ISSN: 0973-7510
E-ISSN: 2581-690X
Pseudomonas aeruginosa is an important opportunistic pathogen which causes bacterial keratitis, cyctic fibrosis and other hospital acquired infections. Its ability to form biofilm provides resistance to conventional antibiotics and further corroborates the global health concerns. The antibiotic resistance by P. aeruginosa is due to highly complex cellular signaling system called quorum sensing (QS). As QS controls bacterial pathogenicity and plays a crucial role in biofilm formation, it is a promising alternative target to combat the bacterial virulence. The present study aims to determine the inhibitory activity of Plectranthus tenuiflorus extract on QS and biofilm development in P. aeruginosa PAO1. The crude plant extract inhibited the production of pyocyanin, elastase, protease and chitinase by 71.96 ± 1.82, 38.74 ± 1.29, 30.84 ± 1.20 and 44.75 ± 1.40 % respectively at sub-MIC concentration of 500 µg/ml. The production of biofilm aggravating phenotypes such as exopolysaccharides, alginate and rhamnolipid were also significantly reduced. The biofilm inhibition capability of P. tenuiflorus was further supported by light microscopic and confocal laser scanning microscopic analysis. The phytochemicals such as phytol and mosloflavone were identified from the crude extract using gas chromatography–mass spectrometry (GC-MS). The role of these phytochemicals in down regulation of QS in P. aeruginosa was further confirmed by in silico studies targeting transcriptional receptors, LasR and RhlR of the QS regulatory network. The in vitro and docking studies suggest the anti QS potential of P. tenuiflorus in combating the bacterial pathogenesis.
Plectranthus tenuiflorus, Quorum sensing, Biofilm, GC-MS, Pseudomonas aeruginosa, Molecular docking
Quorum sensing (QS) is a cell density dependent signaling system in bacteria that supports the survival and virulence by coordinating the production of virulence factors1. Pseudomonas aeruginosa is a highly pathogenic bacterium causing pulmonary infections in the patients suffering from cystic fibrosis2. The chronic bacterial infections and associated health consequences by P. aeruginosa are due to highly evolved QS regulatory network. The QS phenomenon in P. aeruginosa comprises of highly recognized and intricately co-related cascade of four signaling systems such as LasI/R, RhlI/R, quinolone based PQS and integrated quorum sensing (IQS) systems3. Among the four interconnected QS hierarchy, the Las and Rhl system constitute two autoinducers synthase and their cognate receptors. The signaling molecules (autoinducers) generated from their respective AHL synthases bind with cognate receptors and control the expression of pyocyanin, exotoxins, elastolytic and proteolytic enzymes 2. The QS regulatory network also involves in the formation, development and maturation of highly persistent biofilm matrix. The biofilm matrix is made of exopolysaccharides, rhamnolipids and alginates, which provide resistance to conventional antimicrobial therapy4. As QS mediates expression of virulence factors and controls development of biofilm, it provides novel platform for the development of anti-infective therapy.
In this context, from last few decades extensive research is being carried out in exploiting novel therapeutic agents targeting the bacterial QS and biofilm formation. Among the different strategies used to disrupt bacterial QS network, plant derived phytochemicals with proven biological activities represent an extensive class of quorum sensing inhibitors (QSIs)5. India represents one of the most important hotspots of medicinal plants with biodiversity. North-East India forms an important stretch of hotspot of medicinal plants with widespread distribution and rich variety of medicinal plants which have not been explored yet, thereby providing ample avenues for biomedical and pharmaceutical applications6. P. tenuiflorus is an important medicinal and ornamental shrub belongs to Lamiaceae family with ethnomedicinal importance. The folkloric use of P. tenuiflorus is well established as antiseptic, antioxidant, antimicrobial agent and for the treatment of nausea, abdominal disorders and respiratory infections7. In the present study, the anti QS and anti-biofilm activity of P. tenuiflorus was evaluated against P. aeruginosa PAO1 followed by docking studies.
Chemicals and reagents
For determination of enzymatic activity, azocasein, elastin congo red (ECR) and chitin azure were purchased from Sigma Aldrich, USA. For other biological assays, chemicals like methanol, orcinol, crystal violet and acridine orange, reagents such as hydrochloric acid (HCl), sulphuric acid (H2SO4), calcium chloride (CaCl2), Tris-HCl and dimethyl sulfoxide (DMSO) and the media Luria Bertani broth (LB broth), and LB agar were procured from HiMedia laboratories, India.
