ISSN: 0973-7510
E-ISSN: 2581-690X
The prevalence of antibiotic resistance among Gram-negative bacteria, particularly Enterobacterales, is rising. Extensively drug-resistant (XDR) Enterobacterales demonstrate nonsusceptibility to all except two or fewer classes of antibiotics, where it retains susceptibility to at least one agent. Besides tigecycline, colistin and polymyxin B are often the only available therapeutic options in developing countries. The aim of this study was to determine the susceptibility of XDR Enterobacterales to colistin, polymyxin B, and tigecycline by determining the MIC using microbroth dilution and analyzing the treatment outcome. A descriptive study was done at Mahatma Gandhi Medical College and Research Institute, Pondicherry, from May 2023 to July 2023. The study included non-ICU patients aged 18 years or older, who had infections caused by XDR Enterobacterales isolated from clinical specimens during the study period and provided informed consent. All quantitative measurement values in this study were analyzed using descriptive statistical methods. Colistin and polymyxin B MIC of 109 clinical isolates of XDR Enterobacterales were tested by microbroth dilution. Tigecycline MIC was determined for 73 of these isolates. Forty-eight patients received colistin or polymyxin B monotherapy and their treatment outcomes were documented. Out of the 109 XDR isolates, 16 (14.7%) were resistant to colistin, while 11 (10.1%) were resistant to polymyxin B. Tigecycline MIC values ranged from 0.06 µg/mL to 4 µg/mL. Successful treatment outcome was observed in 23.5% of patients with colistin and/or polymyxin B resistant isolates, whereas it was 70.9% in patients with colistin and polymyxin B intermediate isolates. The present study revealed that K. pneumoniae emerged as the predominant isolate among XDR Enterobacterales in our healthcare facility. Although only a small proportion of strains exhibited resistance to polymyxin B, colistin, and tigecycline, the treatment outcomes were notably poor in the case of colistin and/or polymyxin B resistant strains, underscoring the grave therapeutic limitations posed by these resistant pathogens.
Drug Resistance, Enterobacteriaceae, Lipopeptides
Gram-negative bacteria, especially Enterobacterales are increasingly becoming resistant to antibiotics commonly used in community and healthcare settings throughout the world.1 Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) Enterobacterales have emerged as an indisputable therapeutic challenge and a global concern in current years. As per the European Centre for Disease Prevention and Control, annually over 6,70,000 infections and 33,000 deaths in Europe were associated with MDR strains.2,3 Conventional and high-end antibiotics such as fluoroquinolones, aminoglycosides, beta-lactam/beta-lactamase inhibitor combinations, and carbapenems are progressively losing therapeutic effectiveness against the infections caused by MDR & XDR Enterobacterales. Despite the challenges of drug development, new antibiotics such as tigecycline, eravacycline, cefiderocol, plazomicin, ceftolozane-tazobactam, ceftazidime-avibactam, meropenem-vaborbactam, imipenem-relebactam have been introduced in therapy.4 However, the unknown adverse reaction profile, uncertain therapeutic efficacy as monotherapy or in combination with other drugs, unstandardized dosage regimens, and resistance detection methods besides the exorbitant cost of these newer antibiotics adversely affect patient care.5,6 Moreover, the availability of these new antibiotics is greatly limited in developing countries like India. Therefore, older antibiotics like polymyxin B, colistin, and fosfomycin have been reintroduced in the treatment of drug-resistant Enterobacterales infections. In our hospital, polymyxin B, colistin, and tigecycline are employed as a cost-effective alternative. There is a gap in the literature regarding the therapeutic efficacy of these antibiotics and their impact on prognosis. Furthermore, CLSI guidelines provide no interpretation range for tigecycline, and only intermediate and resistant range values for Colistin & polymyxin B are given. Hence, it is imperative to consider minimum inhibitory concentration (MIC) values while analyzing the therapeutic responses. Only a few studies have adequately examined XDR Enterobacterales infections with their MIC distribution which is essential for formulating an effective infection control policy.7 This study was undertaken to address these aspects. The aim of this study was to determine the susceptibility of XDR Enterobacterales for colistin, polymyxin B, and tigecycline by determination of MIC using microbroth dilution and analyze the treatment outcome.
