ISSN: 0973-7510
E-ISSN: 2581-690X
This investigation was carried out to examine the safety and microbiological quality of products made from meat, dairy and vegetables. Samples of frozen food were examined for the presence of Gram-negative bacteria. A total of 49 frozen food samples were collected, including chicken nuggets, chicken fillets, chicken sticks, breaded breast chicken, fish, beef burger and minced beef roll, white cheese, camembert cheese, and vegetables (okra and green peas). Standard methods were used to determine the diversity of bacterial isolates in various food samples. About 182 isolates of Escherichia coli, 63 isolates of Salmonella typhi, 51 isolates of Pseudomonas, and 63 isolates of Klebsiella were recovered from the various frozen food samples. Meat samples showed a high prevalence of E. coli and Pseudomonas. The antibiotic susceptibility patterns of the isolated bacterial strains were also examined. Out of 12 antibiotics, only ciprofloxacin and ofloxacin showed a high level of susceptibility. According to the study’s findings, the majority of the frozen meat product samples contained a significant number of bacteria and were therefore unsafe for human consumption. These microorganisms can cause infection and are therefore associated with a high risk to the consumers. Therefore, it is important to pay attention to health and education issues in relation to food safety.
Frozen Food, Bacteria, Antibiotic Susceptibility
Foods that are damaged or tainted because they contain either microorganisms, such as bacteria or parasites, or toxic compounds that render them unsafe for consumption are often referred to as contaminated foods. Biological, chemical, or physical contaminants can affect food; the former two are more frequent. These pollutants can enter a food product through a variety of points along the supply chain (from farm to fork), rendering it unsafe for human consumption.1 Common organisms include Yersinia enterocolitica, Listeria monocytogenes, Salmonella spp., Shigella spp., pathogenic Staphylococcus aureus, Bacillus cereus, Campylobacter jejuni, Clostridium botulinum, Clostridium perfringens, pathogenic Escherichia coli, Vibrio cholera, V. parahaemolyticus, and V. vulnificus.2
The food sector constantly deals with numerous regional and worldwide contamination challenges that are both current and emerging.3 Scientific and technical advances are being made to address these issues. The nature of the contamination, its origins, consumer dangers, and strategies to reduce or eradicate contamination levels must all be understood by food safety management. To produce food products with a low risk of contamination or those that are free of contamination, excellent scientific understanding is required.3 In numerous documents and reports, the World Health Organization (WHO) has identified food contamination as a global issue.4 A statement acknowledging this fact states, “Food contamination that occurs in one place may harm the health of customers living on the opposite side of the earth”.5-7 Over the course of their lifetimes, the vast majority of people globally acquire a food- or water-borne illness. As a result, millions of people become sick from eating tainted food, and many die as a result. In this situation, “food contamination” becomes a significant issue, and its problems are numerous and constantly expanding.
Microbial standards relating to product safety and quality are required by the food industry globally. Since safety considerations are of utmost importance, they are decided upon in a straightforward manner. Usually, pathogenic bacteria that could cause consumers to have serious health issues are subject to rigorous limits or no tolerance.8 A Foodborne Diseases Active Surveillance Network recently conducted surveillance in the USA for all diseases caused by certain pathogens that are often spread through food. In 2013,9 it was reported that there were 19,531 infections, 4563 hospitalizations, and 68 fatalities linked to foodborne illnesses.
Every year, foodborne diseases cause a financial burden. The investigation of foodborne illnesses linked to 31 foodborne pathogens and spoilage microorganisms costs the United States 77.7 billion dollars every year, according to Scharff.10-11 Even though a wide variety of foods can result in foodborne illnesses, the meat and dairy products sector remains the major safety concern and a focus in many ways. Thus, the goal of the current study was to assess how common Gram-negative bacteria were in frozen foods towards the end of their shelf life, to shed light on the statistics and to indicate where future educational initiatives by food regulatory authorities should be directed. Furthermore, by avoiding the risks associated with such meals and maintaining public health, as well as by identifying defective frozen food products early on, economic losses can be minimized.
