ISSN: 0973-7510
E-ISSN: 2581-690X
The aim of the present study was to detect the prevalence of Anaplasma marginale in buffaloes in two Iraqi governorates, Al-Qadisiyah and Babylon, by the microscopy as well as the competitive-ELISA that used firstly among the Iraqi buffaloes. A total of 184 buffaloes from both sexes of different age groups of animals were submitted for collection of blood samples to prepare the smears and sera. Overall results were revealed on 10.33% and 36.41% positive animals by microscopy and competitive-ELISA, respectively. In addition, positive rates by both tests were 7.61%; by microscopy only, 2.72%; and by competitive-ELISA only, 28.8%. In Al-Qadisiyah and Babylon governorates, respectively, 8.7% and 11.96% of microscopy samples, and 44.57% and 28.26% of competitive-ELISA were positives with significant differences (P>0.05). Regarding to age factor, the highest prevalence was detected by microscopy in young age group (1-3 years) was 14.15%, whereas by competitive-ELISA, it was 55% in adult buffaloes group (>3 years). Significant increases (P>0.05) in rates of infection were showed in females compared to males, respectively, by microscopy (11.18% and 4.35%) and competitive-ELISA (39.13% and 17.39%).
Anaplasma marginale, Buffaloes, Microscopy, Competitive-ELISA, Iraq.
Anaplasma marginale is rickettsial intra-erythrocytic organism that causes bovine anaplasmosis in tropical, sub-tropical and temperate countries of the world, including Iraq; and being endemic in most animals of these regions1,2. Anaplasma that classified in Alphaproteobacteria class, Rickettsiales order of Anaplasmataceae family, is transmitted to cattle biologically by ticks and mechanically by flies and fomites3,4. Clinical disease is most notable in cattle, but other ruminants including water buffaloes can become persistently infected with A. marginale5. During acute anaplasmosis, A. marginale invades and multiplies within mature erythrocytes, leading to variable degrees of hemolytic anemia, fever, anorexia, weight loss, decreasing in milk production, reproductive problems and death in some cases6, 7. Recovering from acute phase results in persistent infected animal that serve as long-term reservoirs for transmission of infection within a herd8. The disease is a major constraint to bovine production because it affects dairy and beef domestic ruminants at any age resulting in high economic losses that estimated to be over 300 million dollars per year in United States 9, 10.
Microscopy is easy to perform, inexpensive, and considered as a “gold standard” test for confirming the acute phase of disease; however, its labor intensive and tedious for large numbers of samples, less sensitive, and impractical for routine testing of persistently infected ruminants as the bacterium is seldom detected in this phase11, 12. Hence, many serological techniques have been developed to detect specific IgM and IgG antibodies such as complement fixation test (CFT), card-agglutination (CAT), immunofluorescent antibody (IFAT) and enzyme-linked immunosorbent assay (ELISA)13, 14. Competitive-ELISA based on monoclonal antibody to recognize the major surface protein 5 (MSP5) of A. marginale, is used currently for diagnosis of bovine anaplasmosis15. It is highly accurate in diagnosis of acute and chronic infections with sensitivity and specificity that can reach to 95.6% and 98.6% of, respectively16, 17.
The purpose of this study was to evaluate the prevalence A. marginale infections in buffaloes through microscopic diagnosis of intra-erythrocytic A. marginale-inclusion bodies in slides of blood smears, and serological detection of specific anti-A. marginale antibodies in sera, for first time in Iraq, by a competitive-ELISA. In addition, the association of positive samples obtained by both assays to some epidemiological factors (residence, age, sex) of study’s buffaloes was evaluated.
Study’s samples
This study was performed in some rural regions related for two Iraqi governorates, Al-Qadisiyah and Babylon, during the period of March to August 2017. A total of 184 buffaloes from both sexes and different age groups were selected for the present study. From each animal, 10 ml of jugular venous blood was drawn and divided into two tubes (AFMA, Jordan); 3ml within an EDTA-anticoagulant tube to prepare of blood smear, and 7 ml within a free-anticoagulant tube that centrifuged at 3000 rpm for 15 minutes for sera. All sera were saved into numbered 1ml eppendorf tubes (China) and frozen at -20°C until be used18.