This descriptive study was conducted at the Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Pondicherry, over a period of three months (from May to July 2023) after obtaining the ethical committee’s approval.
Inclusion criteria
Non-duplicate isolates of XDR Enterobacterales recovered from blood, urine, exudate, and respiratory specimens from non-ICU patients of age ≥18 years during the period of study were included for determining MIC of colistin, polymyxin B, and tigecycline. To evaluate treatment outcomes, all consecutive patients who received colistin and/or polymyxin B therapy for infections caused by XDR Enterobacterales and provided informed consent were enrolled in the study.
Exclusion criteria
Non-XDR Enterobacterales isolates and other pathogens, i.e. non-Enterobacterales isolates were excluded. Patients who were unwilling to participate or not given informed consent and patients with less than 48 hours of hospital stay were excluded from the study. Patients without clinical findings or laboratory parameters or host response against infection (i.e. direct smear examination, quantification of culture, WBC count, ESR, CRP) indicating infection were excluded to rule out colonizers.
Study procedure
A total of 7,150 clinical specimens comprising blood, urine, exudate, and respiratory samples received in the department of microbiology during the study period (time-bound study) were processed and the isolates were identified by standard Microbiology protocols. Antibiotic susceptibility testing was done against a panel of antibiotics comprising cotrimoxazole, ampicillin, ceftriaxone, ceftazidime, cefoperazone-sulbactam, piperacillin-tazobactam, ciprofloxacin, norfloxacin, gentamicin, amikacin, imipenem and meropenem by Kirby-Bauer disc diffusion method as per CLSI 2023 guidelines.7 An isolate was considered as MDR if it showed non-susceptibility to at least a minimum against one drug in three or more antibiotic categories and XDR if it showed non-susceptibility to at least one drug in all but two or fewer antibiotic categories.8 Among these samples, 2,427 showed bacterial growth. Out of the 2,427 samples, 371 (15.2%) gram-negative isolates were identified as multidrug-resistant, of which 140 (5.7%) were classified as extensively drug-resistant (XDR). Among these XDR isolates, 109 were Enterobacterales and 31 were nonfermenters.
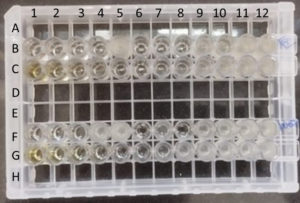
These 109 XDR Enterobacterales were further tested for their MIC breakpoints towards polymyxin B and colistin. Similarly, tigecycline MIC was determined for 73 isolates (except for urine and blood isolates). We used commercially available Micropro-BMD kits (Microexpress, Goa, India) based on the principle of microbroth dilution method to determine the MIC of polymyxin B, colistin (Lot number: BDCP0823), and tigecycline (Lot number: BDTG1123) (Figure). The kit provides an array of 12 wells assembled in a strip. The wells were pre-coated with a two-fold dilution series of an antimicrobial agent. The inoculum is prepared by picking up one or two similar, isolated fresh colonies from the overnight plate and emulsified in 2 ml saline broth. After vortexing gently, 50 µL of this test broth is transferred into the broth provided by the company kit. By gentle mixing, 200 µL of mixed broth from this vial was dispensed into all the wells in each individual MIC strip (polymyxin B and colistin, tigecycline) using sterile tips in an aseptic manner. It was incubated overnight at 35 °C. MIC was determined by observing the lowest concentration of the antimicrobial that inhibits any visible growth of the bacterial isolate. E. coli ATCC 25922 (colistin/ polymyxin B and tigecycline sensitive) was used for the quality control of the microbroth dilution method. MIC breakpoints of polymyxin B and colistin were interpreted as per the CLSI 2023 guidelines and MIC of tigecycline as per US Food and Drug Administration (FDA)-Identified Interpretive Criteria for Tigecycline-Injection products; 2019.9,10
Figure. Microbroth dilution assay for colistin, polymyxin B, and tigecycline.