Collection of food samples
A total of 49 frozen food samples were randomly collected from 4 different markets in Delhi, India. About 23 meat product samples, 8 dairy samples, and 8 vegetable products were included in the study. These were chicken nuggets, chicken fillets, chicken sticks, breaded breast chicken, fish, beef burger and minced beef roll, white cheese, camembert cheese, and vegetables (okra and green peas). The samples were tagged and analyzed after being collected in sterile plastic bags and transported in ice boxes.
Media and reagents
The media used for bacteriological analysis were MacConkey agar, blood agar, Mueller-Hinton agar and Nutrient broth (NB). The reagents used during the study were crystal violet, Gram’s iodine, safranin, 95% ethyl alcohol, 3% hydrogen peroxide, Kovacs indole reagent, normal physiological saline, phosphate buffered saline (PBS), and distilled water. Commercially available antibiotic discs were used to test the drug sensitivity pattern. The commercially available media were prepared according to the manufacturer’s instructions, while the non-commercial media were prepared in the laboratory.
Sample processing
To create adequate dilutions for the microbiological examination, 30 g of each sample were combined and homogenized with 225 ml of sterile saline solution. Homogenates were diluted ten-fold before being introduced into the appropriate media.
Isolation of bacterial pathogens
By streaking 0.1 ml of each food sample at a suitable dilution onto blood agar and McConkey agar, bacteria were isolated and counted before being cultured at 37°C for 24 hours. Pure cultures on nutrient agar slants at 37°C for 24 hours were used to isolate typical colonies for subsequent identification.12 The identification of isolated bacteria was carried out based on their microscopic, cultural, and biochemical properties.13
Antibiotic sensitivity test for bacterial isolates
To test their effectiveness against bacterial isolates, 12 antibiotic discs from different groups that are readily available were selected. The culture broth was transferred aseptically into sterile distilled water overnight and forcefully stirred to produce turbidity that matched the 0.5 McFarland standard (about 108 CFU/ml), as described in Bauer’s14-15 standard Kirby-Bauer disk diffusion procedure. The surface of solidified Mueller-Hinton agar plates was then inoculated with the swab dipped in the culture solution.16 To achieve complete contact with the agar, antibiotic discs were placed on the infected plate surface and gently pressed down with sterile forceps. The plates were then incubated at 37°C for 24 hours in air supplemented with 5% carbon dioxide. Clinical Laboratory Standards “CLS” were used to assess and interpret the inhibition zones surrounding the antibiotic discs. The classification of the results was R (resistant), IS (intermediate sensitive), and S (sensitive).17
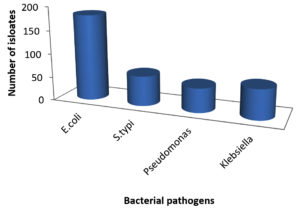
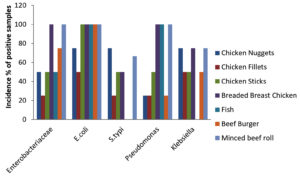
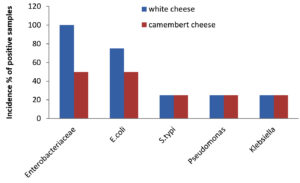
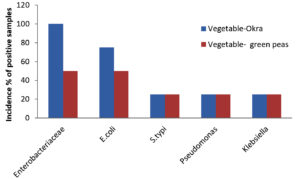
A total of 49 samples of frozen food were collected, which included samples of meat (23), dairy (8), and vegetables (8). The prevalence of bacterial pathogens in different food samples are illustrated in Table 1 and Figure 1. The total occurrence of E. coli in different food samples was 50.0%, S. typhi 17.5%, Pseudomonas aeruginosa 14.2% and Klebsiella 17.5%. Out of 23 samples of meat, 15 were positive for Enterobacteriaceae, while for dairy and vegetable samples only 6 samples were found to be positive. The prevalence of Enterobacteriaceae was 50% for chicken nuggets (2), 25% for chicken fillets (4), 50% for chicken sticks (2), 100% for breaded breast chicken (1), 50% for fish (2), and 100% for beef burger (1) and minced beef roll (1) (Table 2 & Figure 2). A total of 6 positive samples for dairy and vegetable products were recorded for Enterobacteriaceae. The Enterobacteriaceae prevalence for dairy (Table 3 & Figure 3) and vegetables (Table 4 & Figure 4) included 100% for white cheese (1), 50% for camembert cheese (2), 100% for okra (1), and 50% for green peas (2).