Blood smears (Preparation and examination)
Acutely infected buffaloes with A. marginale were diagnosed by using a rapid staining of Diff-Quick set (Modified Giemsa). According to manufacturer instructions (Vetlab Supplies, United Kingdom), the slides of thin blood smears were prepared, fixed with fixative solution, stained with solution I then solution II, rinsed with distilled water and, finally, dried by air. By light microscope (Trinoculr-MEIJI, Japan), the stained slides were examined under oil immersion to detect the positive samples that having intra-erythrocytic corpuscles of A. marginale as small dark spots, of peripheral location, and ranging from 0.1-0.8 mm19, 20.
Serological Survey
Competitive-ELISA was established for detection of specific IgG antibodies in persistently infected buffaloes with A. marginale. According to manufacturer instructions (VMRD, USA), the sera tested, and the results read using a microplate absorbance spectrophotometer reader (BioTekג, USA) at an optical density (OD) of 650nm. The test validation has been made as the mean of negative control must have an OD>0.40 and ≤2.10, whereas, mean of positive control must have an inhibition of ≥30%. Regarding to interpretation of samples values, samples having ≥30% inhibition rate were considered positive.
Statistical analysis
All obtained data were tabled and classified using of Microsoft Office Excel program (2013), and analyzed by a computerized IBM/SPSS program (V.23) through application of descriptive statistics and Chi-square test (x2). The significant differences between positive results of microscopic and serologic assays, and within residence, age, and sex factors of study’s animals, were compared and detected at a level of P≤0.0521.
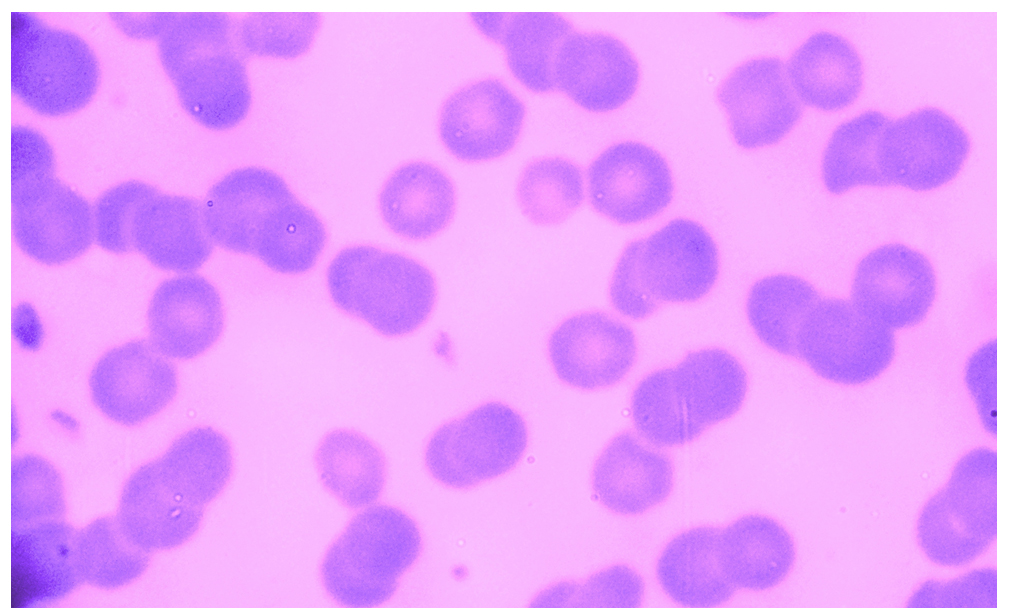
Microscopic examination of blood smears obtained from 184 study’s buffaloes revealed that 19 (10.33%) buffaloes were positives with specific intra-erythrocytic inclusion bodies of A. marginale, (Fig. 1). In addition, sera samples of 184 buffaloes were tested by a serologic competitive-ELISA that detected 67 (36.41%) seropositive buffaloes with anti-A. marginale IgG antibodies, (Table 1).