Six wells of row B & F were used for determination of colistin & another six wells for polymyxin B MIC from left to right side for two bacterial isolates. Row C & G were used for determination of tigecycline MIC of two isolates
No patients received tigecycline during the study period, hence the assessment therapeutic outcome was limited to patients receiving colistin or polymyxin B. Patients treated with colistin and/or polymyxin B were followed up until their discharge, referral to another hospital, or death. Therapeutic outcomes were assessed after 15 days of therapy.
Statistical analysis
All data were entered in an MS Excel sheet and quantitative measurement values in this study were analyzed by descriptive statistical method.
During the period of study, a total of 109 XDR isolates of Enterobacterales, i.e. 96 (88%) Klebsiella pneumoniae, 7 (6.4%) Escherichia coli, and 6 (5.5%) Citrobacter spp., were recovered from various clinical samples. Pus/exudate 36 (33%) and urine 30 (27.5%) were identified as the most frequently encountered sample types associated with the XDR strains, followed by endotracheal aspirates, sputum, blood, and tissue samples. The specimen-wise distribution of the XDR Enterobacterales from various sites of infections is listed in Table 1.
Table (1):
Specimen-wise distribution of the XDR Enterobacterales from various sites of infections
Exudate |
Urine |
Endotracheal aspirate |
Sputum |
Blood |
Tissue |
|
|---|---|---|---|---|---|---|
K. pneumoniae |
34 (35.4%) |
19 (19.8%) |
26 (27.1%) |
9 (9.4%) |
6 (6.3%) |
2 (2.1%) |
E. coli |
1 (14.3%) |
6 (85.7%) |
0 |
0 |
0 |
0 |
Citrobacter spp. |
1 (16.7%) |
5 (83.3%) |
0 |
0 |
0 |
0 |
Total |
36 (33%) |
30 (27.5%) |
26 (23.9%) |
9 (8.3%) |
6 (5.5%) |
2 (1.8%) |
In our study, XDR Enterobacterales constituted 4.5% of the samples exhibiting bacterial growth. The majority of XDR isolates were K. pneumonia 96 (88.1%), followed by E. coli 7 (6.4%) and Citrobacter spp. 6 (5.5%). These XDR isolates were predominantly recovered from pus/exudate 36(33%) and urine samples 30 (27.5%), followed by endotracheal aspirates 26 (23.9%), sputum 9 (8.3%), blood 6 (5.5%), and tissue specimens 2 (1.8%) (Table 1).
The 109 XDR Enterobacterales isolates were further tested by microbroth dilution method for polymyxin B and colistin. 16 (14.6%) isolates were found to have colistin resistance, and 11 (10.1%) isolates had polymyxin B resistance. Among the 16 colistin-resistant isolates, 10 were also resistant to polymyxin B and the remaining 6 isolates had polymyxin B intermediate susceptibility. The 16 colistin-resistant strains included 14 (87.5%) isolates of K. pneumoniae and 2 (12.5%) isolates of Citrobacter spp. from urine samples (Table 2). The colistin MIC50 and MIC90 values were 0.5 µg/mL and 4 µg/mL for K. pneumoniae, 0.5 µg/mL and 2 µg/mL for E. coli, and 1 µg/mL and 4 µg/mL for Citrobacter spp. respectively. Similar patterns were observed in polymyxin B-resistant strains which included 10 (90.1%) K. pneumonia and 1 (9.1%) E. coli isolate. The MIC50 and MIC90 for polymyxin B were 0.5 µg/mL and 4 µg/mL for K. pneumoniae and E. coli, and 0.5 µg/mL and 1 µg/mL for Citrobacter spp. respectively.