Collection of Plants and Extract Preparation
The leaf samples of P. tenuiflorus were collected from Manipur, India. The collected samples were cleaned and shade dried. The dried plant materials were grounded into fine powder. The powdered sample (10 g) was extracted with methanol (100 ml) by continuous stirring for 48 h. After incubation, the solution was filtered, rotaevaporated and the obtained crude extract was stored for biological activities 8.
Maintenance of culture
The anti QS activity of P. tenuiflorus was evaluated against biomarker strain, Chromobacterium violaceum (MTCC 2656) and test microrganism, P. aeruginosa PAO1 (MTCC 2453). The test cultures were obtained from IMTECH, Chandigarh, India.
Screening of crude extracts for anti QS activity
The preliminary anti QS activity of P. tenuiflorus crude extract was tested against C. violaceum (biomarker strain) and P. aeruginosa PAO1 using standard agar well diffusion method. Briefly, overnight bacterial culture was spread on top of the agar plates using sterile cotton swabs. Wells of 8mm diameter were created and plant extract (500 µg/ml) was loaded. The zone of inhibition was measured after 24 h of incubation9.
Determination of Minimal inhibitory concentration (MIC)
According to Clinical and Laboratory Standards Institute (CLSI, 2014), broth macrodilution method was used to determine the MIC of P. tenuiflorus extract against P. aeruginosa PAO1. All the biological activities were carried out at sub-MIC concentration10.
Violacein inhibition assay
Briefly, C. violaceum was incubated in presence of P. tenuiflorus extract at 30°C for 24 h. After incubation, insoluble violacein was precipitated by centrifugation. DMSO was added to the pellet to solubilize the violacein pigment. The cell debris was removed by recentrifugation and the absorbance of violacein containing supernatant was determined at 585 nm11.
Inhibition of QS controlled virulence in P. aeruginosa PAO1
The production of pyocyanin, elastase, protease and chitinase are the important virulence factors which are regulated by QS. The inhibitory effect of P. tenuiflorus methanolic extract on the production of these virulence factors was evaluated. For pyocyanin inhibition, P. aeruginosa PAO1 was incubated in presence of P. tenuiflorus extract at 30°C for 18 h. Pyocyanin pigment from cell-free supernatant was extracted using chloroform (5:3 ratio). The pyocyanin containing chloroform phase (blue organic phase) was re-extracted with 0.2 M HCl. The absorbance of the upper aqueous layer was determined at 520 nm12. The ablity of P. tenuiflorus extract on Staphylolytic activity of P. aeruginosa PAO1 was examined as per the standard protocol13.
For elastolytic activity, bacterial cell-free supernatant was mixed with ECR buffer (100 mM Tris, 1 mM CaCl2, pH 7.5) and incubated at 37°C for 3 h. After incubation, insoluble ECR was separated and the absorbance of the supernatant was measured at 495 nm14. For LasA protease activity, the cell free supernatant of P. tenuiflorus treated PAO1 was mixed with 0.3% azocasein (50 mM Tris-HCl, pH 7.8) and incubated for 30 min. After incubation, undigested substrate was pelleted and the absorbance was determined at 400 nm15. For chitinase activity, P. aeruginosa PAO1 was added with sodium citrate buffer (0.1 M, pH 4.8) containing chitin azure (0.5 mg/ml). The reaction mixture was incubated for 7 days with continuous stirring condition (100 rpm). After incubation, the undigested chitin azure was removed and absorbance was measured at 570 nm11.
Inhibition of bacterial motility
In P. aeruginosa PAO1, motility plays a crucial role during biofilm formation. The inhibitory potential of P. tenuiflorus extract on swimming and swarming motility was evaluated. Overnight grown P. aeruginosa PAO1 treated with plant extract was point inoculated into swimming medium (agar 0.3%, tryptone 1%, NaCl 0.5%) and swarming medium (0.5% agar, 0.5% filter sterilized glucose, 1% peptone, 0.5% NaCl) and incubated at 37°C16.
Inhibition of biofilm formation in P. aeruginosa PAO1
The process of biofilm formation and development is regulated by QS network. The biofilm is composed of carbohydrate residues like exopolysaccharides, rhamnolipids and alginate which are responsible for antibiotic resistance. The effect of methanolic extract on biofilm formation was evaluated. For biofilm inhibition, crystal violet assay was performed with slight modification. Overnight grown P. aeruginosa PAO1 was incubated in the freshly prepared media on treatment with plant extract for 16-18 h. After incubation, planktonic cells containing media were removed and the biofilm containing microtiter plate was washed with PBS to remove excess of planktonic cells. The biofilm cells were then stained with crystal violet (0.4%) for 10 min. After incubation, the crystal violet bound biofilms were dissolved in 95% ethanol and the optical density was measured at 540 nm16.
