ISSN: 0973-7510
E-ISSN: 2581-690X
Cyperus rotundus (Family Cyperaceae) is used as a natural drug with low cost by different local tribes in the Middle East. In this study, the antioxidant and antimicrobial activity of the fermented C. rotundus extract was determined using different assays including DPPH and FRAP, and antibacterial activity. The 1H-NMR analysis was carried out for the profiling of bioactive metabolites in the fermented C. rotundus extract. The results demonstrated strong antioxidant activity for DPPH (49.08 µg/mL) and for FRAP (46.95 mmol Fe (II)/g). The 1H-NMR–based metabolomics analysis showed the presence of 19 metabolites including lactic acid, alanine, acetic acid, succinic acid, pyruvic acid, citric acid, choline, beta glucose, betaine, acetoacetate, caffeine, lysine, ascorbic acid, fructose, xylose, alpha Glucose, sucrose, anserine and fumaric acid. The identified metabolites were in correlation to the antioxidant and antimicrobial activity measured. This study indicated the high potential of the C. rotundus fermented extract for natural remedy applications.
antimicrobial, antioxidant, lactic acid bacteria, 1H-NMR metabolomics.
There is an increasing trend in the use of traditional remedies for the basic health care worldwide. It is widely used against drug-resistant bacteria in medicine, where it is considered one of the most important reasons for unsuccessful treatment in infectious diseases. Medicinal plants are the main sources of new drugs and may substitute for regular medicines1. Moreover, medicinal plants are getting much acceptance as antioxidant agents, to enhance physical performance of the human body via preventing excessive formation of free radicals (oxidative stress)1,2.
Cyperus rotundus L. (family: Cyperaceae) is regarded as a traditionally medicinal herb that has been used for treating numerous clinical ailments such as diarrhea, diabetes, inflammation, malaria, and belly and bowel disorders. Phytochemical and pharmacological research printed that C. rotundus includes an excessive concentration of vital oils, ascorbic acids, phenolic acids, and flavonoids demonstrating its antibacterial, antioxidant, anti-inflammatory, anti-cancerous, and antimalarial4.
Nuclear magnetic resonance (NMR) considered the most advanced technique for the profiling and identifying the metabolites in complex matrices such as plant extracts. The technique is broadly used due to its ease in pattern instruction and the outcomes received are reproducible5. The NMR technique in combination with multivariate statistics evaluation (MVA) has been employed appreciably in recent studies for metabolites profiling for the discrimination of samples of same origin subjected to different processes6. Therefore, this study objective was to determine the antibacterial and antioxidant activities of C. rotundus L Fermented by lactic acid bacteria. Moreover, the metabolites in charge for the antibacterial and antioxidant activities of C. rotundus were identify by using 1H-NMR spectroscopy.
Microorganisms and culture conditions
Lactobacillus plantarum R12 obtained from Faculty of Food Science and Technology, University Putra Malaysia (UPM). The strain was maintained at -80°C in MRS broth supplemented with glycerol (60% v/v). L, plantarum was growing in 30 mL MRS and incubated at 37°C for 48 h to be activated, and the inoculum was adjusted to 10% (v/v) used for C. rotundus fermentation.
Preparation of Cyperus rotundus
C. Rotundus were bought from Alkadhemia city, Baghdad. The tubers were washed with water, air dried and cut into strips. A quantity of 300 g Cyperus rotundus and 1000 mL water were mixed and the mixture blended using home blander. The mixture was then filtered using a cotton cloth filter, autoclaved at 121°C for 30 min.
Fermentation process
The initial pH of the mixture from Section 100 mL was inoculated with 10% (v/v) of L. plantarum and incubated at 37°C for 48 h. The cells counts and pH values were determined after 48 h of fermentation. Non-fermented samples were subjected to same conditions but without starter culture.