Table (2):
Colistin & Polymyxin B MIC distribution of XDR Enterobacterales strains
| Colistin MIC (µg/mL) | Polymyxin B MIC (µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intermediate | Resistant | Intermediate | Resistant | |||||||||
| 0.12 | 0.25 | 0.5 | 1 | 2 | ≥4 | 0.12 | 0.25 | 0.5 | 1 | 2 | ≥4 | |
| K. pneumoniae (n = 96) | 11 | 32 | 15 | 15 | 9 | 14 | – | 23 | 29 | 32 | 2 | 10 |
| • Exudate (n = 34) | 1 | 15 | 8 | 4 | 5 | 1 | – | 10 | 13 | 10 | – | 1 |
| • Urine (n = 19) | – | 5 | 3 | 4 | 1 | 6 | – | 3 | 2 | 7 | 2 | 5 |
| • ET aspirate (n = 26) | 6 | 7 | 3 | 5 | 2 | 3 | – | 5 | 11 | 10 | – | – |
| • Sputum (n = 9) | 2 | 2 | – | 1 | – | 4 | – | 2 | – | 3 | – | 4 |
| • Blood (n = 6) | 2 | 2 | – | 1 | 1 | – | – | 3 | 3 | – | – | – |
| • Tissue (n = 2) | – | 1 | 1 | – | – | – | – | – | – | 2 | – | – |
| E. coli (n = 7) | – | 2 | 3 | – | 2 | – | – | 2 | 3 | – | 1 | 1 |
| • Urine (n = 6) | – | 2 | 3 | – | 1 | – | – | 2 | 3 | – | 1 | – |
| • Exudate (n = 1) | – | – | – | – | 1 | – | – | – | – | – | – | 1 |
| Citrobacter spp. (n = 6) | – | – | – | 3 | 1 | 2 | – | 1 | 1 | 4 | – | – |
| • Urine (n = 5) | – | – | – | 2 | 1 | 2 | – | 1 | 1 | 3 | – | – |
| • Exudate (n = 1) | – | – | – | 1 | – | – | – | – | – | 1 | – | – |
Tigecycline MIC was determined by the microbroth dilution method for 73 isolates. Thirty isolates from urine and 6 from blood were excluded from tigecycline MIC testing as it has questionable utility in urinary and bloodstream infections owing to its low concentrations achieved in serum and urine.11 Only one K. pneumoniae had MIC in the intermediate range (MIC of 4 µg/mL) for tigecycline and no other Enterobacterales displayed resistance to tigecycline. The tigecycline MIC distributions of these strains are displayed in Table 3. Among the 73 Enterobacterales isolates tested i.e. 71 (97.2%) K. pneumoniae, 1 (1.3%) E. coli, and 1 (1.3%) Citrobacter spp., none of the isolates displayed tigecycline resistance (MIC ≥ 8 µg/mL), and their MIC values ranged from 0.06 µg/mL to 4 µg/mL. The tigecycline MIC50 and MIC90 among the K. pneumoniae isolates were 0.25 µg/mL and 0.5 µg/mL, respectively. One K. pneumoniae isolate from an exudate sample showed an intermediate MIC of 4 µg/mL.
Table (3):
Tigecycline MIC distribution of XDR Enterobacterales strains
| Sensitive | Interm-ediate | Resistant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 µg/mL | 0.06 µg/mL | 0.12 µg/mL | 0.25 µg/mL | 0.5 µg/mL | 1 µg/mL | 2 µg/mL | 4 µg/mL | 8 µg/mL | 16 µg/mL | 32 µg/mL | |
| K. pneumoniae (n = 71) | – | 1 | 17 | 27 | 20 | 5 | – | 1 | – | – | – |
| • Exudate (n=34) | – | 1 | 8 | 11 | 9 | 4 | – | 1 | – | – | – |
| • Endotracheal aspirate (n = 26) | – | – | 9 | 10 | 7 | – | – | – | – | – | – |
| • Sputum (n = 9) | – | – | – | 6 | 3 | – | – | – | – | – | – |
| • Tissue (n = 2) | – | – | – | – | 1 | 1 | – | – | – | – | – |
| E. coli (n = 1) | – | – | 1 | – | – | – | – | – | – | – | – |
| Citrobacter spp. (n = 1) | – | – | – | 1 | – | – | – | – | – | – | – |
Among the 109 patients diagnosed with XDR Enterobacterales infections, 48 (44%) individuals, i.e. 39 (81%) males, and 9 (18.7%) females, received either colistin or polymyxin B therapy in this study. Out of 48 patients, 17 Enterobacterales isolates were colistin and/or polymyxin B resistant and 31 were colistin and polymyxin B intermediate. The majority of the patients were in the above 60 years age group (n = 39, 81.2%), followed by 40-60 years (n = 5, 10.4%), 20-40 years (n = 2, 4.1%), and 18-20 years age group (n = 2, 4.1%). All these patients were symptomatic, had signs of infection, and had elevated CRP.