For EPS inhibition assay, P. tenuiflorus extract treated P. aeruginosa PAO1 was centrifuged and the resulting pellet was resuspended in high salt buffer. The solution was recentrifuged and to the supernatant, chilled ethanol was added and incubated overnight (4°C). The extracted EPS was then quantified by standard phenol-sulphuric acid method17. The effect of P. tenuiflorus extract on alginate production was evaluated as per the standard carbazole method. In brief, P. aeruginosa PAO1 culture treated with P. tenuiflorus extract was mixed with boric acid-sulphuric acid solution. The reagent mixture was vortexed followed by addition of carbazole solution (0.2%) and incubated at 55°C for 30 min. After the incubation, optical density was measured at 530 nm18. For rhamnolipids inhibition, modified orcinol method was followed according to the method described by Banerjee et al. (2017)19.
Cell surface hydrophobicity is crucial for bacterial adhesion during biofilm formation. The effect of P. tenuiflorus extract on bacterial CSH was evaluated by microbial adhesion to hydrocarbon (MATH) assay. Briefly, overnight culture of P. aeruginosa PAO1 cultivated in the presence of crude plant extract was centrifuged at 12,000 rpm for 5 min. The pellet was washed three times with phosphate buffer and finally resuspended in ice-cold phosphate buffer. The absorbance of the resuspension was measured at 600 nm (A0). To the resuspended mixture, toluene (an aromatic hydrocarbon) was added and vigorously vortexed. After vortexing, the reaction mixture was allowed for phase separation and the absorbance of the aqueous phase was determined (A1) and hydrophobicity (%) was calculated 20.
Hydrophobicity (%) = [1 – (A1/A0)] × 100
The effect of P. tenuiflorus extract on biofilm formation in P. aeruginosa PAO1 was evaluated by light and confocal laser scanning microscopic (CLSM) analysis. For microscopic studies, overnight P. aeruginosa PAO1 culture was inoculated into fresh growth medium containing cover glass of 1 × 1 cm in 24-well Microtiter plate along with crude plant extract and incubated for 16 h. After incubation, the cover glasses were rinsed with distilled water to remove the planktonic cells. For light microscopy, the adhered biofilms on the cover glasses were stained with 0.4% crystal violet and then visualised under a light microscope at a magnification of 40×. Meanwhile, for CLSM analysis, acridine orange was used to stain the biofilm and visualised under CLSM (Model LSM710, Carl Zeiss, Jena, Germany) 17.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
The phytochemical profile of methanolic extract of P. tenuiflorus extract was identified by GC-MS analysis. The phytochemicals were separated in the column using Helium as carrier gas at a constant flow of 1 ml/min. The phytochemicals present in the extract were identified based on the obtained spectrum and retention time using GC-MS NIST (2008) library.
Molecular docking studies
The docking studies were carried out in Schrodinger Maestro software v.11.5 (Schrodinger, LLC, New York, NY, 2018) and the binding affinity of identified phytochemicals from GC-MS analysis and natural autoinducers to transcriptional receptors, LasR and RhlR were analyzed. The ligand binding domain of LasR protein’s 3D-strcture file (PDB ID: 2UV0) was retrieved from Protein Data Bank. As there is no experimental protein 3D-structure available for RhlR, protein sequence was retrieved from Uniprot database (ID: P54292.1) and the 3D-structures were predicted using ROBETTA web server. The obtained protein structures were validated using RAMPAGE web server (http://mordred.bioc.cam.ac.uk/<“rapper/rampage.php) and the best model was selected for docking studies.
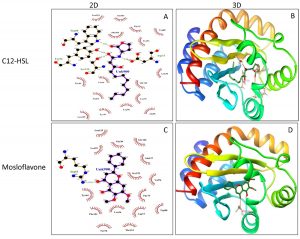
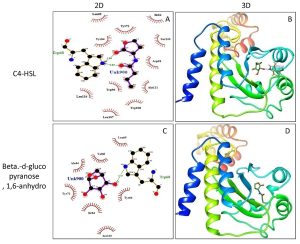
The LasR and RhlR proteins were subjected to preparation in protein preparation wizard of Maestro software v.11.5.Grid generation was performed in Glide, version 7.8 in Maestro software v.11.5, for LasR protein grid were defined around the active site residues (Tyr-56, Trp-60, Asp-73, Thr-75 and Ser-129) where autoinducer C12-homoserine lactone (C12-HSL) interacts with LasR protein whereas for RhlR, the grid was generated around the active site residue Trp-6821, 22.The above prepared grids were used for docking. The ligand compounds were obtained from PubChem database and submitted for preparation in LigPrep module in Maestro softwarev.11.5 and the prepared protein and ligand were subjected for docking. The 2D and 3D structures were generated using LigPlot+v.1.4.5 and Chimera v.1.6.2 respectively.