Antioxidant Activity of Fermented Cyperus rotundus
DPPH Assay
The Radical Scavenging Activity (DPPH) determines how potent the antioxidant capacity of the fermented mixture8. The assay was performed based on the method from previous study using 2, 2-diphenyl-1-picrylhydrazyl radicals9. Briefly, in 96 wells plate, 0. 25 mL of C. rotundus extract was mixed with 1. 75 mL DPPH solution that was prepared by diluting DPPH in methanol. The plate was incubated in dark place at room temperature for 30 min and absorption was taken at 515 nm using microtiter plate reader (Biotek, EL 800) and DPPH percentage was calculated as the following:
% DPPH = [(control 515 – sample 515) / control 515] *100
FRAP Assay
Ferric reducing antioxidant power (FRAP) assay is a method used to estimate the antioxidant capacity of the fermented mixture that count on reducing the ferric-tripyridyltriazine10. FRAP reagent was prepared by mixing 2, 4, 6-tripyridyl-s-triazine (TPTZ) and FeCl3 solutions in (pH 3. 6) buffer acetic acid at the ratio of 1: 1: 10 (v / v / v). In 96 microtiter plate, 20µL sample was mixed with FRAP reagent (200µL) and incubated for 30 min at room temperature. The absorbance was measured at 593 nm and the calculations were determined using different concentrations from 0. 1 to 1 mM of ferrous sulfate as standard, and expressed as mmol Fe (II) / g DW.
Antibacterial Activity of Fermented Cyperus rotundus
The antibacterial activity of the fermented mixture was determined in following the method described by Aween et al.,11. The fermented mixture was tested against Pseudomonas aeruginosa ATCC10415, Bacillus subtilis ATCC11778 and Escherichia coli ATCC11229. Fermented mixture 100 µL was added to the wells and 100 µL nutrient broth containing 106 of each selected pathogens. The control was nutrient broth with the pathogenic bacteria only. The plates were incubated at 37°C for 24 h and the growth inhibition was measured at 600 nm by using microtiter plate reader. The antibacterial activity was calculated by subtracting the 0 h readings from the 24 h readings, then using the following mathematical equation.
1H-NMR metabolomics analysis
The method used for the extraction was adopted from Abas et al., (2013)12. Ten mg of the fermented and samples were mixed with CH3OH-d4 (0. 375 mL) and KH2PO4 buffer (0. 375 mL) in D2O (pH 6) containing 0. 1 % TSP as standard. The mixed samples were subjected to sonication for 15 min at 30°C. The samples were centrifuged at 13,000 rpm for 5 min and 600µL transferred into NMR tubes13. The NMR works on the INOVA 500 MHz variable spectrometer and at 499. 887 MHz at room temperature 26°C with 64 scans were done on every sample and verified, the acquisition time was 193 s, 3. 75µL pulse width and 1. 0 sec relaxation delay. Each sample was measurement was repeated six times. The residual water region (d 4. 70 to 4. 96) and methanol region (d 3. 28 to 3. 33) were excepted from the analysis.
Statistical analysis
MINITAB version 16 was used to analysis the Data. Statistical measures were used to evaluate the results of these samples and their controls, where one-way analysis of variance (ANOVA). The multivariate analysis was done using SIMCA-P software (Umetrics, Sweden).
DPPH Assay
Antioxidant helps to prevent, inhibit or delay oxidation in oxidizable resources and thus reduce oxidative stress and helps in free radicals scavenging14. The principle of the method is based on the ability of an antioxidant compound to scavenge DPPH free radicals produced in the reaction mixture. The DPPH of the fermented C. rotundus extract is shown in Fig. 1. Fermented C. rotundus showed very high antioxidant activity in comparison to the non-fermented extract. The antioxidant activity of the fermented C. rotundus extract was very high as indicated by the inhibition values (26. 109µg/mL), while non-fermented extract demonstrated the lowest antioxidant activity (15. 069µg/mL). It is indicated that the fermentation with L. plantarum improved the antioxidant activity in comparison to non-fermented extract. According to15. The results are in line with previous reports that showed the antioxidant activity of C. rotundus L. in water and ethanol extract16.