Table (1):
Prevalence of A. marginale in an overall 184 buffaloes
Test |
No. |
Positives |
Negatives |
|---|---|---|---|
Light Microscope |
184 |
19 (10.33%) B |
165 (89.67%) |
Competitive-ELISA |
67 (36.41%) A |
117 (63.59%) |
Variation in large letters, vertically, refers to significant differences at level of P≤0.05
The results of (Table 2) showed that 14/184 (7.61%) of buffaloes were positives by both microscopy and competitive-ELISA, and 112/184 (60.87%) were negatives by both tests. On other hand, 5/184 (2.72%) of buffaloes were positives with microscopy, only; whereas, 53/184 (28.8%) were positives by competitive-ELISA, only.
Table (2):
Cross-classification results of microscopy and competitive-ELISA
| Competitive-ELISA | Total | ||
|---|---|---|---|
| Microscopy | Positives | Negatives | |
| Positives | 14 (7.61%)Ba | 5 (2.72%)Bb | 19 |
| Negatives | 53 (28.8%)Ab | 112 (60.87%)Aa | 165 |
| Total | 67 | 117 | 184 |
Variation in large vertical and small horizontal letters refers to significant differences
Animals of this study were comprised 92 buffaloes from some areas of each governorate. Whereas, 11 (11.96%) and 41 (44.57%) of buffaloes were positives, respectively, by microscopy and competitive-ELISA in Al-Qadisiyah; 8 (8.7%) and 26 (28.26%) were positives by both tests, respectively, in Babylon (Table 3).
Table (3):
Association of positive A. marginale infections to residence factor
Residence |
No. |
Microscopy |
Competitive-ELISA |
|---|---|---|---|
Al-Qadisiyah |
92 |
11 (11.96%) Ab |
41 (44.57%) Aa |
Babylon |
92 |
8 (8.7%) Ab |
26 (28.26%) Ba |
Total |
184 |
19 |
67 |
Variation in large vertical and small horizontal letters refers to significant differences
Among three age groups, positive buffaloes of microscopy and competitive-ELISA were distributed, respectively, as follow: in <1 year age group, 1/38 (2.63%) and 6/38 (15.79%); 1-3 years age group, 15/106 (14.15%) and 39/106 (36.79%); and in >3 years age group, 3/40 (7.5%) and 22/40 (55%), (Table 4).
Table (4):
Association of positive A. marginale infections to age factor/span>
Age |
No. |
Microscopy |
CompetitiveELISA |
|---|---|---|---|
38 |
1 (2.63%) Cb |
6 (15.79%) Ca |
|
1-3 years |
106 |
15 (14.15%) Ab |
39 (36.79%) Ba |
>3 years |
40 |
3 (7.5%) Bb |
22 (55%) Aa |
Total |
184 |
19 |
67 |
Variation in large vertical and small horizontal letters refers to significant differences
Among 161 female buffaloes, 18 (11.18%) and 63 (39.13%) were positives by microscopy and competitive-ELISA; while in 23 males, 1 (4.35%) and 4 (17.39%) were positives by both diagnostic methods, respectively, (Table 5).
