ISSN: 0973-7510
E-ISSN: 2581-690X
Pregnant women are at high risk of urinary tract infections (UTIs). There is growing concern about the rise of Enterobacteriaceae that are resistant to drugs, including, more recently, those that produce carbapenemase. The study aimed to perform molecular detection and antibiograms of Enterobacteriaceae that produce carbapenemase in pregnant women with UTIs. Using clinical specimens taken from the general hospital in Qurrayat, Saudi Arabia, we identified 83 isolates of Enterobacteriaceae. Microscan WalkAway Plus and Phoenix automated analyzers were used to carry out bacterial isolation using standard microbiological procedures. DNA sequencing was employed to identify the carbapenemase bla genes, while phenotypic techniques and PCR were employed to characterize bacterial strains. The carbapenemase bla gene was detected among the 30 members of the Enterobacteriaceae. Of these 30, bla gene variants were found in 13 isolates (41%) blaOXA-23; 11 (35%) blaNDM-1; 10 (32%) blaNDM-5; 7 (22%) blaOXA-24; 4 (12%) blaVIM and 3 (9%) blaOXA-48. A statistically non-significant relationship between the blaNDM-1 and Klebsiella pneumoniae (p = 0.33) was seen, and the correlation between the blaNDM variants was not significantly associated with Pseudomonas aeruginosa (p = 0.5) and Escherichia coli (p = 0. 14). Antibiotic resistance was extremely common, as evidenced by the minimum inhibitory concentrations (MICs) in vitro of carbapenemase-producing Enterobacteriaceae against a number of antibiotic groups. These bacterial strains exhibited minimal resistance to amikacin (14; 46.6%) and were not resistant to two aminoglycosides, namely Ertapenem (30; 100%) and Meropenem (30; 100%). Our investigation shows that many Enterobacteriaceae that produce carbapenemases are a serious risk for pregnant women and others in the community. As a result, alternatives for therapy are limited to the aminoglycosides Ertapenem and Meropenem.
Carbapenemase, Enterobacteriaceae, Multidrug-resistant, Urinary Tract Infection
The most prevalent illnesses among pregnant women are urinary tract infections (UTIs). Common forms of UTI are upper or lower tract infections (acute pyelonephritis or acute cystitis, respectively).1 Due to changes in the immunological and urinary systems, women are more susceptible to urinary tract infections during pregnancy. Both ureteral pressure from the gravid uterus and progesterone-related smooth muscle relaxation cause the ureter and renal calyces to expand. These are physiological changes to the urinary system, and urinary tract infections become more common as a result. The signs of a symptomatic UTI include haematuria, fever, suprapubic pain, urgency, increased urine frequency, and flank pain.2 Cystitis is defined as a positive urine culture accompanied by lower UTI symptoms in the absence of systemic symptoms.3 In contrast, women with high-burden bacterial growth who do not exhibit UTI symptoms are diagnosed with asymptomatic bacteriuria (ASB). Maternal and neonatal morbidity and mortality are significantly increased by pregnancy-related UTIs.4 Preterm labour, hypertensive disorders of pregnancy, such as pregnancy-induced hypertension and preeclampsia, anaemia (haematocrit level less than 30%), and amnionitis are among the maternal problems associated with UTIs.5 Two neonatal complications associated with UTIs are sepsis and pneumonia; more precisely, group B streptococcus infection is the cause of both conditions. Low birth weight newborns (less than 2,500 g) and stillbirths are also more common in babies born to mothers with UTIs.6,7
Pregnant women are susceptible to UTIs caused by the same bacteria that affect non-pregnant women. Eighty-nine percent of infections are caused by Escherichia coli.8 Additional microbes include Staphylococcus saprophyticus, Group B Streptococcus (GBS), Proteus mirabilis, Klebsiella pneumoniae (K. pneumoniae) and rare organisms such Ureaplasma ureolyticum, Gardnerella vaginalis, and Enterococci.9 When treating UTIs in clinical settings, empirical principles are followed until a urinalysis determines the specific organism.10
Earlier, the synthesis of enzymes such as penicillinases, cephalosporinases, and extended-spectrum lactamases was the primary cause of MDR among Enterobacteriaceae (ESBL).11 More recently, it has been clear that the production of carbapenemase plays a significant role in the emergence of drug resistance in the Enterobacteriaceae family. Due to their high levels of antibiotic resistance, carbapenemase-producing Enterobacteriaceae (CPE) are challenging to treat since they may degrade all -lactam drugs, including carbapenems, rendering them useless.12 As an additional choice, carbapenem antibiotics, including imipenem, meropenem, ertapenem, and doripenem have been suggested when treating Enterobacteriaceae that cause ESBLs.13,14