Before the initiation of colistin or polymyxin B therapy, 18 (37.5%) patients had already undergone empirical antibiotic treatment and the antibiotics were ceftriaxone, cefepime, amoxicillin-clavulanate, piperacillin-tazobactam, imipenem, meropenem, norfloxacin levofloxacin, gentamicin, clindamycin, and vancomycin. Polymyxin B was administered in 13 (27%) and colistin in 35 (72.9%) patients. We found successful treatment outcomes in 54% (26 out of 48) of cases in patients treated with colistin or polymyxin B. As per the International Consensus Guidelines on Polymyxins, colistimethate sodium IV 300 mg CBA loading dose followed by maintenance doses and polymyxin B 2.0-2.5 mg/kg loading dose followed by 1.25-1.5 mg/kg every 12 hours was administered to patients with normal renal function.12 Among the patients with colistin and/or polymyxin B-resistant Enterobacterales infections (MIC ≥ 4 µg/mL), a successful treatment outcome was observed in 23.5% (4 out of 17) of cases. In contrast, patients with colistin and polymyxin B intermediate isolates (MIC ≤ 2 µg/mL) experienced successful treatment outcomes in 70.9% (22 out of 31) cases. Most of these (91%) cases with successful outcomes had colistin and polymyxin B MIC between 0.25 and 0.5 µg/mL.
With the worldwide emergence of carbapenemase-producing strains, multidrug resistance and extensive drug resistance among Enterobacterales have surfaced as a significant concern in recent years. Although rational use of antibiotics, antibiotic stewardship, and infection control measures are strictly observed in most hospitals, the use of antibiotics in poultry and livestock farming and veterinary medicine is still unrestricted.3 Colistin has been employed not only for its therapeutic and prophylactic applications in veterinary medicine but also as a growth promoter in animal feed. Ceftazidime-avibactam, plazomicin, eravacycline, and cefiderocol are among the newer treatment options introduced to resolve the therapeutic crisis.13 However, the greater healthcare expenditure and non-availability of these newer antibiotics have limited their use in low and middle-income countries where the treatment of MDR & XDR Enterobacterales is still largely dependent on old classic antibiotics like polymyxin B, colistin, and fosfomycin.6 Polymyxins belong to the lipopeptides class of antibiotics. Among the different members of this class, only polymyxin B and colistin (polymyxin E) are in clinical use. Colistin was discovered in Bacillus polymyxa in 1949 and utilized for therapeutic purposes since 1959.5 However, the use of colistin and polymyxin B gradually declined due to their high potential to cause nephrotoxicity. The acute scarcity of novel antibiotics effective against MDR gram-negative bacteria has led to the revival of the attention on polymyxin B and colistin as treatment options.5 The antibacterial action of polymyxin B and colistin is based on their positive charge which allows them to bind to the negatively charged phosphate groups of lipid A and displace Ca2+ and Mg2+ ions disrupting the integrity of gram-negative bacterial lipopolysaccharide (LPS) and outer membrane.3 Furthermore, colistin through its hydrophobic domain gets integrated into the outer membrane which leads to increased membrane permeability, leakage of periplasmic molecules, and cell death. Unlike polymyxins, tigecycline, a glycylcycline antibiotic approved by the FDA in 2005, inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit, thus preventing the integration of amino acids into the growing peptide chain.14 Colistin resistance is attributed to plasmid-mediated colistin resistance (mcr-1-5) genes as well as mutation of chromosomal genes associated with LPS modifications, efflux pumps, or two-component regulatory systems.3 Whereas, tigecycline resistance is mostly due to the high expression of efflux pumps like AcrAB, MexXY, and AdeABC.14
The incidence of XDR Enterobacterales exhibits considerable variability across different geographic regions, specimen types, and temporal periods. It was 4.5% in the present study. Surveillance data from Canada reported the incidence of XDR Enterobacterales was 0.13 per 1,000 admissions in 2019.15 The highest isolation of XDR strains was from stool/rectal swabs (73%), followed by urine (11%), blood (6.2%), wound (1.5%), and respiratory samples (3.1%).15 In another cross-sectional study from India, Basak et al. described a 12.1% incidence of XDR.16 Among these isolates, Pseudomonas aeruginosa and K. pneumoniae accounted for 32.2% and 27.8%, respectively, making them the predominant species identified.16 In contrast, a multicentric study detected a lower incidence of XDR Enterobacterales in the US between 2018 and 2020. The incidence was 0.4% in non-ICU areas and 1.2% in ICU.17