Statistical Analysis
All the experiments were performed in triplicates and the data was presented as mean ± standard deviation (SD). For each assay, a control experiment (without the treatment of crude extract) was performed. All the obtained results were calculated as compared to the control.
Screening of the plant extracts for anti QS activity
From the preliminary agar well diffusion assay, P. tenuiflorus extract exhibited zone of inhibition of 18 and 16 mm against biomarker strain, C. violaceum and test organism, P. aeruginosa PAO1 respectively at a concentration of 500 µg/ml.
Determination of MIC
The MIC of P. tenuiflorus extract against P. aeruginosa PAO1 was found to be 750 µg/ml and 500 µg/ml was selected as the sub-MIC.
Violacein inhibition assay
On treatment with P. tenuiflorus extract, the production of violacein in C. violaceum was significantly reduced by 80.23 ± 2.73% as compared to control.
Inhibition of QS regulated virulence factors in P. aeruginosa PAO1
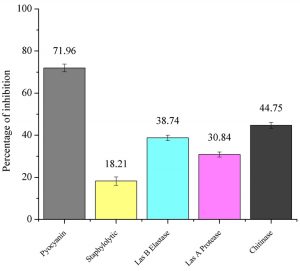
The pyocyanin production was significantly inhibited by 71.96 ± 1.82% when treated with P. tenuiflorus extract (Figure 1). It also showed promising ability in inhibiting Staphylolytic activity of P. aeruginosa PAO1 by 18.21 ± 2.01% (Figure 1). On treatment with plant extract, LasB elastase activity was inhibited by 38.74 ± 1.29%. A significant decrease in the Las A protease and chitinase activity was observed in P. aeruginosa PAO1 on treatment with P. tenuiflorus extract with an inhibition of 30.84 ± 1.20 and 44.75 ± 1.40% respectively (Figure 1).
Fig. 1. Effect of sub-MIC dose (500 µg/ml) of P. tenuiflorus extract on production of QS regulated virulence factors such as pyocyanin, Staphylolytic activity, LasB elastase, LasA protease and chitinase activity
Inhibition of bacterial motility
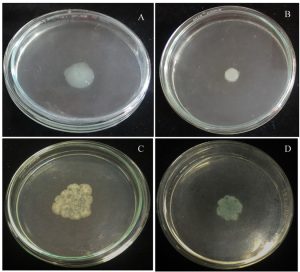
On treatment with P. tenuiflorus extract, a significant decrease in swimming and swarming motility of P. aeruginosa was observed with a reduction of 37.93 and 54.54% respectively (Figure 2).
Fig. 2. Effect of sub-MIC dose (500 µg/ml) of P. tenuiflorus extract on the inhibition of bacterial motility (swimming and swarming) as compared to untreated control. (A) Swimming motility of untreated P. aeruginosa PAO1, (B) Swimming motility of P. tenuiflorus treated P. aeruginosa PAO1, (C) Swarming motility of untreated P. aeruginosa PAO1, (D) Swarming motility of P. tenuiflorus treated P. aeruginosa PAO1
Inhibition of biofilm formation in P. aeruginosa PAO1
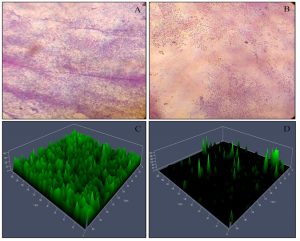
From the crystal violet based biofilm inhibition activity, it was observed that the biofilm formation was reduced by 44.03±2.00% on treatment with P. tenuiflorus extract. A significant reduction (41.08 ± 2.67%) in the production of EPS was observed in P. aeruginosa PAO1 when treated with P. tenuiflorus extract (Table 1). Meanwhile, on treatment with sub-MIC dose of P. tenuiflorus extract, alginate production in P. aeruginosa PAO1 was significantly inhibited by 37.13 ± 3.93%. The rhamnolipid production was also significantly altered on treatment with P. tenuiflorus extract with an inhibition of 34.95 ± 2.49% as compared to untreated control. The treatment with the crude extract resulted in decrease of cell to cell surface attachment of P. aeruginosa PAO1 with a hydrophobicity percentage of 29.19 ± 3.29% (Table 1). From light microscopic and CLSM analysis, a significant reduction in biofilm formation was observed in presence of P. tenuiflorus extract with dispersed bacterial cells, compared to thick and compact biofilm in untreated control (Figure 3).