Currently, both K. pneumoniae and E. coli are the main sources of carbapenem-resistant genes, which increase the risk of UTIs caused by MDRE. Pregnant women are particularly at risk from these strains due to their high fatality rate and potential for widespread transmission.15 Treatment options for infections are limited, in some cases absent, and have been associated with fatality rates as high as 50%. Hospitals in close proximity frequently have issues with Enterobacteriaceae that produce carbapenemase due to patient transfers within the healthcare system.16,17 One of the main current reasons for concern is the prevalence of Enterobacteriaceae that generate carbapenemase, which is mainly detected in pregnant women.18 Both in community- and healthcare-associated infections, the etiology of UTIs and the antibiotic resistance of uropathogens have progressed in recent years. Since Enterobacteriaceae are the primary cause of UTIs and have multiple methods to destabilize currently available antibiotics, including carbapenems, the isolation of this bacterial group is essential for optimal management.19 Furthermore, the situation of Qurayyat has not yet been evaluated. Therefore, this study was intended to investigate whether uropathogens in pregnant women with UTIs lead to the development of carbapenemase.
Specimen collection and study design
Pregnant women attending the outpatient departments (OPDs) at Qurayyat General Hospital in Qurayyat, Saudi Arabia, as well as those on the campus of AL Jouf University, served as the source of the study population. The patient’s current age, the age at which they became pregnant, and the symptoms and indicators of UTI were recorded. Eligibility for the study depended on the treating physicians’ assessment of the clinical manifestations of urinary tract infections. Before collecting samples, those who were pregnant women received thorough instructions on how to collect urine samples aseptically to prevent urethral contamination.20 Samples from the midstream were collected, labelled, transported to the laboratory, and processed for aerobic bacterial culture within one hour. The samples were refrigerated if they were scheduled to be delayed and processed within 4 hours.
Isolation and identification of Enterobacteriaceae
Urine specimens were directly inoculated onto Cystine-Lactose-Electrolyte-Deficient Agar (CLED) agar, and the petri-dishes were then incubated for 24 hours at 37°C. The culture was deemed positive if it contained more than 105 CFU/mL. Enterobacteriaceae were identified from positive urine cultures using standard procedures based on their distinctive appearance on the medium, their Gram staining reaction, and their pattern of metabolic profiles. Enterobacteriaceae isolates were identified by biochemical examination, including oxidase, urease, citrate consumption, indole formation, sugar fermentation, and the generation of H2S and gas.21
Susceptibility and carbapenemase testing
The Kirby Bauer disk diffusion method was used to assess antibiotic susceptibility using Mueller Hinton agar in accordance with the guidelines established by the Clinical and Laboratory Standards Institute.22 Employing the automated analyzers system (Phoenix and Microscan WalkAway Plus), the minimum inhibitory concentration (MIC) in vitro of various antibiotics was determined in phenotypically confirmed MBL-producing Enterobacteriaceae. The bacterial strains were classified as resistant, sensitive, and with intermediate sensitivity using the MIC breakpoints detailed in the Clinical and Laboratory Standards Institute manual.23 All isolates with MDRE (resistance to two or more classes of antibiotics), were collected and subcultured onto CHROM agar to measure the synthesis of carbapenemase following antimicrobial susceptibility testing. Colonies exhibiting typical colouring properties were recorded to evaluate carbapenemase-producing isolates following a 24-hour overnight incubation period. Other strains of Enterobacteriaceae generated metallic blue colonies, whereas E. coli that produced carbapenemase showed dark pink to reddish colony characteristics.24 The colonies of carbapenemase-producing organisms that produced a metallic blue colour were subsequently identified using various standard biochemical tests.
Molecular analysis of carbapenemase-the producing gene variants
The boiling method was employed to isolate DNA template from bacteria.25 We employed several colonies of bacteria obtained from an overnight culture of Enterobacteriaceae grown on nutritional agar, combined with 200 µl TE buffer, vortexed, and heated for ten minutes in a water bath. The samples were then centrifuged at 14,000 rpm for five minutes to remove any remaining cellular pieces from the suspension. The clear supernatant was kept as a source of DNA at -70°C. Using 0.5 µM forward and reverse primers, we used PCR to amplify the target gene (815 bp).