Table (1):
Distribution of bacterial pathogens in different food samples
Bacterial isolates |
Chicken Nuggets |
Chicken Fillets |
Chicken Sticks |
Breaded Breast Chicken |
Fish |
Beef Burger |
Minced beef roll |
White cheese |
Camembert cheese |
Vegetables- Okra |
Vegetables- Green peas |
Number and occurrence (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
E. coli |
10 |
11 |
15 |
20 |
1 |
10 |
22 |
25 |
20 |
24 |
24 |
182 (50.6%) |
S. typhi |
1 |
7 |
15 |
5 |
– |
– |
3 |
7 |
8 |
8 |
9 |
63 (17.5%) |
Pseudomonas |
1 |
5 |
11 |
11 |
– |
3 |
3 |
7 |
1 |
5 |
4 |
51 (14.2%) |
Klebsiella |
8 |
3 |
5 |
5 |
– |
9 |
9 |
8 |
3 |
7 |
6 |
63 (17.5%) |
Total |
20 |
26 |
46 |
41 |
1 |
22 |
37 |
47 |
32 |
44 |
43 |
359 |
Table (2):
Incidence of Gram-negative bacteria in Meat samples
| Types of samples | No. of samples | Enterobacteriaceae | E. coli | S. typhi | Pseudomonas | Klebsiella | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | ||
| Chicken Nuggets | 4 | 2 | 50 | 2 | 3 | 75 | 1 | 2 | 75 | 2 | 1 | 25 | 4 | 1 | 75 | 4 |
| Chicken Fillets | 4 | 1 | 25 | 4 | 2 | 50 | 2 | 1 | 25 | 4 | 1 | 25 | 4 | 2 | 50 | 2 |
| Chicken Sticks | 2 | 1 | 50 | 2 | 2 | 100 | 1 | 1 | 50 | 1 | 1 | 50 | 2 | 1 | 50 | 2 |
| Breaded Breast Chicken | 4 | 4 | 100 | 1 | 4 | 100 | 1 | 2 | 50 | 2 | 2 | 100 | 2 | 1 | 75 | 1 |
| Fish | 2 | 1 | 50 | 2 | 2 | 100 | 2 | ND | – | 2 | ND | 100 | – | ND | – | – |
| Beef Burger | 4 | 3 | 75 | 1 | 4 | 100 | 1 | ND | – | 1 | 1 | 25 | 4 | 2 | 50 | 2 |
| Minced beef roll | 3 | 3 | 100 | 1 | 3 | 100 | 1 | 2 | 66.6 | 1 | 1 | 100 | 1 | 2 | 75 | 1.5 |
The positive samples were tested for the presence of E. coli in meat (20), dairy products (6), and vegetables (6). The prevalence of E. coli was 75% for chicken nuggets (1), 50% for chicken fillets (2), 100% for chicken sticks (1), 100% for breaded breast chicken (1), 100% for fish (2), 100% for beef burger (1), and 100% for minced beef roll (1) (Table 2 & Figure 2). Six samples of dairy and vegetables found to be positive for E. coli. The E. coli prevalence for dairy products (Table 3 & Figure 3) and vegetables (Table 4 & Figure 4) was 100% for white cheese (1), 50% for camembert cheese (2), 100% for okra (1), and 50% for green peas (2).