Table (1):
Effect of sub-MIC dose (500 µg/ml) of P. tenuiflorus extract on production of QS regulated biofilm phenotypes in P. aeruginosa PAO1.
Sl no. |
QS regulated biofilm phenotypes |
Inhibition (%) |
|---|---|---|
1. |
Cell Surface Hydrophobicity |
29.19 ± 3.29 |
2. |
Rhamnolipid inhibition |
34.95 ± 2.49 |
3. |
Biofilm inhibition |
44.03 ± 2.00 |
4. |
EPS inhibition |
41.08 ± 2.67 |
5. |
Alginate inhibition |
37.13 ± 3.93 |
Fig. 3. Microscopic (Light microscopy and CLSM analysis) observation of P. aeruginosa PAO1 biofilm. (A) Light microscopic observation of untreated P. aeruginosa PAO1 biofilm, (B) Light microscopic observation of P. tenuiflorus treated P. aeruginosa PAO1 biofilm, (C) CLSM analysis of untreated P. aeruginosa PAO1 biofilm, (D) CLSM analysis of P. tenuiflorus treated P. aeruginosa PAO1 biofilm
GC-MS analysis
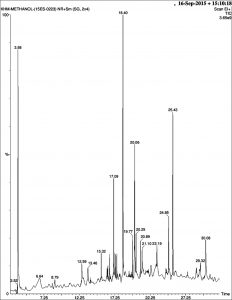
The phytochemical components identified from the methanol extract of P. tenuiflorus using GC-MS analysis were phytol, mosloflavone, N-hexadecanoic acid, Beta-D-glucopyranose, 1,6-anhydro and gamma sitosterol along with other minor phytoconstituents (Figure 4, Table 2).
Table (2):
List of phytochemicals identified from methanolic extract of P. tenuiflorus using GC-MS analysis.
Sl no. |
Phytochemicals |
Retention time (min) |
Molecular formula |
Molecular weight |
Peak area (%) |
|---|---|---|---|---|---|
1. |
Beta.-D-glucopyranose, 1,6-anhydro |
13.463 |
C6H10O5 |
162 |
3.212 |
2. |
N-Hexadecanoic acid |
18.400 |
C16H32O2 |
256 |
23.731 |
3. |
Phytol |
19.765 |
C20H40O |
296 |
4.324 |
4. |
Octadecanoic acid |
20.246 |
C18H36O2 |
284 |
2.535 |
5. |
Mosloflavone |
25.433 |
C17H14O5 |
298 |
12.526 |
6. |
Gamma sitosterol |
30.080 |
C29H50O |
414 |
4.502 |
Molecular docking studies
Molecular docking of P. teniflorus revealed that, mosloflavone exhibited a docking score of -7.55 and -6.521 kcal/mol for LasR and RhlR respectively. The binding affinity of mosloflavone for RhlR was observed to be comparatively higher than its natural ligand suggesting the molecular target of mosloflavone (Table 3, Figure 5). Apart from mosloflavone, Beta-D-glucopyranose, 1,6-anhydro also showed greater affinity towards RhlR with a docking score of -7.499 kcal/mol as compared to -5.756 kcal/mol in case of natural ligand, C4-HSL (Figure 6).
Table (3):
Interaction of bioactive constituents of P. tenuiflorus extract with QS regulators, LasR and RhlR of P.aeruginosa PAO1 expressed in terms of molecular docking energy (kcal/mol), hydrogen bonds and interacting hydrophobic residues.