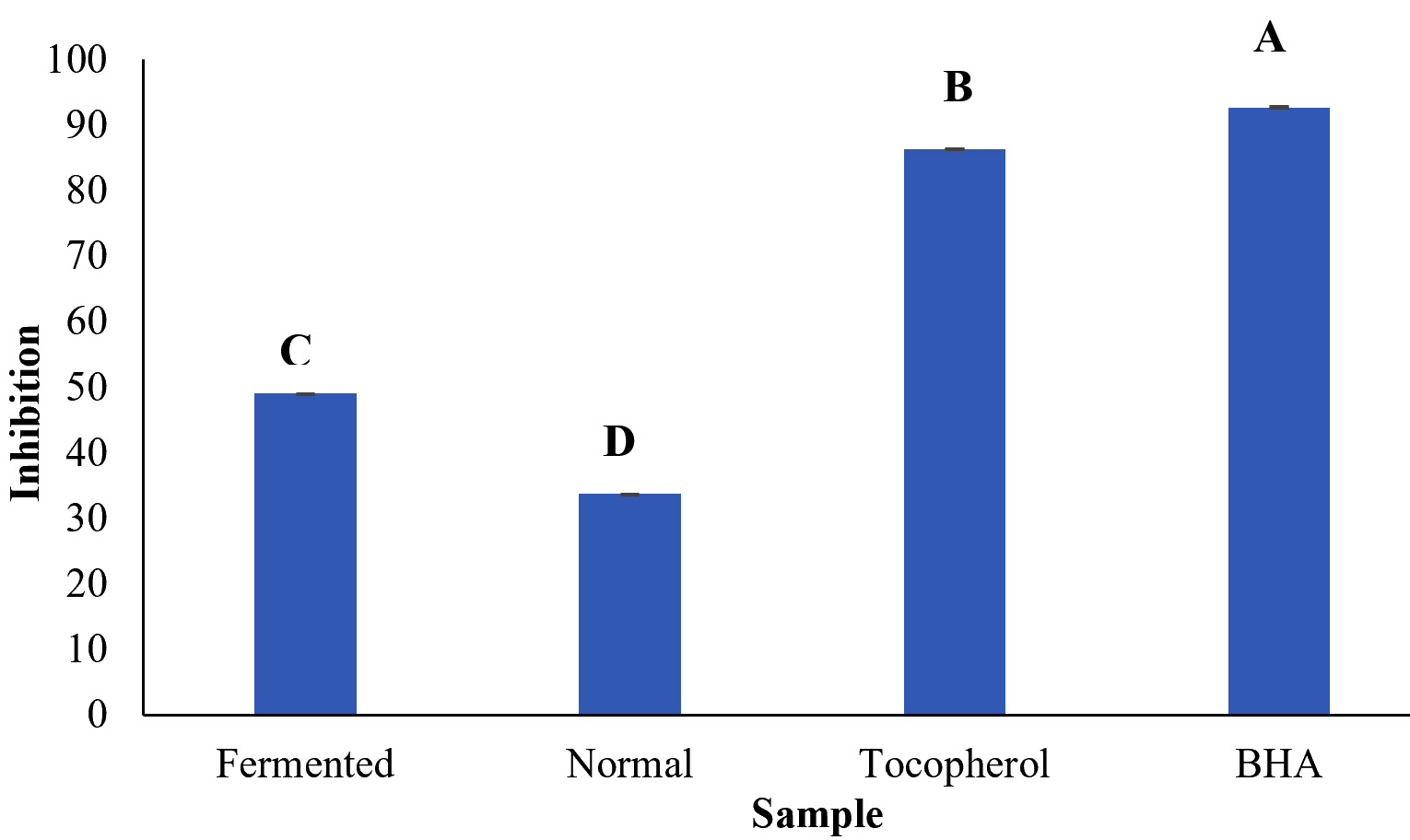 Fig. 1. DPPH radical scavenging activity of the fermented and normal Cyperus rotundus extract Mean of different letters are significantly different at p < 0.05
Fig. 1. DPPH radical scavenging activity of the fermented and normal Cyperus rotundus extract Mean of different letters are significantly different at p < 0.05FRAP Assay
Another antioxidant method used test the antioxidant capacity of compound is FRAP assay that relay on the reduction of Fe3+ to Fe2+ 17. The FRAP antioxidant assay a popular method for analysing the antioxidant strength of samples in comparison to other methods18. The stronger antioxidant effects of the substance used show High FRAP values19. The correlation between FRAP and DPPH activities has been reported in several previous studies20. The FRAP values for the C. rotundus extracts were in the range of 26. 95 to 10. 19 mmol Fe (II)/g DW (Fig. 2). The highest rate for FRAP observed was 26. 95 mmol Fe (II)/g DW in the fermented C. rotundus extract while the lowest was for non-fermented C. rotundus extract.
