolated by using Qiagen DNeasy Mini Prep Kit. Detection of VIM/IMP/KPC/NDM/OXA-23/40/58 genes was done based on amplification of signals in the respective quencher dyes along with the detection of internal controls.
Bacterial Isolates
Among the 340 VAP cases, maximum isolates were found of Acinetobacter baumannii 132 (44.14%). 61 (46.21%) isolates of Acinetobacter baumannii were identified in early VAP patients and remaining 71 (53.79%) were identified from late VAP patients. 71 (53.78%) isolates were found as multidrug resistant (MDR).
Antimicrobial Sensitivity
Colistin & Tigecycline (Table) were found most sensitive drug of choice showed 100% and 96% sensitivity respectively. Imipenem was sensitive in 64 (48.48%) isolates of Acinetobacter baumannii.
Table:
Antibiotic sensitivity pattern of Acinetobacter baumannii (n=132) isolates, isolated from ET secretions
Antimicrobial Agent |
Sensitive |
Resistant |
|---|---|---|
Amikacin |
7 (5.3%) |
125 (94.69%) |
Gentamicin |
8 (6.06%) |
124 (93.93%) |
Tobramycin |
9 (6.8%) |
123 (10.73%) |
Ampicillin sulbactam |
12 (9.09%) |
120 (90.90%) |
Piperacillin- Tazobactam |
16 (12.12%) |
116 (87.87%) |
Ceftazidime |
4 (3.03%) |
128 (96.96%) |
Cefotaxime |
4 (3.03%) |
128 (96.96%) |
Ceftriaxone |
5 (3.78%) |
127 (96.21%) |
Cefepime |
5 (3.78%) |
127 (96.21%) |
Ciprofloxacin |
6 (4.54%) |
126 (95.45%) |
Doxycycline |
6 (4.54%) |
126 (95.45%) |
Trimethoprim+ Sulfamethoxazole |
4 (3.03%) |
128 (96.96%) |
Imipenem |
64 (48.48%) |
68 (51.51%) |
Tigecycline |
128 (96.96%) |
4 (3.03%) |
Colistin |
132 (100%) |
0 (0%) |
Distribution of MBL by Phenotypic Method
62 (47%) isolates showed metallo -β-lactamase activity by Combined Disc Test and 68 (51.5%) by Modified Hodge Test. All Modified Hodge Test positive cases were also found resistant to Imipenem by E-Test.
Distribution of Carbapenem Resistance Gene by Genotypic Method
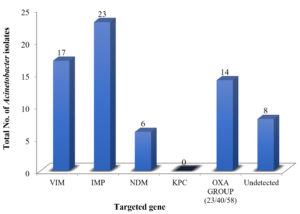
The results of RT-PCR are shown in Figure 1 and amplification process of gene in Figure 2. Resistance gene blaIMP (33.82%), blaVIM (25%), blaNDM (8.82%), blaOXA (20.58%), KPC (0%) was detected in 60 isolates.
Figure 1. Distribution of Acinetobacter spp. based on Genotypic Resistance (VIM IMP NDM KPC & OXA-Group 23/40/58 gene)
Ventilator-Associated Pneumonia (VAP), is a frequent hospital-acquired infection that is acquired in patients admitted to ICU after 48 hrs of mechanical ventilation. It is associated with high morbidity and mortality, and become one of the most important public health issues with increased cost of treatment. High frequency of MDR pathogens in ICU is associated with increased incidence of VAP due to Acinetobacter spp. Acinetobacter spp. is one of the causes of severe infections in patient on ventilator, resistant to most of the available antimicrobial agents. This poses a serious problem in choosing appropriate antimicrobial therapy for the treatment of critically ill patients as only few agents as Colistin and Tigecycline remains as therapeutic options. So, it is necessary to construct various strategies for infection control, Antimicrobial Resistance Surveillance Programme (AMSP), judicious prescription of antibiotics and cycling of antibiotics to reduce emergence of resistant strain. Distribution of Acinetobacter spp. in present setting was found high and similar findings were observed by other authors.15,16 This indicates high frequency of MDR pathogens in ICU is associated with increased incidence of VAP due to Acinetobacter spp. Acinetobacter spp. was found to be more common with late onset VAP. Late onset of VAP associated with higher rate of infection with MDR pathogen. Prior antimicrobial therapy before admission in ICU’s can lead to alteration in normal flora. As Acinetobacter spp. has higher potential to stay in hospital environment for longer duration leading to nosocomial outbreak and other host factors such as underlying diseases also contribute in higher incidence of Acinetobacter infection. Imipenem resistance was found 51.5% in the present study. The results were in concordance with Goel et al.17 (44%). In other studies15,18 the resistance is more as compared to present study. Colistin and tigecycline were found to be the most effective drugs against Acinetobacter spp. showing 100% sensitivity. Similar findings were found in the other author’s studies.17-19 Acinetobacter spp. has become MDR, XDR and Pan drug resistant due to widespread usage of antimicrobials worldwide in ICU. Carbapenems have been considered the final resort to treat multi-drug resistant strain infections because of their capacity to treat ESBL and AmpC β-lactamase in a steady manner. The excessive usage of these antibiotics has now produced carbapenem-resistant strains. Production of Metallo β-lactamase (MBL) was found most common resistance mechanism in Acinetobacter. MBL gene are harboured as mobile elements and easily disseminate among the isolates. We found differences in the prevalence of MBL producing Acinetobacter spp. as compare to other studies.20-22 It may be due to variations among different patient studied, different rates of antibiotic use in different hospitals and differences in geographical distribution which might have led to variations in the prevalence of the β-lactamases with varied resistance pattern.