As per the ICMR AMR annual report 2019, the susceptibility to imipenem in E. coli has shown a consistent decline from 86% in 2016 to 64% in 2021, whereas in K. pneumonia imipenem susceptibility declined from 65% in 2016 to 43% in 2021 in India.18 This decline is mostly due to the production of various carbapenemases such as KPC, NDM, and OXA-48, resulting in the emergence of XDR Carbapenem-resistant Enterobacterales (CRE).3 All these study isolates were also found to be carbapenem-resistant. Polymyxin B, colistin, and tigecycline are considered the antibiotics of final recourse in treating such infections in developing nations. Hence, it is imperative to routinely determine and judicially report the susceptibility of these antibiotics for XDR isolates. The microbroth dilution method is the reference method of antibiotic susceptibility testing for polymyxin B, colistin, and tigecycline.19,20 We determined polymyxin B and colistin susceptibility for 109 isolates and Tigecycline susceptibility for 73 isolates by the microbroth dilution method. Since Tigecycline is not recommended for the bloodstream and urinary infections,11 30 isolates from urine and 6 from blood were excluded from Tigecycline MIC testing.
In the present study, CLSI breakpoints were followed for the interpretation of colistin and polymyxin B MIC breakpoints. MIC ≥ 4 µg/mL was considered resistant. Colistin resistance was detected in 14 K. pneumoniae and 2 Citrobacter spp., whereas polymyxin B resistance was found in 10 K. pneumoniae and 1 E. coli. The MIC50 and MIC90 represent the concentrations of an antibiotic that can effectively inhibit the growth of 50% and 90% of the isolates, respectively. The MIC50/ MIC90 value for colistin varies based on the local prevalence of resistant strains, hospital settings, and the method used for testing.20 In a study from Pakistan, colistin MIC50 and MIC90 among 251 CRE strains were found to be 0.5 µg/mL and 16 µg/mL, respectively, by broth microdilution and VITEK 2.6 However, Bir et al. reported a lower MIC for colistin.20 In their study, MIC50/MIC90 was 0.5/8 µg/mL in K. pneumoniae and 0.5/4 µg/mL in E. coli isolates. Despite these variations, a higher MIC50/MIC90 has been observed in K. pneumoniae in comparison to other Enterobacterales in most studies.20,21
Due to the absence of MIC breakpoints for tigecycline for Enterobacterales in the CLSI guidelines, the US FDA breakpoints were followed, which classify isolates as susceptible if MIC ≤ 2 µg/mL, intermediate if MIC = 4 µg/mL, and resistant if MIC ≥ 8 µg/mL.10 Among 73 isolates, there was no tigecycline resistance and the MIC values ranged from 0.06 µg/mL to 4 µg/mL. Sader et al. found tigecycline MIC50 of 0.12 µg/mL and MIC90 of 1 µg/mL among 8341 Enterobacteriaceae isolates from 13 European countries, Turkey, and Israel.22 Higher resistance was observed in a study by Li et al. They found tigecycline MIC50 2 µg/mL and MIC90 4 µg/mL among Carbapenemase-producing Enterobacteriaceae.23
In this study, 48 (44%) out of the 109 patients diagnosed with XDR Enterobacterales infections were treated with either colistin or polymyxin B. In adherence to our institutional antibiotic policy, the selection of targeted therapy for each patient with XDR Enterobacterales infections was individually determined, taking into consideration their renal parameters, co-morbidity status, and treatment cost. Tigecycline opted for patients who had infection with XDR isolates resistant to colistin or polymyxin B therapy or showed no evidence of improvement with colistin or polymyxin B therapy. However, no patients received tigecycline during the period of this study due to various factors like advanced age, co-morbidity, high cost of treatment, and lack of consent from patients or next of their kin. Ustundag et al. reported clinical success in 43.9% of patients who received colistin.24 This is in accordance with our study. However, a higher rate (about 83%) of successful treatment outcomes with colistin has been reported in another study.25 There is not much clinical evidence available on the treatment outcome of XDR infections treated with newer molecules such as plazomicin, eravacycline, and cefiderocol.4 Successful