Table (5):
Association of positive A. marginale infections to sex factor
<th “>CompetitiveELISA
Sex |
No. |
Microscopy |
|
|---|---|---|---|
Female |
161 |
18 (11.18%) Ab |
63 (39.13%) Aa |
Male |
23 |
1 (4.35%) Bb |
4 (17.39%) Ba |
Total |
184 |
19 |
67 |
Variation in large vertical and small horizontal letters refers to significant differences
According to FAO report in 1997, buffaloes are recognized as the “Black gold of Asia”, however, few neglected studies have examined the occurrence of A. marginale among buffaloes if compared to other field animals22. In this study, the total rate of positive buffaloes with A. marginale was 10.33% by slides of blood smears microscopy and 36.41% by serological competitive-ELISA (Table1). In previous studies, the occurrence rate of A. marginale among buffaloes by blood smears microscopy was reported 5.71% in Iraq23, 10.3% in Philippines (24), 4.29-22% in Pakistan22, 25, 33.5% in South Africa26, 59.3% in Egypt27; whereas, the seroprevalence of anti-A. marginale antibodies among buffaloes was 63% in Brazil28, and 78.1% in Egypt27. Also, the study reported that 2.72% of buffaloes were positives, only, by light microscopy, which might be explained by the persistence of recent infection and IgG-antibodies were not developed, completely, to be detected by competitive-ELISA29; whereas, 7.61% of buffaloes were positives by both tests, which can be explained that these animals with acute infection and have high level of IgG-antibodies from previous exposure30, at late stage of acute infection where the number of parasitemia decreased clearly and the immunity was increased, drastically31, or presence of high immunity with severe infection4. Major surface protein (MSP5) is a highly conserved surface protein among different strains of A. marginale, which has been proven as effective diagnostic antigen and used in a competitive-ELISA32. MSP5-competitive-ELISA demonstrated a high sensitivity and specificity for determining the true-positive and true-negative animals (bovine, ovine, caprine, camelidae) in endemic areas2, 33. In addition, the test is excellent for detection of specific IgG antibodies in sera of naturally or experimentally infected hosts and in vaccinated animals, so that, it can be applied for eradication programs, regulation of interstate and international movement of reproductive field hosts15,34. Many studies reported that the test has an ability to detect of individually infected animals accurately. Hence, it can be utilized for epidemiological investigations where the infections expanding through the movement of infected animals into disease-free regions35, 36.
Although, the worldwide seroprevalence of bovine anaplasmosis in buffaloes was reported to be less than that detected in cattle, the seropositive results of this study were higher than those reported previously in Iraqi cattle by2, 37. This could be attributed to that study’s buffaloes were exposed for unsuitable environmental conditions such as stress factors and ticks38. Other reasons are the bad management systems that include problems in feeding, drinking, housing and disease control or medication, which leading to decrease or waning of immunity. In general, buffaloes can play a role for harboring A. marginale and act as a potent carrier for other animals39, 40.
In microscopy, although the positive prevalence of buffalo’s A. marginale in Al-Qadisiyah (11.96%) was higher than reported in Babylon (8.7%) governorates; no significant differences (P£0.05) have been detected relatively between them, (Table 3). Whereas, the seroprevalence of infection by competitive-ELISA in Al-Qadisiyah (44.57%) much more than showed in Babylon (28.26%), and this could belong to variations in either owner’s subculture, topography or to some risk factors such as stocking density, type of dipping, introduction of cattle to the farm, farm type, herd size, tick density, and dipping intervals41, 42.
Positive results among different buffalo’s age groups (Table 4) detected that the highest prevalence by microscopy was showed in young buffaloes (1-3 years age group), whereas by competitive-ELISA, it’s seen in adults (>3 years age group). In young animals, these results might be explained by the age resistance and lack of maternal immunity gained by colostrums, which may last up 6 months to 1 year, hence more exposure for infections; whereas in adults, the seroprevalence of IgG-anti A. marginale antibodies was interpreted by the facts that the disease is of adults and the high titer levels of antibodies might be reflection for previous frequent multiple exposure to Anaplasma or recent infection 26, 27.
In relation to sex factor (Table 5), significant increases in A. marginale infections were detected in female buffaloes by both the microscopy and competitive-ELISA, which might belong to the low samples of study males in comparison to females, exposing of females to high stress conditions (gestation, parturition, milking), and/or that males received an attention more than females concerned to housing, feeding and medication43, 44, 45.
The present study resumed that the prevalence of A. marginale in buffaloes have been increased, clearly, when compared to previous Iraqi studies; as well as, the seropositive results by competitive-ELISA were much more than reported by microscopy. In addition, differences in positivity among residence, age and sex factors could provide a benefit data for a futurism studies that recommended to be depended on competitive-ELISA or molecular techniques as a high sensitive and specific diagnostic methods.
Acknowledgements
None.
Conflicts of Interests
The author declare that there are no conflicts of interest.