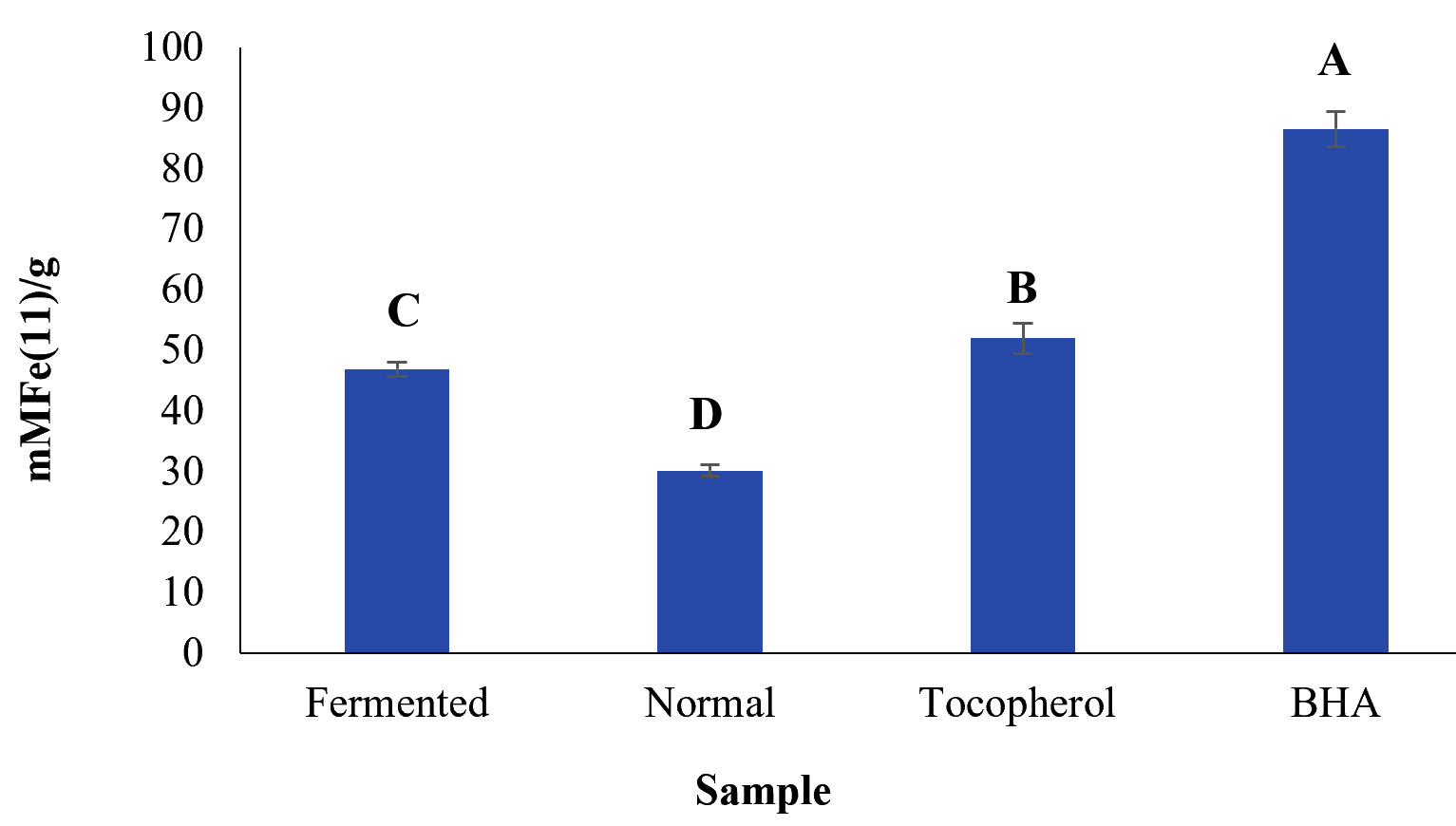 Fig. 2.FRAP ferric reducing antioxidant power of the fermented and normal Cyperus rotundus extract Mean of with the same letters are not significantly different at p > 0.05
Fig. 2.FRAP ferric reducing antioxidant power of the fermented and normal Cyperus rotundus extract Mean of with the same letters are not significantly different at p > 0.05Antibacterial activity
Antibacterial activity of C. rotundus extract is well known by the great numbers of studies carried out previously, However, there is no data available about the antibacterial activity of C. rotundus fermented with L. plantarum. This study is the first to consider the potential of fermented C. rotundus for natural remedy to inhibit the infections caused by pathogenic bacteria. The antibacterial activity of the fermented C. rotundus was very high in comparison to the non-fermented extract (Table 1). The fermentation process has significantly increased the antibacterial activity against the selected pathogens. The inhibition percentage of fermented C. rotundus extract was 85. 50±0. 12, 92. 32±0. 20, and 83. 58±0. 24 for P. aeruginosa, B. subtilis and E. coli, respectively. The non-fermented extract showed low antibacterial activity at 57. 17±0. 07, 69. 80±0. 48, and 40. 72±0. 11 against P. aeruginosa, B. subtilis and E. coli, respectively. In previous study, fermentation of the plant-based materials demonstrated increased antibacterial activity due to the bioactive compounds generated by the starter culture applied21,22. The results of this study are in line with the previous findings reported for plant-based extracts fermented by lactic acid bacteria. Fermentation with L. plantarum increased the antibacterial activity to 1 fold in comparison to the non-fermented extract.