Table (3):
Incidence of Gram-negative bacteria in Dairy samples
| Types of samples | No. of samples | Enterobacteriaceae | E. coli | S. typhi | Pseudomonas | Klebsiella | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | ||
| White cheese | 4 | 4 | 100 | 1 | 4 | 75 | 1 | 1 | 25 | 4 | 1 | 25 | 4 | 1 | 25 | 4 |
| Camembert cheese | 4 | 2 | 50 | 2 | 2 | 50 | 2 | 1 | 25 | 4 | 1 | 25 | 4 | 1 | 25 | 4 |
Table (4):
Incidence of Gram-negative bacteria in Vegetable samples
| Types of samples | No. of samples | Enterobacteriaceae | E. coli | S. typhi | Pseudomonas | Klebsiella | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | Positive samples | Incidence percentage (%) | Average log. no | ||
| Vegetables- Okra | 4 | 4 | 100 | 1 | 4 | 75 | 1 | 1 | 25 | 4 | 1 | 25 | 4 | 1 | 25 | 4 |
| Vegetables- Green peas | 4 | 2 | 50 | 2 | 2 | 50 | 2 | 1 | 25 | 4 | 1 | 25 | 4 | 1 | 25 | 4 |
The presence of S. typhi in positive samples of meat (7), dairy products (2), and vegetables (2) was also assessed. The figures were 75% for chicken nuggets (2), 25% for chicken fillets (4), 50% for chicken sticks (1), 50% for breaded breast chicken (2), 0% for fish (2), 0% for beef burger (1), and 66.6% for minced beef roll (1) (Table 2 & Figure 2). Out of 8 samples of dairy products and vegetables, only two samples were found to be positive for S. typhi. The S. typhi percentage was 25% for dairy products (white cheese (4) and camembert cheese (4)), as shown in Table 3 & Figure 3, and 25% for vegetables (okra (4) and green peas (4)) as shown in Table 4 & Figure 4.
The figures for Pseudomonas and Klebsiella in positive samples of meat (7,8), dairy products (2,2), and vegetables (2,2) were also recorded. The Pseudomonas and Klebsiella average count for chicken nuggets (4,4), chicken fillets (4,2), chicken sticks (2,2), breaded breast chicken (2,1), fish (0,0), beef burger (4,2) and minced beef roll (1,1.5) were recorded with a prevalence of 25, 25, 50, 100, 100, 25 and 100% for Pseudomonas and 75, 50, 50, 75, 0, 50 and 75% for Klebsiella (Table 2 & Figure 2). Out of 8, two of each sample of dairy and vegetables were positive for Pseudomonas and Klebsiella. The Pseudomonas and Klebsiella count for dairy products (Table 3 & Figure 3) and vegetables (Table 4 & Figure 4) were recorded. The Pseudomonas and Klebsiella count for white cheese (2, 2), camembert cheese (2,2), okra (2,2) and green peas (2,2) and the percentages for dairy (25%) and vegetables (25%) products were also recorded.
Research by Bezeraa et al., indicates that in 31.4% of samples with positive E. coli results, hamburgers were deemed unfit for human consumption.18 The presence of E. coli in combination with unsanitary conditions, such as a poor waste disposal system, may be a potential source of food contamination. The presence of members of the Enterobacteriaceae family, which have been recognized as significant contributors to foodborne outbreaks,19 presents a health concern to children and those with underlying illnesses. The overall number of positive samples was 359 and the percentage of E. coli concentration was (182) 50.6% (Table 1 & Figure 1). The use of contaminated water at various stages of processing may be responsible for the occurrence of E. coli. In this regard, the water used for washing raw meat is also used for washing hands and production-related utensils.20 Salmonella typhi and E. coli were both discovered to be contaminants in the beef items.21 Salmonella outbreaks are typically caused by inappropriate food handling and preparation at food service establishments, with infected food handlers not washing their hands. These findings are consistent with those of Zhao et al.,22 who isolated Salmonella in 19–54% of cow carcasses, 1.9% of beef samples purchased at retail, and 4.2% of poultry samples purchased at retail. As of January 16, 2003, Trinidad and Tobago had 49 cases of salmonellosis from weeks 1 to 52 of 2002.23 Gram-negative facultative anaerobes such Klebsiella (K. pneumonia), Salmonella (S. typhi), and Proteus (P. vulgaris) were found in the food samples under investigation, according to Lengeler et al.,24 According to Samson et al.,25 many meat samples under investigation contained Gram-negative aerobes such as Campylobacter (C. jejuni and C. coli), Pseudomonas (P. aeruginosa, P. fluorescens, and P. putida). Since these bacteria are spread by equipment or contact with raw foods, the presence of Enterobacteriaceae and Coliform group is a helpful predictor of cleanliness of post-processing contamination of processed foods. Hassan et al.,26 reported similar findings, demonstrating that the mean value of the Enterobacteriaceae count ranged from 3.9×102 to 1×103 for each sample of yogurt and feta cheese. Furthermore, Mohammed et al.,27 studies is not consistent with the coliform group’s infection % with our investigation results. The pathogenic E. coli O157:H7 was isolated from 19% of the total white cheese samples in the earlier tests conducted in Egypt.28
The bacterial isolates from meat and dairy dietary samples were identified on the basis of their morphological, cultural, and broth consumption manual tests, as described in Bergey’s manual.29 Three main types of foodborne bacterial isolates were identified on the basis of biochemical findings (Figure 5).