Authors’ Contribution
Author listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
None
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
- Araתjo FR., Costa CM., Ramos CA., Farias TA., de Souza IF., Melo ES., and Fonseca, AH. . IgG and IgG2 antibodies from cattle naturally infected with Anaplasma marginale recognize the recombinant vaccine candidate antigens VirB9, VirB10, and elongation factor-Tu. Memףrias do Instituto Oswaldo Cruz. 2008, 103(2), 186-190.
Crossref - Al-gharban HA., and Dhahir, SH. Serological diagnosis of persistent infection with Anaplasma marginale bacteria in cattle. The Iraqi Journal of Veterinary Medicine. 2015, 39(1): 33 39.
- Dumler JS., Barbet AF., Bekker CP., Dasch GA., Palmer GH., Ray SC., and Rurangirwa, FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. International journal of systematic and evolutionary microbiology.2001, 51(6), 2145-2165.
Crossref - Kocan KM., De la Fuente J., Guglielmone AA., and Melיndez, RD. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clinical Microbiology Reviews.2003, 16(4), 698-712.
Crossref - De la Fuente J., Naranjo V., Ruiz-Fons F., Hofle U., Fernandez De Mera IG., Villanתa D., and Gortazar, C.Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector-Borne & Zoonotic Diseases. 2005a, 5(4), 390-401.
Crossref - Jaswal H., Bal MS., Singla LD., Gupta K., and Brar, AS. Pathological observations on clinical Anaplasma marginale infection in cattle. Journal of parasitic diseases.2015, 39(3), 495-498.
Crossref - Regitano LA., and Prayaga, KE. Ticks and tick-borne diseases in cattle. Breeding for disease resistance in farm animals, 2010; 3.
- Coetzeea JF., Apleya MD., Kocanb KM., Rurangirwac FR., and Van Donkersgoedd, J. Comparison of three oxytetracycline regimens for the treatment of persistent Anaplasma marginale infections in beef cattle. Veterinary parasitology, 2005; 127(1): 61-73.
Crossref - Jonsson NN., Bock RE., and Jorgensen, WK. Productivity and health effects of anaplasmosis and babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Veterinary parasitology. 2008, 155(1): 1-9.
Crossref - Rymaszewska A., and Grenda, S. Bacteria of the genus Anaplasma-characteristics of Anaplasma and their vectors: a review. Vet Med. 2008; 53(11): 573-584.
Crossref - Fosgate GT., Urdaz-Rodrםguez JH., Dunbar MD., Rae DO., Donovan GA., Melendez P., and Alleman, A. R. Diagnostic accuracy of methods for detecting Anaplasma marginale infection in lactating dairy cattle of Puerto Rico. Journal of veterinary diagnostic investigation. 2010; 22(2): 192-199.
Crossref - Reinbold JB., Coetzee JF., Hollis LC., Nickell JS., Riegel CM., Christopher JA., and Ganta, RR. Comparison of iatrogenic transmission of Anaplasma marginale in Holstein steers via needle and needle-free injection techniques. American journal of veterinary research. 2010; 71(10): 1178-1188.
Crossref - Madruga CR., Marques AC., Leal CB., Carvalho CM., Araתjo FR., and Kessler, RH. Evaluation of an enzyme-linked immunosorbent assay to detect antibodies against Anaplasma marginale. Pesquisa Veterinבria Brasileira, 2000; 20(3): 109-112.
Crossref - Barros SL., Madruga CR., Araתjo FR., Menk CF., de Almeida MO., Melo EP., and Kessler, RH. Serological survey of Babesia bovis, Babesia bigemina, and Anaplasma marginale antibodies in cattle from the semi-arid region of the state of Bahia, Brazil, by enzyme-linked immunosorbent assays. Memorias do Instituto Oswaldo Cruz., 2005; 100(6): 513-517.
Crossref - De Echaide ST., Knowles DP., McGuire TC., Palmer GH., Suarez CE., and McElwain, TF. Detection of Cattle Naturally Infected with Anaplasma marginale in a Region of Endemicity by Nested PCR and a Competitive Enzyme-Linked Immunosorbent Assay Using Recombinant Major Surface Protein 5. Journal of clinical microbiology., 2001; 39(3): 1207.