| Compounds/Ligands | LasR | RhlR | ||||
|---|---|---|---|---|---|---|
| Docking score Kcal/mol |
Hydrogen bond | Hydrophobic interactions | Docking score | Hydrogen bond | Hydrophobic interactions | |
| C12-HSL (Natural ligand for LasR) | -8.489 | Tyr 56, Trp 60, Asp 73, Ser 129. | Leu 36, Leu 40, Try 47, Arg 61, Tyr 64, Val 70, Val 76, Trp 88, Tyr 93, Ala105, Phe 101, Leu 110, | — | — | — |
| C4-HSL (Natural ligand for RhlR) |
— | — | — | -5.756 | Trp 68 | Ala 44, Tyr 64, Tyr 72, Tyr 73, Asp 81, Trp 96, Leu 107, Trp 108, Ala 111 |
| Beta.-d-glucopyranose, 1,6-anhydro |
-7.035 | Typ 56, Trp 60 Ser 129 | Tyr 64, Asp 73, Phe 101, Leu 110 and Thr 115 | -7.499 | Trp 68 | Ala 44, Val 60, Leu 69, Tyr 72, Ile 84 |
| Mosloflavone | -7.555 | Arg 61 | Leu 36, Gly 38, Leu 39, Tyr 64, Asp 73, Thr 75, Phe 101, and Ser 129 | -6.521 | — | Ala 44, Tyr 64, Leu 69, Trp 68, Asp 81, Ile 84, Phe 101 and Ser 135 |
| N-hexadecanoic acid |
-3.206 |
Tyr 93 |
Leu 36, Leu 39, Leu 40, Tyr 56, Trp 60, Ala 70, Trp 88 |
-3.626 |
His 61 |
Ala 44, Tyr 45, Val 60, Tyr 64, Trp 68, Leu 69, Tyr 72, Ala 83, Trp 96 |
| Octadecanoic acid
|
-3.563 |
Leu 110 |
Leu 36, Leu 40, Ala 50, Ile 52, Tyr 56, Trp 60, Tyr 64, Pro 74, Asp 73 |
-0.600 |
His 61 |
Val 60, Tyr 64, Pro 65, Leu 69 |
Fig. 5. Molecular docking studies of mosloflavone from crude extract of P. tenuiflorus to study the binding affinity with the active site of the receptor protein, LasR compared to the natural ligand (C12-HSL). (A) 2D docked conformation of C12-HSL (natural ligand) into the active site of LasR; (B) 3D docked conformation of C12-HSL into the active site of LasR, (C) 2D docked conformation of mosloflavone into the active site of LasR, (D) 3D docked conformation of mosloflavone into the active site of LasR.
Fig. 6. Molecular docking studies of Beta-D-glucopyranose, 1, 6-anhydro from crude extract of P. tenuiflorus to study the binding affinity with the active site of the receptor protein, RhlR compared to the natural ligand (C4-HSL). (A) 2D docked conformation of C4-HSL (natural ligand) into the active site of RhlR; (B) 3D docked conformation of C4-HSL into the active site of RhlR, (C) 2D docked conformation of Beta-D-glucopyranose, 1,6-anhydro into the active site of RhlR, (D) 3D docked conformation of Beta-D-glucopyranose, 1,6-anhydro into the active site of RhlR.
Traditional medicinal plants are known for their ethnomedicinal value from several decades as the most affordable and easily accessible folkloric medicines for the treatment of various kinds of diseases including microbial infections 23. India represents a rich heritage of medicinal plants with enormous ethnomedicinal properties of which majority of medicinal plants remain unnoticed and unexplored 24. The methanolic extract of P. tenuiflorus was evaluated for its anti QS and anti-biofilm activity against P. aeruginosa PAO1. The plant extract significantly attenuated virulence factors production and biofilm formation regulated by QS regulatory network in P. aeruginosa with different potency. The production of violacein pigment, an important virulence factor of biomarker strain, C. violaceum is regulated by QS hierarchy. The methanolic extract of P. tenuiflorus significantly inhibited the production of violacein suggesting the efficacy in combating bacterial virulence 25. Pyocyanin is an important member of the phenazine compounds produced by P. aeruginosa and represents a major virulence determinant by generating reactive oxygen species (ROS) 26. In the present study, P. tenuiflorus inhibited the pyocyanin production by 71.96%. The synergistic activity of phytochemicals like phytol and mosloflavone present in the crude extract could be responsible for pyocyanin inhibition 27. The production of lytic enzymes such as LasA protease and LasB elastase by P. aeruginosa gives an aided advantage to the bacteria during host infection by enhancing the ability to degrade the host tissues 28. In the present sudy, LasA protease and LasB elastase activity were significantly altered on treatment with P. tenuiflorus extract. These results suggested the ability of crude extract in imparing the ability of P. aeruginosa PAO1 to invade and degrade host tissues during infection. Chitinase is also an extracellular enzyme produced by P. aeruginosa and plays a crucial role during host invasion and survival within the host 29. P. tenuiflorus extract significantly inhibited the chitinase activity of P. aeruginosa PAO1.