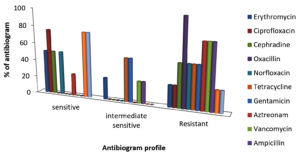
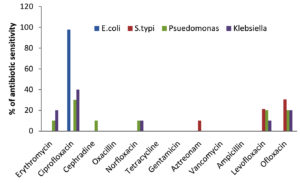
As shown in Table 5 and Figure 6a, the antibiotic sensitivity of the examined bacterial isolates varied, ranging between sensitive (S), intermediate sensitive (IS), and resistant (R) for the various antibiotics tested. The antibiotic susceptibility patterns of representative isolates are shown in Figure 6b. The total numbers of isolates based on their antibiotic sensitivity pattern (antibiogram) against all 12 antibiotics are shown in Table 5. In the present investigation, erythromycin, ciprofloxacin, cephradine, oxacillin, norfloxacin, tetracycline, gentamicin, aztreonam, vancomycin, ampicillin and levofloxacin were employed to screen bacterial pathogens for antibiotic susceptibility. E. coli was found to be especially sensitive to ciprofloxacin (97.83%), S. typhi to ofloxacin (41%), levofloxacin (30.5%) and aztreonam (21.4%), while Pseudomonas was sensitive to erythromycin (10%), ciprofloxacin (30%), cephradine (10%), norfloxacin (10%) and levofloxacin (20%). Klebsiella was sensitive to ciprofloxacin (40%), norfloxacin (10%) and levofloxacin (10%) as well as erythromycin (20%) and ofloxacin (20%) (Figure 7).
Table (5):
Antibiogram profile of foodborne pathogens
| Antibiotics | Bacterial isolates | |||
|---|---|---|---|---|
| E. coli | S. typhi | Pseudomonas | Klebsiella | |
| Erythromycin | R | I | S | S |
| Ciprofloxacin | S | R | S | S |
| Cephradine | R | S | S | R |
| Oxacillin | R | R | R | R |
| Norfloxacin | R | R | S | S |
| Tetracycline | R | R | I | I |
| Gentamicin | R | I | R | I |
| Aztreonam | R | S | R | R |
| Vancomycin | R | R | I | R |
| Ampicillin | R | I | R | R |
| Levofloxacin | I | S | S | S |
| Ofloxacin | R | S | S | S |
The findings showed that frozen food samples had high levels of E. coli bacterial contamination. Over 50% of meat samples contained E. coli and Salmonella, which is much higher than the stated percentage for other countries.30-35 Alarming multi-resistance frequencies for Salmonella and E. coli isolates from food were also shown by the antibiotic susceptibility tests. Antibiotic-resistant E. coli isolates have been found, probably reflecting the unchecked use of antibiotics in food-producing animals.36-39 Salmonella species isolated from pork and poultry were found to be resistant to one or more antibiotics in 78 to 89% of the cases.