- Howden KJ., Geale DW., Parי J., Golsteyn-Thomas EJ., and Gajadhar, AA. An update on bovine anaplasmosis (Anaplasma marginale) in Canada. The Canadian Veterinary Journal., 2010; 51(8): 837.
- Co’kun A., Ek c ײD., G zelbekte’ H., Aydoנdu U., and ‘en,. Acute phase proteins, clinical, hematological and biochemical parameters in dairy cows naturally infected with Anaplasma marginale. Kafkas niversitesi Veteriner Fak ltesi Dergisi., 2012; 18(3): 497-502.
Crossref - Onwuegbuzie AJ., and Leech, NL. Sampling Designs in Qualitative Research: Making the Sampling Process More Public. Qualitative Report., 2007; 12(2): 238-254.
- Vidotto O., and Marana, EM. Diagnosis in bovine anaplasmosis. Ciךncia Rural., 2001; 31(2): 361-368.
Crossref - Noaman V., and Shayan, P. Comparison of microscopy and PCR-RFLP for detection of Anaplasma marginale in carrier cattle. Iranian journal of microbiology., 2010; 2(2): 89.
- Petrie A., and Watson, P. Statistics for Veterinary and Animal Science, Second Edition. Ames: Blackwell Publishing.2006, Pp: 12-43.
- Rajput ZI., Hu SH., Arijo AG., Habib M., and Khalid, M. Comparative study of Anaplasma parasites in tick carrying buffaloes and cattle. Journal of Zhejiang University. Science. B., 2005, 6(11): 1057.
- Alhtheal, ED. Studies on Morphological Classification of Anemia and Clinical Examination in Iraqi Buffaloes. Master thesis, College of Veterinary Medicine, University of Baghdad.2012.
- Mingala CN., Konnai S., Cruz LC., Onuma M., and Ohashi, K. Comparative moleculo-immunological analysis of swamp-and riverine-type water buffaloes responses. Cytokine., 2009; 46(2): 273-282.
Crossref - Sajid MS., Siddique RM., Khan SA., Iqbal Z., and Khan, MN. Prevalence and risk factors of anaplasmosis in cattle and buffalo populations of district Khanewal, Punjab. Pakistan Global Vet., 2014; 12: 146-53.
- Debeila, EM. Occurrence of Anaplasma and Ehrlichia species in African buffalo (Syncerus caffer) in Kruger National Park and Hluhluwe-iMfolozi Park in South Africa Doctoral dissertation, University of Pretoria.2013.
- Abou-Elnaga TR, Mahmoud MA, Osman WA, and Goda, AS. Serological survey of Anaplasma marginale (Rickettsia) antibodies in animals by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. Suez Canal Vet. Med. J., 2009; 19: 309-320.
- da Silva JB., Vinhote WS., Oliveira CC., Andrי MR., Machado RZ., da Fonseca AH., and Barbosa, JD. Molecular and serological prevalence of Anaplasma marginale in water buffaloes in northern Brazil. Ticks and tick-borne diseases., 2014, 5(2): 100-104.
Crossref - Brown WC., Palmer GH., Lewin HA., and McGuire, TC. CD4+ T Lymphocytes from Calves Immunized with Anaplasma marginale Major Surface Protein 1 (MSP1), a Heteromeric Complex of MSP1a and MSP1b, Preferentially Recognize the MSP1a Carboxyl Terminus That Is Conserved among Strains. Infection and immunity., 2001; 69(11): 6853-6862.
Crossref - Lopez JE., Siems WF., Palmer GH., Brayton KA., McGuire TC., Norimine J., and Brown, WC. Identification of novel antigenic proteins in a complex Anaplasma marginale outer membrane immunogen by mass spectrometry and genomic mapping. Infection and immunity., 2005; 73(12): 8109-8118.
- Nazifi S., Razavi SM., Kaviani F., and Rakhshandehroo, E. Acute phase response in cattle infected with Anaplasma marginale. Veterinary microbiology., 2012; 155(2): 267-271.