Emergence of carbapenem mediated resistance in India is of serious concern. The detection of carbapenem mediated resistance is of utmost importance in deciding the most appropriate therapeutic regimen for the treatment. In this study, carbapenem mediated resistance was mainly found due to class B and class D carbapenemase. Among class B blaIMP and blaVIM the results are in concordance with other studies,23,24 while in some studies25-28 lower percentage of isolates harbouring blaIMP gene was observed. These variations were found due to change in pattern of resistance over different years and geographical difference within the countries over a period. Our study showed blaNDM producer 8.82% while high percentage of blaNDM was reported by many studies.27 In present study we could not differentiate between the OXA genes as per our kit protocol. It could detect OXA-gene in total (23/40/58) rather than individual OXA gene as in other studies.15,26 Oxacillinase encoding gene can be intrinsic (blaOXA-51) or acquired (blaOXA23, blaOXA40 and blaOXA58). The oxacillin enzymes weakly hydrolyse carbapenems. In 8 isolates no gene was detected. Resistance in these strains can be attributed to presence of other mechanism such as defects of porins, decreased expression of outer membrane proteins and alteration in the binding site of penicillin binding protein. Early detection of these genes is useful in identification of carbapenem resistance Acinetobacter spp. as we observed that transfer of antimicrobial resistance gene to other bacterium and further spread of theses in hospital environment is common. So, Epidemiological surveillance of resistance gene in Acinetobacter spp. is essential for outbreak detection. Carbapenem resistance gene are able to hydrolyse most of the β-lactams, including imipenem and meropenem especially in MDR strains. Therefore, detection of carbapenem producing Acinetobacter spp. is essential for treatment of patients and for controlling nosocomial spread of these genes. Our aim should be implementations of quality assurance management for infection control.
Acinetobacter spp. has been an opportunistic pathogen with high virulence factor in hospital-acquired infections. It is particularly multidrug resistant strain which causes severe infections in patients admitted in various hospitals. Treatment of such infections is problematic due to their broad resistance to antimicrobial drugs. The early detection of carbapenem resistance gene producing strain, would be necessary to check dissemination of these strains in hospital which will help to reduce mortality rate. This study may help clinicians in prescribing antibiotics by knowing the predominant resistance mechanisms allows for targeted therapy by choosing antibiotics less susceptible to those mechanisms and predict the likelihood of encountering this multidrug-resistant organism in their ICU and adjust their empiric antibiotic choices accordingly. By knowing the molecular diversity of circulating A. baumannii isolates in ICU, can inform targeted environmental decontamination and surveillance strategies to prevent further spread within the hospital.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Chastre J. and Fagon J.Y. Ventilator-associated pneumonia. American J of Res and Crit Care Medi. 2002;165(7): 867–903.
- Kalanuria AA, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208.
Crossref - Garanacho MJ, Ortiz LC, Jimenez FJ, et al. Treatment of multidrug resistant Acinetobacter baumannii Ventilator associated Pneumonia with intravenous colistin: A comparision with imipenem susceptible VAP. Clin Infect Dis. 2003;36(9):11-1118.
Crossref - Patil VH, Mohite TS, Patil CV. Multidrug Resistant Acinetobacter in patient with ventilator associated pneumonia: Review Article. J Krishna Inst Medical Sci Univ. 2019;8(3):1-18.
- Elbrolosy AM, Labeeb AZ, Hassan DM. New Delhi metallo-β-latamases-producing Acinetobacter isolates among late-onset VAP patients: multidrug resistant pathogen and poor outcome. Infection and drug resistance. 2019; 12:373-384
- Chaudhary M and Payasi A. Molecular Characterization and Antimicrobial Susceptibility Study of Acinetobacter baumannii Clinical Isolates from Middle East, African and Indian Patients. J Proteom Bioinform. 2012;5(11):265-269.
Crossref - Zhou H, Zhang T, Yu D, et al. Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob. Agents Chemother. 2011;55(10):4506-4512.