The quality of food products in relation to public health standards has attracted the attention of researchers. In this regard, the prevalence of pathogenic bacteria in various frozen food samples was investigated. Strict guidelines for food safety should be observed, along with instructions on safety principles and proper sanitary practices. It is highly recommended that consumers are made aware of the quality standards for frozen food. If these aspects are taken into consideration, infection and toxicity caused by the consumption of these products can be controlled.
ACKNOWLEDGMENTS
The authors would like to thank A-DBT Research Laboratory, India, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Pateiro M, Munekata PES. Sant’Ana AS, Dominguez R, Rodriguez-Lazaro D, Lorenzo JM. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int J Food Microbiol. 2021;337:108966.
Crossref - Candeliere F, Raimondi S, Spampinato G, et al. Draft genome sequences of 12 Leuconostoc carnosum strains isolated from cooked ham packaged in a modified atmosphere and from fresh sausages. Microbiol Resour Announce. 2020;9(2):e01247-19.
Crossref - Zwirzitz B, Wetzels SU, Dixon ED, et al. Co-Occurrence of Listeria spp. and spoilage associated microbiota during meat processing due to cross-contamination events. Front Microbiol. 2021;12:632935.
Crossref - Greig JD, Ravel A. Analysis of foodborne outbreak data reported internationally for source attribution. Int J Food Microbiol. 2009;130(2):77-87.
Crossref - Todd E. Food-Borne Disease Prevention and Risk Assessment. International Journal of Environmental Research and Public Health. Int. J. Environ. Res. Public Health 2020;17: 5129.
Crossref - Fukuda K. Food safety in a globalized world. Bull WHO. 2015;93(4):212.
- Shrivastava SR, Shrivastava PS, Ramasamy J. Global Food Safety: Challenges and Recommended Public Health Strategies. Int J Prev Med. 2016; 13;7:8.
Crossref - Arvinderpal Singh, Maninder Singh, Mohd Ashraf Malik, Sonali Padha “Is There a Shift in Salmonella Diversity Among Poultry in Northern India?,” Avian Diseases, 67(1);108-113.
Crossref - CDC. Incidence and trends of infection with pathogens transmitted commonly through food – foodborne diseases active surveillance network, 10 U.S. sites, 1996-2012. Wkly Rep. 2013;62(15):283-287.
- CDC. Preliminary Food Net data on the incidence of infection with pathogens transmitted commonly through food-10 states 2009. Morb Mortal Wkly Rep. 2010;59:418-422.
- Scharff RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot. 2012;75(1):123-131.
Crossref - “FDA” Food and Drug Administration. Bacteriological analytical manual. 9th Ed., AOAC Int., Arlington, VA, USA. 2002.
- Roberts D, Greenwood M. Practical food microbiology. 3rd edition. Blackwell Publishing Ltd, UK. 2003:273-274.
Crossref - Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Tech Bull Regist Med Technol. 1966;45(4):493-496.
- D’Amato RF, Hochstein L. Evaluation of a rapid inoculum preparation method for agar disc diffusion susceptibility testing. J Clin Microbiol. 1982;15(2):282-285.
Crossref - Mohamed IA, Safaa ME, Badawi AO, Khaled AEl-D. Antibiotics resistance phenomenon and virulence ability in bacteria from water environment. Water Science, 2017; 31(2): 109-121.
Crossref - Morency-Potvin P, Schwartz DN, Weinstein RA. Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship. Clin Microbiol Rev. 2016; 30(1):381-407.
Crossref - Bezerra ACD, Reis RB, Bastos DHM. Microbiological quality of hamburgers sold in the streets of Cuiaba-MT, Brazil and vended hygiene-awareness. Cienc Tec Aliment. 2010;30(2):121-132.
Crossref - Centinkaya F, Cibik G, Soyuteniz E, Ozkin C, Kayali R, Levent B. Shigella and Salmonella contamination in various foodstuffs in Turkey. J Food Control. 2008;19(11):1059-1063.
Crossref - Ologhobo AD, Omojola AB, Ofongo ST, Moiforay S, Jibir M. Safety of street vended meat productschicken and beef suya. African J Biotec. 2010;9(26):4091-4095.