In addition to virulence factors production, the QS regulatory network also controls the formation, development and maturation of biofilm matrix, which is mainly responsible for increased antibiotic resistance 30. The effect of crude extract on biofilm formation was quantified by crystal violet staining depicting the reduction in biofilm formation. This result was in accordance with the earlier report 31. EPS and alginates not only provide assistance during bacterial adhesion but also shield the bacterial cells from oxidative stress, host immunity and antimicrobial treatment 32. The production of EPS and alginate were inhibited when treated with P. tenuiflorus extract suggesting its role in disrupting the biofilm matrix. The production of biosurfactant, rhamnolipid plays a critical role in different stages of biofilm formation. In this context, combating rhamnolipid production could be a promising target for biofilm inhibition 33. The rhamnolipid production was significantly altered on treatment with P. tenuiflorus extract suggesting its ability to inhibit biofilm formation. The bacterial motility plays an important role in the bacterial pathogenicity and biofilm formation. The swimming and swarming motility of P. aeruginosa PAO1 were significantly inhibited when treated with the plant extract suggesting its efficacy in interrupting the process of biofilm formation. These results were in accordance to the earlier report 34. The in vitro anti-biofilm efficacy of crude plant extract was further corroborated by microscopic analysis depicting the reduction in the biofilm architecture as compared to compact biofilm architecture in the untreated control.
The presence of phytol in the methanolic extract of P. tenuiflorus, as identified from GC-MS analysis suggested the efficacy of crude extract in combating QS regulated virulence and biofilm formation in P. aeruginosa PAO1 27. The in vitro results were further validated using molecular docking studies of the identified phytochemicals for their affinity towards LasR and RhlR as compared to their respective autoinducers. Among the identified phytochemicals, mosloflavone and Beta.-D-glucopyranose, 1,6-anhydro exhibited promising affinity towards LasR and RhlR respectively suggesting their role in inhibiting bacterial QS system in P. aeruginosa PAO1.
The present study illustrated the importance of P. tenuiflorus as a potent QS inhibitor and biofilm inhibitor of P. aeruginosa PAO1. The promising anti QS and anti biofilm activity exhibited by P. tenuiflorus extract was the result of synergistic activity of different phytochemicals such as phytol, mosloflavone and Beta.-D-glucopyranose, 1,6-anhydro present in the extract. The in vitro results were further validated by molecular docking studies depicting the binding ability of phytochemicals to LasR and RhlR of QS regulatory network. The present study will provide a lead in the antimicrobial drug discovery for the development of anti-infectives by targeting the QS regulated bacterial virulence and biofilm formation.
Acknowledgements
The authors are thankful to Bharathidasan University, Tiruchirappalli for using the CLSM facility. The authors would also like to thank Sophisticated Instrumentation Facility, VIT University, VIT-SIF Lab, SAS, Chemistry Division for GC-MS Analysis.
Conflict of Interest
The author(s) declare that there is no conflict of interest.
- Schuster M, Sexton D J, Diggle S P, Greenberg E P. Acyl-homoserine lactone quorum sensing: From evolution to application. Annu Rev Microbiol, 2013; 67: 43-63.
- Lee J, Wu J, Deng Y, Wang J, Wang C, Wang J, Chang C, Dong Y, Williams P, Zhang L H. A cell-cell communication signal integrates quorum sensing and stress response. Nature Chem Biol, 2013; 9: 339-343.
- Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell, 2015; 6(1): 26-41.
- Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Phramaceut Des, 2015; 21: 5-11.
- Bouyahya A, Dakka N, Et-Touys A, Abrini J, Bakri Y. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pacific J Trop Med, 2017; 10(8): 729-743.
- Shankar R, Rawat M S. Conservation and cultivation of threatened and high valued medicinal plants in North East India. Int J Biodiv Conserv, 2013; 5(9): 584-591.
- Waly N M, El Gayed S H. Botanical and biological studies of Plectranthus tenuiflorus (Vatke) Agnew. (Lamiaceae) growing in Saudi Arabia. Int J Life Sci Pharma Res, 2012; 2 (2): L52-L64.
- Mostafa A A, Al-Askar A A, Almaary K S, Dawoud T M, Sholkamy E N, Bakri M M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci, 2018; 25: 361-366.
- Burt S A, Ojo-Fakunle V T A, Woertman J, Veldhuizen E J A. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE, 2014; 9(4): e93414.
- El-Shaer S, Shaaban M, Barwa R, Hassan R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl â-naphthylamide. J Med Microbiol, 2016; 65: 1194-1204.
- Husain F M, Ahmad I, Al-thubiani A S, Abulreesh H H, AlHazza I M, Aqil F. Leaf extracts of Mangifera indica L. inhibit quorum sensing – regulated production of virulence factors and biofilm in test bacteria. Front Microbiol, 2017; 8: 727.
- Aybey A, Demirkan E. Inhibition of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa by human serum paraoxonase. J Med Microbiol, 2016; 65: 105-113.
- Mohabi S, Kalantar-Neyestanaki D, Mansouri S. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by Quercus infectoria gall extracts. Iran J Microbiol, 2017; 9(1): 26-32.