outcomes were recorded in 54.2% of cases of CRE infections treated with fosfomycin in combination with colistin or tigecycline.26 However, resistance to fosfomycin may develop during the therapy.26,27 Several studies have recognized the therapeutic efficacy of colistin and tigecycline, especially as a combination therapy with other antibiotics. Tumbarello et al. described the superiority of colistin-tigecycline-meropenem combination therapy in CRE infections.28 It has been suggested that despite in vitro resistance, meropenem, when used as a combination therapy could exert antibacterial activity at a higher dose, i.e. 2 g per 8 hours, especially if the MIC is <8 µg/mL.29 While certain studies have shown remarkable in vitro activity (82% to 92.1%) of tigecycline against CRE,23,30 a higher dose of tigecycline is also prescribed for XDR isolates.31 Although it is generally well tolerated, with occasional reports of vomiting and diarrhoea, the effectiveness of a higher dose of tigecycline remains uncertain. It is important to note that using tigecycline as monotherapy or in the bloodstream and urinary infections where suboptimal tigecycline concentrations at the infection site are expected, can lead to treatment failure and an increased risk of mortality.13
Limitations
The scope of our observation is constrained by the relatively brief duration and small sample size of the study. Moreover, the isolates in the present study originated from a single centre, implying that these findings may vary from those of other studies due to geographical differences.
XDR Enterobacterales are increasingly becoming common in Indian healthcare settings. Although the prevalence of XDR Enterobacterales in our hospital was less, K. pneumonia emerged as a predominant isolate, showing a high tendency to acquire resistance to most antibiotics. A low proportion of strains exhibited resistance to polymyxin B, colistin, and tigecycline. Despite its potential to cause nephrotoxicity and neurotoxicity, colistin and polymyxin B were employed to treat XDR Enterobacterales infections due to the high treatment cost of newer antibiotics. The treatment outcome of colistin and polymyxin B therapy was poor in patients with colistin and/or polymyxin B resistant strains in comparison to colistin and/or polymyxin B intermediate strains. However, the findings of this study are limited by its short duration and relatively small sample size. More research on this topic is essential to provide valuable insights into the potential preventive measures to treat these conditions.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The study was approved by the Institutional Human Ethics Committee, Mahatma Gandhi Medical College and Research Institute, Pondicherry, India, via letter No. MGMCRI/2022/IRC/95/04/IHEC/37.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Potter RF, D’Souza AW, Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updat. 2016;29:30-46.
Crossref - European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe – Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2018. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf. Accessed January 12, 2024.
- Binsker U, Kasbohrer A, Hammerl JA. Global colistin use: a review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol Rev. 2022;46(1):1-37.
Crossref - Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25(8):943-950.
Crossref - Falagas ME, Kasiakou SK, Saravolatz LD. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin Infect Dis. 2005;40(9):1333-1341.
Crossref - Qamar S, Shaheen N, Shakoor S, Farooqi J, Jabeen K, Hasan R. Frequency of colistin and fosfomycin resistance in carbapenem-resistant Enterobacteriaceae from a tertiary care hospital in Karachi. Infect Drug Resist. 2017;10:231-236.
Crossref - Wildfire J, Waterlow NR, Clements A, Fuller NM, Knight GM. MIC distribution analysis identifies differences in AMR between population sub-groups. Wellcome Open Res. 2024;9:244.
Crossref - Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-281.
Crossref - CLSI. Performance standards for antimicrobial susceptibility testing. 33rd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne. 2023. https://clsi.org/standards/products/microbiology/documents/m100/. Accessed January 12, 2024.
- US Food and Drug Administration. FDA-Identified Interpretive Criteria for Tigecycline- Injection products 2019. https://www.fda.gov/drugs/development-resources/tigecycline-injection-products. Accessed January 12, 2024.