Data analysis
For data analysis, we utilized Chi-square test, GraphPad Prism 6 and IBM Corp.’s SPSS v. 24. P-values below 0.05 in Fisher’s exact test were considered significant.
Demographics of pregnant females with urinary tract infections
A total of 115 urine samples from pregnant women with potential UTIs were collected, and carbapenemase-producing uropathogens were identified. The characteristics and medical aspects of each participant are shown in Table 1. From 115 urine samples, 83 showed no growth while 32 was positive. Eighty-three bacterial strains were isolated from the 32 positive specimens in which two were gram-positive bacteria that were excluded from the study. In addition, 30 bacterial isolates tested positive for producing carbapenemase, whereas the remaining 51 did not. The demographic and clinical information from the pregnant females with urinary tract infections showed a significant correlation between carbapenemase production and age, with the age range of 31–35 years (Mean 35.5, Std Deviation-2.73) being the most important (11/30; 36.7%) whereas isolates that did not generate carbapenemase were associated with a patient age of 26–30 years (20/51; 39.2%). The majority of the isolates (30; 37.0%) that produced carbapenemase were obtained during the third semester of pregnancy (19/30; 63.3%), followed by the second trimester (6/30; 20.0%), and the first semester (5/30; 16.7%) respectively. However, the second trimester yielded the greatest number of noncarbapenemase-producing organisms (20/50; 39.2%), with the first trimester producing 16 (16/50; 31.4%) and the third trimester producing 15 (15/50; 29.9%). The distribution of organisms, obtained from urinary tract-infected pregnant women, that produced carbapenemase and those that did not is shown in Table 2.
Table (1):
Demographics of the pregnant females with urinary tract infections (n = 81)
| Age | Carbapenemase producers (n = 30) | % | Non-carbapenemase producers (n = 51) | % | p-value |
|---|---|---|---|---|---|
| Age distribution | |||||
| 18 – 20 | 1 | 3.3 | 6 | 11.8 | 0.19 |
| 21 – 25 | 7 | 23.3 | 12 | 23.5 | 0.98 |
| 26 – 30 | 10 | 33.3 | 20 | 39.2 | 0.59 |
| 31 – 35 | 11 | 36.7 | 10 | 19.6 | 0.09 |
| 36 – 40 | 1 | 3.3 | 3 | 5.9 | 0.6 |
| Pregnancy trimester | |||||
| 1st trimester | 5 | 16.7 | 16 | 31.4 | 0.16 |
| 2nd trimester | 6 | 20.0 | 20 | 39.2 | 0.08 |
| 3rd trimester | 19 | 63.3 | 15 | 29.4 | 0.004 |
Fisher’s exact test
Table (2):
Distribution of carbapenemase and no-carbapenemase producers isolated in urinary tract infections from pregnant females (n = 81)
Bacterial isolate |
Carbapenemase producers (n = 30) |
% |
Non-carbapenemase producers (n = 51) |
% |
p-value |
|---|---|---|---|---|---|
E. coli |
7 |
23.3 |
20 |
39.2 |
0.14 |
Klebsiella pneumoniae |
10 |
33.3 |
12 |
23.5 |
0.33 |
Pseudomonas aeruginosa |
7 |
23.3 |
9 |
17.6 |
0.5 |
Proteus mirabilis |
3 |
10.0 |
5 |
9.8 |
0.97 |
Enterobacter cloacae |
2 |
6.7 |
3 |
5.9 |
0.88 |
Acinetobacter baumannii |
1 |
3.3 |
2 |
3.9 |
0.89 |
Fisher’s exact test
A urinary tract infection was found in 32 out of 115 (27.8%) of the culture-positive cases. Two types of bacteria were isolated: Gram-positive (2/115) and Gram-negative rods 81/115 (70.4%). The research studies included only Gram-negative bacteria and were classified into two groups as carbapenemase producers and non-carbapenemase producers. Thirty (37.0%) of the 81 isolates of Gram-negative rods tested positive for producing carbapenemase, while 51 (115/63%) were not carbapenemase producers. Klebsiella pneumoniae (10/30, 33.3%), Escherichia. coli (30/7, 23.3%), Pseudomonas aeruginosa (7/30, 23.3%), Proteus mirabilis (3/30 10/0%), and Acinetobacter baumannii (1/30, 33%) were the isolates of bacteria that produced carbapenemase, while Escherichia coli (20/51, 39.2%), Klebsiella pneumoniae (12/51, 23.5%), Pseudomonas aeruginosa (12/51, 7.6%), Proteus mirabilis (9.8%), Enterobacter (3/51 5.9%), and Acinetobacter (2/51, 3%) were non carbapenemase producers.