Table (1):
Antibacterial activity of fermented and non-fermented Cyperus rotundus extract
Pathogenic bacteria |
Antibacterial activity (fermented) |
Antibacterial activity (non-fermented) |
|---|---|---|
P. aeruginosa |
85.50±0.12a |
57.17±0.07b |
B. subtilis |
92.32±0.20a |
69.80±0.48b |
E. coli |
83.58±0.24a |
40.72±0.11b |
Metabolites identification by 1H-NMR spectra
Many studies indicated that C. rotundus possesses frequent health benefits official to its different biological activities. However, there has been restricted information on the metabolites profile of the C. rotundus extracts. As such, in this study, metabolomics profiling for antibacterial and antioxidant activity in the fermented C. rotundus was performed. A combination of utilizing the one-dimensional (1D) NMR spectra compared with the sample under similar test conditions previously reported. Fig. 1 show representative spectra from the fermented and non-fermented C. rotundus extract. Full spectra in figure 3A and B showed the chemical shifts from 1 to 8 ppm of the fermented and non-fermented C. rotundus extract. The 1H-NMR spectra showed minor differences between the normal and fermented C. rotundus extract. However, the relative amount of lactate at d 1. 31 (d, J = 6. 5 Hz) increased and the peaks in the sugar region declined for the fermented samples. Fermentation by lactic acid bacteria utilize the sugars such as sucrose, glucose, and fructose and converted to lactic acid23.
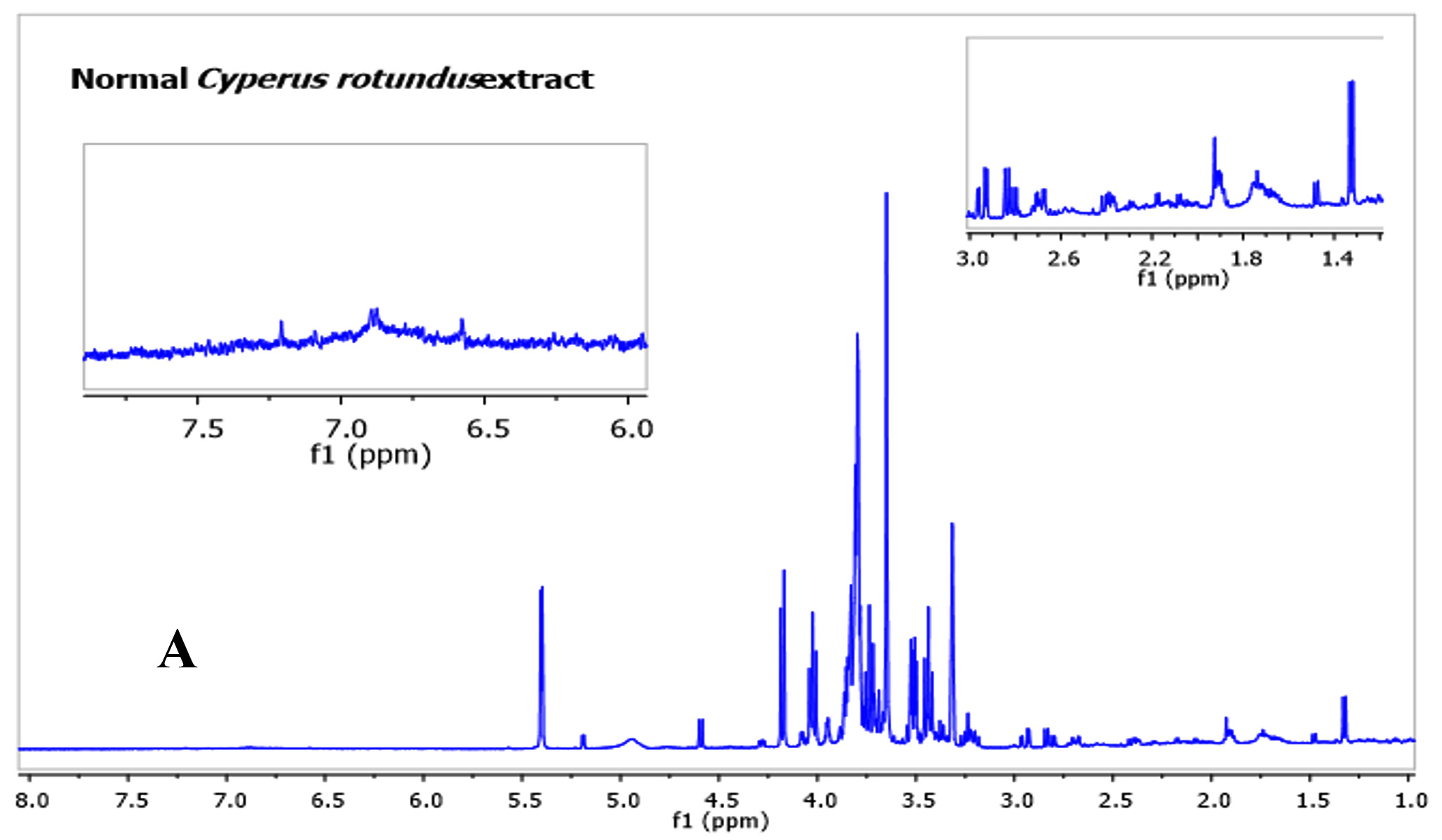
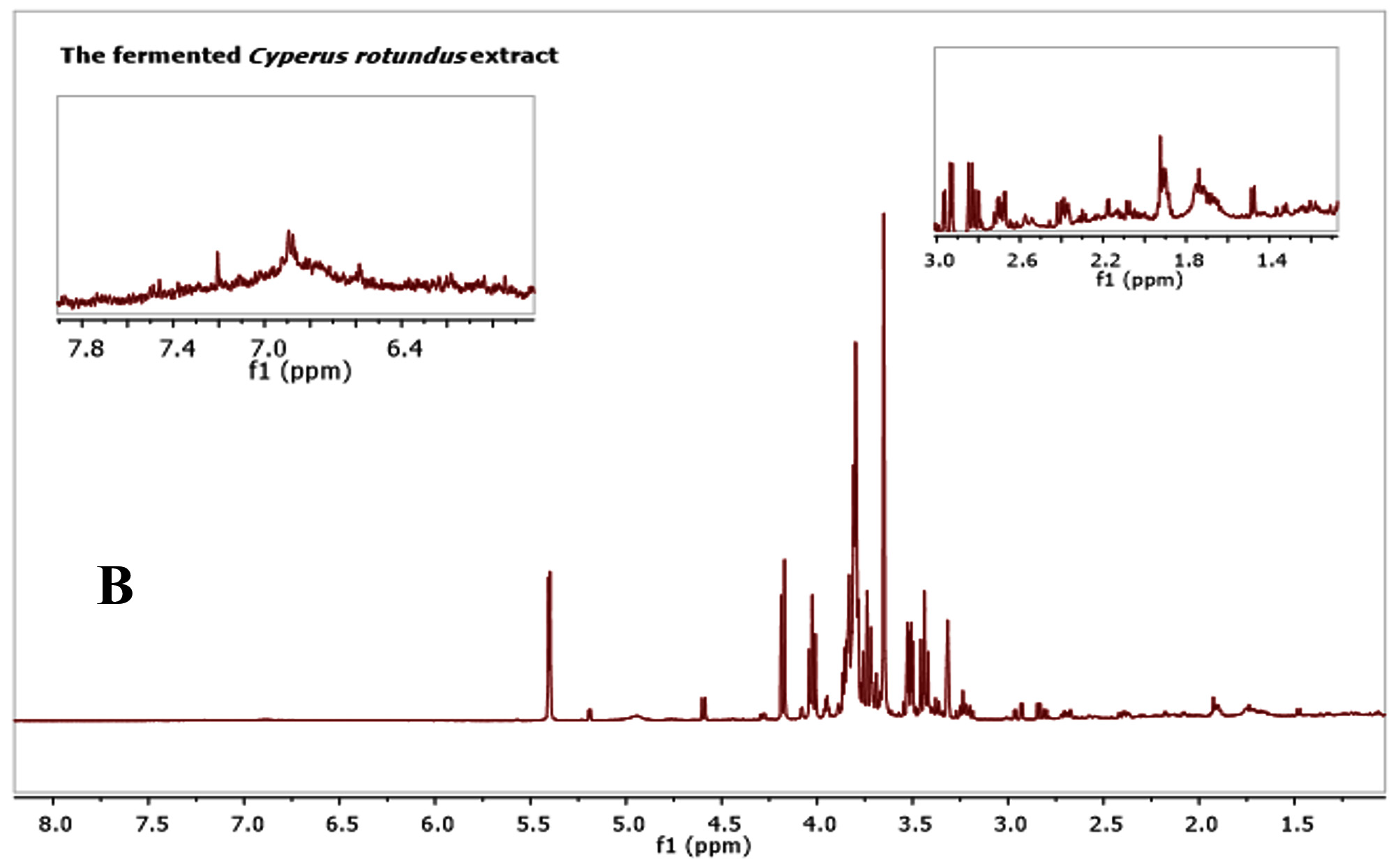 Fig. 3. 1H-NMR full spectra of d 1.0 to 8.0 ppm. (A) The fermented. (B) The non-fermented C. rotundus extract
Fig. 3. 1H-NMR full spectra of d 1.0 to 8.0 ppm. (A) The fermented. (B) The non-fermented C. rotundus extractFig. 4 showed the NMR spectra in the range from 1 to 3 ppm that contain amino acid region (Fig. 4A), 3 to 6 ppm sugar region (Fig. 4B) and 6 to 8 ppm aromatic region (Fig. 3C), respectively. Different groups were found in the samples including phenolic compounds, carbohydrates, organic acids, and amino acids. The major compounds in the fermented C. rotundus extract were glucose, fructose, xylose, sucrose, acetoacetate, alanine, betaine, choline, lysine, lactic acid, acetic, succinic, pyruvic, citric, ascorbic, fumaric acids, and anserine. The 1H-NMR spectra for the fermented C. rotundus extract were compared with those found in previous studies, 19 metabolites were identified24. The metabolites had been identified by comparing to the chemical shifts spectra at Chenomx database. Table 2 summarizes the identified bioactive metabolites, which consist of amino acids, organic acids, and several secondary metabolites. The protons of lactic acid, acetic acid, citric acid, pyruvic acid and ascorbic acid were observed at d 1. 31 (d, J = 6. 5 Hz), d 1. 91(s), d 2. 55 (d, J=3. 113 Hz), d 2. 41 (s), and d 4. 01(m) respectively. However, sugar region were difficult to determine as it contains overlapped signals that can make the identification very complicated process25. Moreover, lactic acid, acetic acid, citric acid, pyruvic acid and ascorbic acid concentrations were observed to be increased in the fermented extract due to the activity of lactic acid bacteria while the concentrations of sugar decreased. In previous study, fermented plant extracts exhibited higher free radical scavenging activities, due to high phenolic compounds and avonoids content compared to non-fermented samples23.