- Anthony KB, Fishman NO, Linkin DR, Gasink LB, Edelstein PH, Lautenbach E. Clinical and Microbiological Outcomes of Serious Infections with Multidrug-Resistant Gram-Negative Organisms Treated with Tigecycline. Clin Infect Dis. 2008;46(4):567-570.
Crossref - Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacother J Hum Pharmacol Drug Ther. 2019;39(1):10-39.
Crossref - Thaden JT, Pogue JM, Kaye KS. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8(4):403-416.
Crossref - Yaghoubi S, Zekiy AO, Krutova M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis. 2022;41(7):1003-1022.
Crossref - Bartoszko JJ, Mitchell R, Katz K, Mulvey M, Mataseje L. Characterization of Extensively Drug-Resistant (XDR) Carbapenemase-Producing Enterobacterales (CPE) in Canada from 2019 to 2020. Microbiol Spectr. 2022;10(4):e0097522.
Crossref - Basak S, Singh P, Rajurkar M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J Pathog. 2016;2016(1):4065603.
Crossref - Sader HS, Mendes RE, Streit JM, Carvalhaes CG, Castanheira M. Antimicrobial susceptibility of Gram-negative bacteria from intensive care unit and non-intensive care unit patients from United States hospitals (2018-2020). Diagn Microbiol Infect Dis. 2022;102(1):115557.
Crossref - Indian Council of Medical Research. Annual report January 2021 to December 2021 – Antimicrobial resistance research and surveillance network. 2021. https://www.icmr.gov.in/icmrobject/custom_data/pdf/resource-guidelines/AMR_Annual_Report_2021.pdf. Accessed January 12, 2024.
- Pournaras S, Koumaki V, Spanakis N, Gennimata V, Tsakris A. Current perspectives on tigecycline resistance in Enterobacteriaceae: susceptibility testing issues and mechanisms of resistance. Int J Antimicrob Agents. 2016;48(1):11-18.
Crossref - Bir R, Gautam H, Arif N, et al. Analysis of colistin resistance in carbapenem-resistant Enterobacterales and XDR Klebsiella pneumoniae. Ther Adv Infect Dis. 2022;9:20499361221080650.
Crossref - Galani I, Adamou P, Karaiskos I, Giamarellou H, Souli M. Evaluation of ComASP™ Colistin (formerly SensiTest™ Colistin), a commercial broth microdilution-based method to evaluate the colistin minimum inhibitory concentration for carbapenem-resistant Klebsiella pneumoniae isolates. J Glob Antimicrob Resist. 2018;15:123-126.
Crossref - Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12). J Antimicrob Chemother. 2014;69(10):2713-22.
Crossref - Li H, Zhou M, Chen X, et al. Comparative Evaluation of Seven Tigecycline Susceptibility Testing Methods for Carbapenem-Resistant Enterobacteriaceae. Infect Drug Resist. 2021;14:1511-1516.
Crossref - Ustundag G, Oncel EK, Sahin A, Keles YE, Aksay AK, Ciftdogan DY. Colistin Treatment for Multidrug-Resistant Gram-Negative Infections in Children: Caution Required for Nephrotoxicity. Med Bull Sisli Etfal Hosp. 2022;56(3):427-434.
Crossref - Babar ZU, Dodani SK, Nasim A. Treatment outcome and adverse effects of colistin in adult patients with carbapenem-resistant gram-negative bacteremia from Pakistan. Int J Infect Dis. 2021;106:171-175.
Crossref - Pontikis K, Karaiskos I, Bastani S, et al. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents. 2014;43(1):52-59.
Crossref - Sojo-Dorado J, Lopez-Hernandez I, Rosso-Fernandez C, et al. Effectiveness of Fosfomycin for the Treatment of Multidrug-Resistant Escherichia coli Bacteremic Urinary Tract Infections: A Randomized Clinical Trial. JAMA Netw Open. 2022;5(1):e2137277-e.
- Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133-2143.
Crossref - Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391-400.
Crossref - Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents. 2010;35(3):240-243.
Crossref - Zha L, Pan L, Guo J, French N, Villanueva EV, Tefsen B. Effectiveness and Safety of High Dose Tigecycline for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis. Adv Ther. 2020;37(3):1049-64.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.