Molecular detection of carbapenemase-producing gene variants
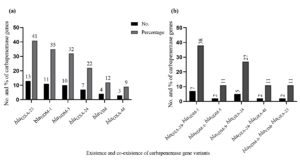
A total of 30 isolates that tested positive for producing carbapenemase were included in the molecular analysis. The results showed that 48 variants had the bla gene; 13 had blaOXA23; 11 contained blaNDM-1; 10 had blaNDM-5; 7 blaOXA-24; 4 blaVIM; 3 blaOXA-48 and 7 had blaOXA-23, blaNDM-1; 2 blaNDM-1, blaNDM-5; 5 blaNDM-5, blaOXA-24; 2 blaOXA-24, blaOXA-48 and 2 had blaNDM-1, blaVIM, blaOXA-23 were the isolates that co-existed with carbapenase bla gene variants. Figure 1 (a and b) show the distribution of carbapenemase bla gene variants overall and as a proportion among isolates that produce carbapenemase.
Figure 1. Molecular detection of carbapenemase gene variants. (a) Overall number and percentage of carbapenemase bla gene variants among the carbapenamase producing isolates. (b) The co-existence (number and percentage) of carbapenemase bla gene variants among the carbapenemase-producing isolates
Antimicrobial resistance of carbapenemase producers isolated from pregnant females with urinary tract infections
Based on minimum inhibitory concentrations (MICs) in vitro, all of the isolates of Enterobacteriaceae that produced carbapenemase were 100% resistant to Ertapenem, Imipenem, and Meropenem. With reference to other antibiotics, they showed varying minimum inhibitory concentrations. The drugs that showed the highest level of resistance were co-amoxiclav (8;80%), cefepime (8;80%), cefixime (8;80%), cefotaxime (8;80%), ceftazidime (8;80%), ceftriaxone (8;80%), and cefuroxime (8;80%). Escherichia coli and Klebsiella pneumoniae had the highest resistance. The bacteria that revealed the least amount of antibiotic resistance were Pseudomonas aeruginosa, Proteus mirabilis, Enterobacter cloacae and Acinetobacter baumanii. The species of bacteria illustrated in Table 3 shows the pattern of antimicrobial activity Urinary tract infections are the most common illness during pregnancy and can have serious effects if left untreated. Thirty bacterial isolates of the 83 strains in our investigation tested positive for carbapenemase production, while 51 were non-carbapenemase producers.