- Vasavi H S, Arun A B, Rekha P D. Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol Immunol, 2014; 58: 286-293.
- Rajkumari J, Borkotoky S, Murali A, Suchiang K, Mohanty S K, Busi S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb Pathog, 2018; 118: 48-60.
- Pattnaik S S, Ranganthan S, Ampasala D R, Syed A, Ameen F, Busi S. Attenuation of quorum sensing regulated virulence and biofilm development in Pseudomonas aeruginosa PAO1 by Diaporthe phaseolorum SSP12. Microb Pathog, 2018; 118: 177-189.
- Packiavathy I A S V, Priya S, Pandian S K, Veera Ravi A. Inhibition of biofilm development of uropathogens by curcumin – An anti-quorum sensing agent from Curcuma longa. Food Chem, 2014; 148: 453-460.
- Gopu V, Meena C K, Shetty P H. Quercetin influences quorum sensing in food borne bacteria: in-vitro and in-silico evidence. PLoS ONE, 2015; 11(1): e0148471.
- Lather P, Mohanty A K, Jha P, Garsa A K. Contribution of cell surface hydrophobicity in the resistance of Staphylococcus aureus against antimicrobial agents. Biochem Res Int, 2016; 2016: Article ID 1091290.
- Banerjee M, Moulick S, Bhattacharya K K, Parai D, Chattopadhyay S, Mukherjee S K. Attenuation of Pseudomonas aeruginosa quorum sensing, virulence and biofilm formation by extracts of Andrographis paniculata. Microb Pathog, 2017; 113: 85-93.
- Bottomley M J, Muraglia E, Bazzo R, Carfì A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem, 2007; 282: 13592-13600.
- Hussain F M, Ahmad I, Baig M H, Khan M S, Khan M S, Hassan I, Al-Shabib N A. Broad-spectrum inhibition of AHL-regulated virulence factors and biofilms by sub-inhibitory concentrations of ceftazidime. RSC Adv, 2016; 6: 27952-27962.
- Maroyi A. Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J Ethnobiol Ethnomed, 2013; 9: 31.
- Pandey M M, Rastogi S, Rawat A K S. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid base Compl Alternative Med, 2013; 2013: Article ID 376327.
- Vasavi H S, Arun A B, Rekha P D. Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pac J Trop Biomed, 2013; 3(12): 954-959.
- Chong Y M, Yin W F, Ho C Y, Mustafa M R, Hadi H A, Awang K, Narrima P, Koh C L, Appleton D R, Chan K G. Malabaricone C from Myristica cinnamomea exhibits anti-quorum sensing activity. J Nat Prod, 2011; 74(10): 2261-2264.
- Pejin B, Ciric A, Glamoclija J, Nikolic M, Sokovic M. In vitro anti-quorum sensing activity of phytol. Nat Prod Res, 2015; 29(4): 374-377.
- Darch S E, McNally A, Harrison F, Corander J, Barr H L, Paszkiewicz K, Holden S, Fogarty A, Crusz S A, Diggle S P. Recombination is a key driver of genomic and phenotypic diversity in a Pseudomonas aeruginosa population during cystic fibrosis infection. Sci Rep, 2015; 5: 7649.
- Frederiksen R F, Paspaliari D K, Larsen T, Storgaard B G, Larsen M H, Ingmer H, Palcic M M, Leisner J J. Bacterial chitinases and chitin-binding proteins as virulence factors. Microbiol, 2013; 159: 833-847.
- Gholami S, Tabatabaei M, Sohrabi N. Comparison of biofilm formation and antibiotic resistance pattern of Pseudomonas aeruginosa in human and environmental isolates. Microb Pathog, 2017; 109: 94-98.
- Issac Abraham S V, Palani A, Ramaswamy B R, Shunmugiah K P, Arumugam V R. Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res, 2011; 42(8): 658-668.
- Periasamy S, Nair H A S, Lee K W K, Ong J, Goh J Q J, Kjelleberg S, Rice S A. Pseudomonas aeruginosa PAO1 exopolysaccharides are important for mixed species biofilm community development and stress tolerance. Front Microbiol, 2015; 6: 851.
- Nickzad A, Deziel E. 2013. The involvement of rhamnolipids in microbial cell adhesion and biofilm development – an approach for control?. Lett Appl Microbiol, 2013; 58: 447-453.
- Al-Haidari R A, Shaaban M I, Ibrahim S, Mohamed G A. Anti-quorum sensing activity of some medicinal plants. African J Trad Compl Alternative Med, 2016; 13(5): 67-71.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.