- Liao RY, Xia Q, Zhou CY, et al. LC-MS/MS-based metabolomics and sensory evaluation characterize metabolites and texture of normal and spoiled dry-cured hams. Food Chem. 2022;371:131156.
Crossref - Zhao C, Ge B, De Villena J, et al. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beeffrom the greater Washington, D.C. area. Appl Environ Microbiol. 2001;67(12):5431-5436.
Crossref - Theodore H. Tulchinsky, Elena A. Varavikova, Chapter 4 – Communicable Diseases, Editor(s): Theodore H. Tulchinsky, Elena A. Varavikova. The New Public Health (Third Edition), Academic Press, 2014: 149-236.
Crossref - Lengeler JW, Drews G, Schlegel HG. Biology of the prokaryotes, Blackwell Science, New York. 2004:80-110.
- Samson RA, Hoekstra ES, Frisvad JC, Filtenborg O. Introduction of food Microbio-logy and Airborne Fungi, 2nd ed., CBS Publications, The Netherlands. 2000.
- Hassan NS, Esmail AS, Mahmoud AH. Bacteriological assessment of some ready-to-eat foods. Kafr El-Sheikh Vet Med J Sci Congress. 2009;7(1):474-487.
Crossref - Mohammed HM, Ali M, Habib M, Servatkhah F, Mohammed RS. Microbiological contamination of traditional chocolate ice cream sold in the Northwest region of Iran. Global Veterinaria. 2001;6(3):269-271.
- El-Kholy, Wafaa & El-Khalek, Azzat & Mohamed, Sahar & Tawfeek, Mohamed & Kassem, Jihan. Tallaga Cheese as a New Functional Dairy Product. American Journal of Food Technology. 2016;11:182-192.
Crossref - Bergey’s Manual. Bergey’s manual of systematic bacteriology. Sneath PHA; Mair Ns; Sharpe M Elizabeth and Holt JG. (Eds.) Pub. Williams and Wilkins. 2009:2605.
- Antunes P, Machado J, Peixe L. Characterization of antimicrobial resistance and class 1 and 2 integrons in Salmonella enterica isolates from different sources in Portugal. J Antimicrob Chemother. 2006;58(2):297-304.
Crossref - Chung YH, Kim SY, Chang YH. Prevalence and antibiotic susceptibility of Salmonella isolated from foods in Korea from 1993 to 2001. J Food Prot. 2003;66(7):1154-1157.
Crossref - Dominguez C, Gomez I, Zumalacarregui J. Prevalence of Salmonella and Campylobacter in retail chicken meat in Spain. Int J Food Microbiol. 2002;72(1-2):165-168.
Crossref - Duffy G, Cloak OM, O’Sullivan MG, et al. The incidence and antibiotic resistance profiles of Salmonella spp. on Irish retail meat products. Food Microbiol. 1999;16:623-631.
Crossref - Harrison WA, Griffith CJ, Tennant D, Peters AC. Incidence of Campylobacter and Salmonella isolated from retail chicken and associated packaging in South Wales. Lett Appl Microbiol. 2001;33(6):450-454.
Crossref - Jordan E, Egan J, Dullea C, et al. Salmonella surveillance in raw and cooked meat and meat products in the Republic of Ireland from 2002 to 2004. Int J Food Microbiol. 2006;112(1):66-70.
Crossref - Van Nhiem D, Paulsen P, Suriyasathaporn W, et al. Preliminary analysis of tetracycline residues in marketed pork in Hanoi, Vietnam. Ann NY Acad Sci. 2006;1081:534-542.
Crossref - Zhou CY, Xia Q, Du LH, et al. Recent developments in off-odor formation mechanism and the potential regulation by starter cultures in dry-cured ham. Crit Rev Food Sci Nutr. 2023;63(7):8781-8795.
Crossref - Zhu YL, Wang W, Zhang YL, et al. Characterization of quality properties in spoiled mianning ham. Foods. 2022;11(12):1713.
Crossref - Zhou CY, Zhan G, Pan DD, et al. Charactering the spoilage mechanism of “three sticks” of Jinhua ham. Food Sci Hum Wellness. 2022;11(5):1322-30.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.