Table (3):
Antimicrobial resistance of carbapenemase producers isolated from urinary tract infections from pregnant females (n = 30)
Antibiotic |
Klebsiella pneumoniae n = 10 |
Escherichia coli n = 7 |
Pseudomonas aeruginosa n = 7 |
Proteus mirabilis n = 3 |
Enterobacter cloacae n = 2 |
Acinetobacter baumannii n = 1 |
|---|---|---|---|---|---|---|
No. (%) |
No. (%) |
No. (%) |
No. (%) |
No. (%) |
No. (%) |
|
Amikacin |
5 (50) |
2 (28.6) |
4 (57.1) |
1 (33.3) |
1 (50) |
1 (100) |
Co-amoxiclav |
8 (80) |
4 (57.1) |
6 (85.7) |
2 (66.7) |
2 (100) |
1 (100) |
Cefepime |
8 (80) |
5 (71.4) |
6 (85.7) |
3 (100) |
2 (100) |
1 (100) |
Cefixime |
8 (80) |
5 (71.4) |
6 (85.7) |
3 (100) |
2 (100) |
1 (100) |
Cefotaxime |
8 (80) |
5 (71.4) |
5 (71.4) |
3 (100) |
2 (100) |
1 (100) |
Ceftazidime |
8 (80) |
5 (71.4) |
4 (57.1) |
3 (100) |
2 (100) |
1 (100) |
Ceftriaxone |
8 (80) |
5 (71.4) |
6 (85.7) |
3 (100) |
2 (100) |
1 (100) |
Cefuroxime |
8 (80) |
5 (71.4) |
6 (85.7) |
3 (100) |
2 (100) |
1 (100) |
Ciprofloxacin |
6 (60) |
5 (71.4) |
5 (71.4) |
3 (100) |
1 (50) |
1 (100) |
Colistin |
2 (20) |
0 (0) |
1 (14.3) |
3 (100) |
0 (0) |
1 (100) |
Co-trimoxazole |
7 (70) |
4 (57.1) |
6 (85.7) |
2 (66.7) |
2 (100) |
1 (100) |
Ertapenem |
10 (100) |
7 (100) |
7 (100) |
3 (100) |
2 (100) |
1 (100) |
Gentamicin |
8 (80) |
2 (28.6) |
5 (71.4) |
2 (66.7) |
2 (100) |
1 (100) |
Imipenem |
10 (100) |
7 (100) |
7 (100) |
3 (100) |
2 (100) |
1 (100) |
Levofloxacin |
5 (50) |
2 (28.6) |
5 (71.4) |
1 (33.3) |
1 (50) |
1 (100) |
Meropenem |
10 (100) |
7 (100) |
7 (100) |
3 (100) |
2 (100) |
1 (100) |
Moxifloxacin |
6 (60) |
2 (28.6) |
6 (85.7) |
2 (66.7) |
1 (50) |
1 (100) |
Nitrofurantoin |
2 (20) |
0 (0) |
2 (28.6) |
0 (0) |
2 (100) |
0 (0) |
Norfloxacin |
6 (60) |
2 (28.6) |
5 (71.4) |
3 (100) |
1 (50) |
1 (100) |
Piperacillin-tazobactam |
3 (30) |
0 (0) |
4 (57.1) |
0 (0) |
0 (0) |
0 (0) |
Antibiotic resistance is a growing concern in modern times, especially with regard to gram-negative bacteria that produce carbapenemase. With an increased frequency of bacteria that produce carbapenemase in pregnant women, one of the most significant problems that they face is that the majority of these bacteria are detected during the third trimester. Here, we discuss the bacteria that produce carbapenemase. Worldwide, the prevalence of bacteria that produce carbapenemase varies by country. According to one study, South America had a greater prevalence of carbapenemase among Klebsiella pneumoniae cases (44%) than Asia, Europe, and North America (7.5–22.4%).26 Research from Uganda and India showed that 57.2% and 62%, respectively, of the population produced carbapenemase. Patients from wards that could provide an explanation for the similarities were included in these investigations. The implementation of programs aimed at detecting Gram-negative bacteria that generate carbapenemase is imperative in light of the current unsolved situation.27
In our investigation, the majority of isolates that produced carbapenemase were Klebsiella pneumoniae (10; 33.3%) and Escherichia. coli (7; 23.3%) 28. Since Klebsiella is the most common organism that causes UTIs in pregnant women, the higher percentage of Klebsiella producing carbapenemase in this investigation related to previous studies was most likely because our patients were pregnant. It should be noted that all uropathogens isolated from pregnant women, both those that produced carbapenemase and those that did not, were completely resistant to Imipenem, Ertapenem, and Meropenen. Imipenem has been identified in previous investigations as the recommended medication for complex bacterial infections including UTIs. This antibiotic works against a broad range of infections, including gram negative bacteria.
Although resistance to colistin and piperacillin-tazobactam has been shown to be infrequent, resistance to nitrofurantoin may be caused by mutations in the DNA gyrase.28 Co-amoxiclav is still the preferred medication for treating bacterial strains that produce carbapenemase regardless of their toxicity.29 According to the findings for colistin in our investigation, reports of sensitivity to colistin vary by region, ranging from 89% to 100%. It has been noted that bacteria with the carbapenemase bla gene exhibit resistance to a number of antibiotic classes, including aminoglycosides and carbapenems.30 A significant number of strains that are intermediately sensitive and a few isolates that are sensitive to one of the carbapenems were also seen. Higher MICs against Imipenem or Ertapenem are not always associated with the carbapenemase bla gene.31 The presence of blaNDM-1 causes cefoxitin to hydrolyze, leading to bacterial resistance. This indicates that an extra resistance gene has been acquired by the population, resulting in an extended resistance phenotype.32 The prolonged drug resistance of blaNDM strains is predominantly caused by the co-existence of extended-spectrum beta-lactamases and AmpC, which increases the range of antibiotic resistance against norfloxacin and other classes of antibiotics.33 It is recommended to closely track hospitals to prevent multidrug resistance, since this can become an endless cycle if further resistance genes are acquired. Many risk factors, including age, gender, sexual activity, pregnancy, contraception, instrumentation, urinary tract blockage, and a history of antibiotic use, can affect the progress of UTIs. According to our research, pregnant women who are in the third trimester are particularly at risk of infection with bacteria that produce carbapenemase.