Crossref - Noori M, Karimi A, Fallah F. High prevalence of metallo-beta-lactamase producing Acinetobacter baumannii isolated from two hospitals of Tehran, Iran. Arch Pediatr Infect Dis. 2014;2(3):e15439.
Crossref - Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63(12):1055-1060.
Crossref - Gupta V. An update on newer β-lactamases. Indian J Med Res. 2007;126(5):417-427.
- Mohanty D, Nayak MK, Raut K, Routray SS, Mishra D. Ventilator Associated Pneumonia in an ICU of a Tertiary Care Hospital in India. Int J Contemp Med. 2016;3(2):139-143.
Crossref - Hudzick J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. ASM. 2016;1-23.
- The Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. CLSI supplement M100S. Wayne, PA: CLSI. 2018.
- Shivaprasad A, Antony B, Shenoy P. Comparative Evaluation of Four Phenotypic Tests for detection of Metallo β- Lactamase and carbapenemase production in Acinetobacter baumannii. J Clin Diagnostic Res. 2014;8(5):DC05-8.
- Samal N, Padhi S, Paty BP. Bacteriological profile and antimicrobial sensitivity pattern of endotracheal tube aspirates of patients admitted in ICU. J NTR Univ Health Sci. 2020;9(15):1-7.
Crossref - Gupta V, Singla N, Gombar S, Palta S, Chandar J. Prevalence of Multidrug- Resistant Pathogens and Their Antibiotic Susceptibility Pattern from Late-onset Ventilator Associated Pneumonia Patients from a Tertiary care Hospital in North India. J Assoc Chest Physicians. 2018;6(1):4-11.
Crossref - Goel N, Punia P, Chaudhary U. Prevalence of ESBL, MBL and AmpC Producing XDR Acinetobacter Isolates from Lower Respiratory Tract Specimens. Int J Contemp Med. 2017;4(10):2091-2095.
- Srivastava G, Ganesh SB, Bajpai T, Patel KB. Sensitivity profile of multidrug resistance Acinetobacter spp isolated at ICUs of tertiary care hospital. Int J Health Syst Disaster Manag. 2013;1(4):200-203.
Crossref - Bhaumik S, Das NK, Gandham NR, et al. Microbiological profile and antibiotic susceptibility pattern of gram-negative isolates from tracheal secretions in a tertiary care setup. Med J DY Patil Vidyapeeth. 2022;15:440-3
- Seral C, Gude MJ, Castillo FJ. Emergence of plasmid mediated AmpC β-lactamases: Origin, importance, detection and therapeutical options. Rev Esp Quimioter. 2012;25(2):89-99.
- Rana G, Sharma S, Hans C. Ventilator Associated Pneumonia in the ICU: Microbiological Profile. J Bacteriol Mycol. 2017;4(5):165-168.
Crossref - Pragasam AK, Vijaykumar S, Bakthavatchalam YD, et al. Molecular Characterization of antimicrobial resistance in Pseudomonas aeruginosa and Acinetobacter baumannii during 2014 and 2015 collected across India. Indian J Med Microbiol. 2016;34(4):433-441.
Crossref - Saranathan R, Vasanth V, Vasanth T, et al. Emergence of carbapenem non-susceptible multidrug resistant Acinetobacter baumannii strains of clonal complexes 103B and 92B harboring OXA-type carbapenemases and metallo-β-lactamases in Southern India. Microbiol Immunol. 2015;59(5):277-84.
Crossref - Abd El-Baky RM, Farhan SM, Ibrahim RA, Mahran KM, Hetta HF. Antimicrobial resistance pattern and molecular epidemiology of ESBL and MBL producing Acinetobacter baumannii isolated from hospitals in Minia, Egypt. Alexandria J Med. 2020;56(1):4-13.
Crossref - Khaledi M, Shahini M, Abadi S, et al. Phenotypic and Genotypic detection of metallo β-lactamases in A. baumanii isolates obtained from clinical samples in Shahrekord, Southwest Iran. BMC Res Notes 2019;12(1):597.
Crossref - Sharma M, Singhal L, Gautam V, Ray P. Distribution of carbapenemase genes in clinical isolates of Acinetobacter baumannii & a comparison of MALDI-TOF mass spectrometry-based detection of carbapenemase production with other phenotypic methods. Indian J Med Microbiol. 2020;151(6):585-591.
Crossref - Niranjan DK, Singh NP, Manchanda V, S Rai, Kaur IR. Multiple carbapenem hydrolyzing gene in clinical isolates of Acinetobacter baumanni. Indian J Med Microbiol. 2013;31(3):237-241.
Crossref - Mahnaz N, Farzaneh F, Farzad B, Kourosh K, Mohammad Z. Molecular Study of metallo-β-lactamases and integrons in Acinetobacter baumannii isolates from burn patients. BMC Infect Dis. 2021;21(1):782.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.