Crossref - de la Fuente J., Lew A., Lutz H., Meli ML., Hofmann-Lehmann R., Shkap V., and Gortבzar, C. Genetic diversity of Anaplasma species major surface proteins and implications for anaplasmosis serodiagnosis and vaccine development. Animal health research reviews., 2005b, 6(1): 75-89.
Crossref - Naqid IA., and Zangana, IZ. Hematological and serological (cELIZA) studies of caprine anaplasmosis in Duhok governorate of Kurdistan region of Iraq. J Duhok Univ., 2010; 13(1): 153-161.
- Al-Adhami B., Scandrett WB., Lobanov VA., and Gajadhar, A. A. Serological cross-reactivity between Anaplasma marginale and an Ehrlichia species in naturally and experimentally infected cattle. Journal of veterinary diagnostic investigation. 2011; 23(6): 1181-1188.
Crossref - Molloy JB., Bowles PM., Knowles DP., McElwain TF., Bock RE., Kingston TG., and Dalgliesh, RJ. Comparison of a competitive inhibition ELISA and the card agglutination test for detection of antibodies to Anaplasma marginale and Anaplasma centrale in cattle. Australian veterinary journal. 1999; 77(4): 245-249.
Crossref - Coetzee JF., Schmidt PL., Apley MD., Reinbold JB., and Kocan, KM. Comparison of the complement fixation test and competitive ELISA for serodiagnosis of Anaplasma marginale infection in experimentally infected steers. American journal of veterinary research. 2007; 68(8): 872-878.
Crossref - Ameen KH., Abdullah BA., and Abdul-Razaq, RA. Seroprevalence of Babesia bigemina and Anaplasma marginale in domestic animals in Erbil, Iraq. Iraqi Journal of Veterinary Sciences. 2012, 26(Suppl. 3), 109-114.
- Gray JS., Dautel H., Estrada-Peסa A., Kahl O., and Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdisciplinary perspectives on infectious diseases, 2009: 1-12.
Crossref - Kocan KM., de la Fuente J., Blouin EF., Coetzee JF., and Ewing, SA. The natural history of Anaplasma marginale. Veterinary parasitology., 2010; 167(2): 95-107.
Crossref - Sharma A., Singla LD., Kaur P., Bal MS., Batth BK., and Juyal, PD. Prevalence and haemato-biochemical profile of Anaplasma marginale infection in dairy animals of Punjab (India). Asian Pacific Journal of Tropical Medicine., 2013; 6(2): 139-144.
Crossref - Rodrםguez-Vivas RI., Mata-Mendez Y., Pיrez-Gutierrez E., and Wagner, G. The effect of management factors on the seroprevalence of Anaplasma marginale in Bos indicus cattle in the Mexican tropics. Tropical animal health and production., 2004; 36(2): 135-143.
- Urdaz-Rodriguez JH., Fosgate GT., Alleman AR., Rae DO., Donovan GA., and Melendez, P. Seroprevalence estimation and management factors associated with high herd seropositivity for Anaplasma marginale in commercial dairy farms of Puerto Rico. Tropical animal health and production. 2009; 41(7): 1439-1448.
Crossref - Kele ., Deנer S., Altuנ N., Karaca M., and Akdemir, C. Tick-borne diseases in cattle: Clinical and haematological findings, diagnosis, treatment, seasonal distribution, breed, sex and age factors and the transmitters of the diseases. YYU Vet Fak Derg., 2001; 12(1-2): 26-32.
- Magona JW., Walubengo J., Olaho-Mukani W., Jonsson NN., Welburn SC., and Eisler, MC. Clinical features associated with seroconversion to Anaplasma marginale, Babesia bigemina and Theileria parva infections in African cattle under natural tick challenge. Veterinary parasitology., 2008: 155(3): 273-280.
Crossref - Swai ES., Karimuribo ED., Ogden NH., French NP., Fitzpatrick JL., Bryant MJ., and Kambarage, DM. Seroprevalence estimation and risk factors for A. marginale on smallholder dairy farms in Tanzania. Tropical animal health and production., 2005; 37(8): 599-610.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.