Our research showed that pregnant women infected with bacteria that produce carbapenemase had a significant prevalence of asymptomatic bacteriuria. Pregnant women should be extremely concerned about their health because a large number of Enterobacteriaceae that produce carbapenemase have been identified throughout our study. These organisms can cause serious illness or even death. The effectiveness of colistin and other extended-spectrum medications is at risk due to an increased co-existence of the bla gene variant with other resistance mechanisms. By implementing more stringent infection control protocols, screening techniques, and timely identification of these bacteria, the endemicity of such resistant pathogens can be decreased.
ACKNOWLEDGMENTS
The authors would like to thank Jouf University, Kingdom of Saudi Arabia, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
The authors are highly grateful to the Deanship of Scientific Research, Jouf University, for funding the project (Grant No. 707/39).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study adhered to the ethical guidelines of the World Medical Association (WMA) and the 2013 Declaration of Helsinki.
- Dugal S, Purohit H. Antimicrobial susceptibility profile and detection of extended spectrum beta-lactamase production by gram negative uropathogens. Int J Pharm Pharml Sci. 2013;4(5):435-438.
- Mazhari BBZ, Saiemaldahr MH. Prevalence of Urinary Tract Infections (UTI) and its etiological agents among pregnant woman at Qurrayat region. Research Journal of Biotechnology, 2019;14(12):88-91
- Huang TD, Berhin C, Bogaerts P, Glupczynski Y. Prevalence and mechanisms of resistance to carbapenems in Enterobacteriaceae isolates from 24 hospitals in Belgium. Antimicrob Agents Chemother. 2013;68(8):1832-1837.
Crossref - Luca A, Migliavacca R, Regattin L, et al. Prevalence of urinary colonization by extended spectrum-beta-lactamase Enterobacteriaceae among catheterised inpatients in Italian long term care facilities. BMC Infect Dis. 2013;13(1):124.
Crossref - van der Meeren BT, Chhaganlal KD, Pfeiffer A, et al. Extremely high prevalence of multi-resistance among uropathogens from hospitalised children in Beira, Mozambique. S Afr Med J. 2013;103(6):382-386.
Crossref - Kibret M, Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in Northeast Ethiopia. Afr Health Sci. 2011;11(3):40-45.
Crossref - Wartiti MAEL, Bahmani F-Z, Elouennass M, Benouda A. Prevalence of Carbapenemase-Producing Enterobacteriaceae in a University Hospital in Rabat, Morocco: a 19-months prospective study. Int Arab J Antimicrob Agents. 2012;2(3):1-6.
- Thakur S, Pokhrel N, Sharma M. Prevalence of multidrug resistant Enterobacteriaceae and extended spectrum β lactamase producing Escherichia Coli in urinary tract infection. Res J Pharm Biol Chem Sci. 2013;4(2):1615-1624.
- Nordmann P, Gniadkowski M, Giske CG, et al. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2012;18(5):432-438.
Crossref - Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791-1798.
Crossref - Day KM, Salman M, Kazi B, et al. Prevalence of NDM-1 carbapenemase in patients with diarrhoea in Pakistan and evaluation of two chromogenic culture media. J Appl Microbiol. 2013;114(6):1810-1816.
Crossref - Fatemi SM, Shokri D, Mohammadi S, Koupahi H. Investigation of NDM metallo-beta-lactamase and CMY-2 AmpC β-lactamase production in Escherichia coli and Enterobacter spp. isolated from human. Comp Clin Pathol. 2018;27(4):1007-1015.
Crossref - Bi R, Kong Z, Qian H, et al. High prevalence of blaNDM variants among carbapenem-resistant Escherichia coli in Northern Jiangsu Province, China. Front Microbiol 2018;9:2704.
Crossref - Williamson DA, Sidjabat HE, Freeman JT, et al. Identification and molecular characterisation of New Delhi metallo-β-lacta-mase-1 (NDM-1)-and NDM-6-producing Enterobacteriaceae from New Zealand hospitals. Int J Antimicrob Agents. 2012; 39(6):529-533.
Crossref - Teo J, Ngan G, Balm M, Jureen R, Krishnan P, Lin R. Molecular characterization of NDM-1 producing Enterobacteriaceae iso-lates in Singapore hospitals. Western Pac Surveill Response J. 2012;3(1):19-24.
Crossref - Rahman M, Shukla SK, Prasad KN, et al. Prevalence and molecu-lar characterisation of New Delhi metallo-β-lactamases NDM-1, NDM-5, NDM-6 and NDM-7 in multidrug-resistant Enterobacte-riaceae from India. Int J Antimicrob Agents. 2014;44(1):30-37.
Crossref - Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. Laboratory detection of Enterobacteriaceae that produce carba-penemases. J Clin Microbiol. 2012;50(12):3877-3880.
Crossref - Hayder N, Hasan Z, Afrin S, Noor R. Determination of the frequency of carbapenemase producing Klebsiella pneumoniae isolates in Dhaka city, Bangladesh. Stam J Microbiol. 2013;2(1):28-30.
Crossref - Yusuf I, Magashi AM, Firdausi FS, et al. Phenotypic detection of Carbapenemases in members of Enterobacteriacea. Int J Sci Technol. 2012;2(11):802-806.
- Andrade SS, Gales AC, Sader HS. Antimicrobial resistance in gram-negative bacteria from developing countries. Antimicrobial Resistance in Developing Countries, 249-266.
Crossref - Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes. 2012;5(1):38.
Crossref - Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 28th Informa-tional Supplement. Vol. M100. CLSI document M100 (ISBN 1-56238-838-X); 2018. https://clsi.org/media/1930/m100ed28_sample.pdf
- Khawcharoenporn T, Vasoo S, Singh K. Urinary tract infections due to multidrug-resistant Enterobacteriaceae: prevalence and risk factors in a Chicago Emergency Department. J Emerg Med. 2013;258517.
Crossref - Lai CC, Wu UI, Wang JT, Chang SC. Prevalence of carbapenemase-producing Enterobacteriaceae and its impact on clinical outcomes at a teaching hospital in Taiwan. J Formos Med Assoc. 2013;112(8):492-496.
Crossref - Javed H, Ejaz H, Zafar A, Rathore AW, Ikram ul Haq. Metallo-beta-lactamase producing Escherichia coli and Klebsiella pneumoniae: A rising threat for hospitalized children. J Pak Med As-soc. 2016;66(9):1068-1072.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythro-mycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046-5054.
Crossref - Wang Y, Zhang R, Li J, et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat Microbiol. 2017;2(4):16260.
Crossref - Hashemi BFMH, Farzanehkhah M, Dolatyar A, et al. A study on prevalence of KPC producing from Klebsiella pneumoniae using Modified Hodge Test and CHROMagar in Iran. Ann Bio Res. 2012;3(12):5659-5664.
- Lascols C, Peirano G, Hackel M, Laupland KB, Pitout JD. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob Agents Chemother. 2013;57(1):130-136.
Crossref - Canton R, Akova M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. J Clinl Microbiol. 2012;18(5):413-431.
Crossref - Beyene G, Tsegaye W. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in JimmaUniversity specialized hospital, Southwest Ethiopia. Ethiop J Health Sci. 2011;21(2):141-146.
Crossref - Huang T-D, Berhin C, Bogaerts P, Glupczynski Y. In vitro susceptibility of multidrug-resistant Enterobacteriaceae clinical isolates to tigecycline. J Antimicrob Chemother. 2012;67(11):2696-2699.
Crossref - Nazik H, Ongen B, Ilktac M, et al. Carbapenem resistance due to Bla(OXA-48) among ESBL-producing Escherichia coli and Klebsiella pneumoniae isolates in a univesity hospital, Turkey. Southeast Asian J Trop Med Pub Health. 2012;43(5):1178-1185.
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.