Table (2):
Assignments of 1H-NMR spectral signals attained from the fermented Cyperus rotundus extract
No |
Compound |
Chemical shift |
|---|---|---|
1 |
Lactic acid |
δ 1.31 (d, J = 6.5 Hz) |
2 |
Alanine |
δ 1.46 (d, J= 7.14 Hz) |
3 |
Acetic acid |
δ 1.91(s) |
4 |
Succinic acid |
δ 2.38 (s) |
5 |
Pyruvic acid |
δ 2.41 (s) |
6 |
Citric acid |
δ 2.55 (d, J=3.113 Hz) |
7 |
Choline |
δ 3.186 (s) |
8 |
Beta glucose |
δ 3.22 (m) |
9 |
Betaine |
δ 3.30 (s) |
10 |
Acetoacetate |
δ 3.44 (s) |
11 |
Caffeine |
δ 3.53 (s) |
12 |
Lysine |
δ 3.74 (t, J = 6.09 Hz) |
13 |
Ascorbic acid |
δ 4.01(m) |
14 |
Fructose |
δ 4.06 (d, J = 12.88 Hz) |
15 |
Xylose |
δ 4.59 (d, J =7.9Hz) |
16 |
Alpha glucose |
δ 5.18 (d, J = 3.76 Hz) |
17 |
Sucrose |
δ 5.41 (d, J = 3.81 Hz) |
18 |
Anserine |
δ 8.16 (s) |
19 |
Fumaric acid |
δ 8.44 (s) |

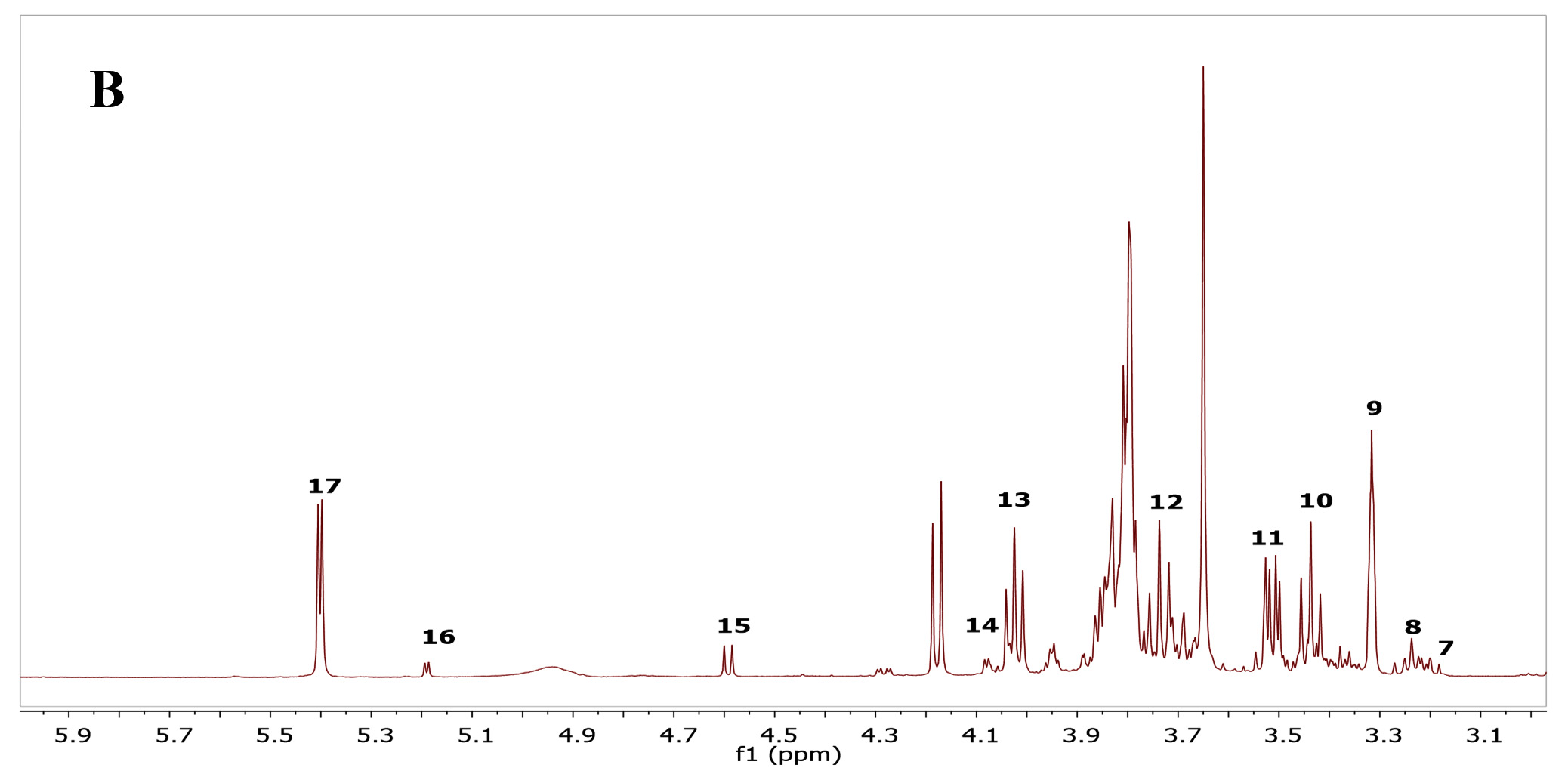
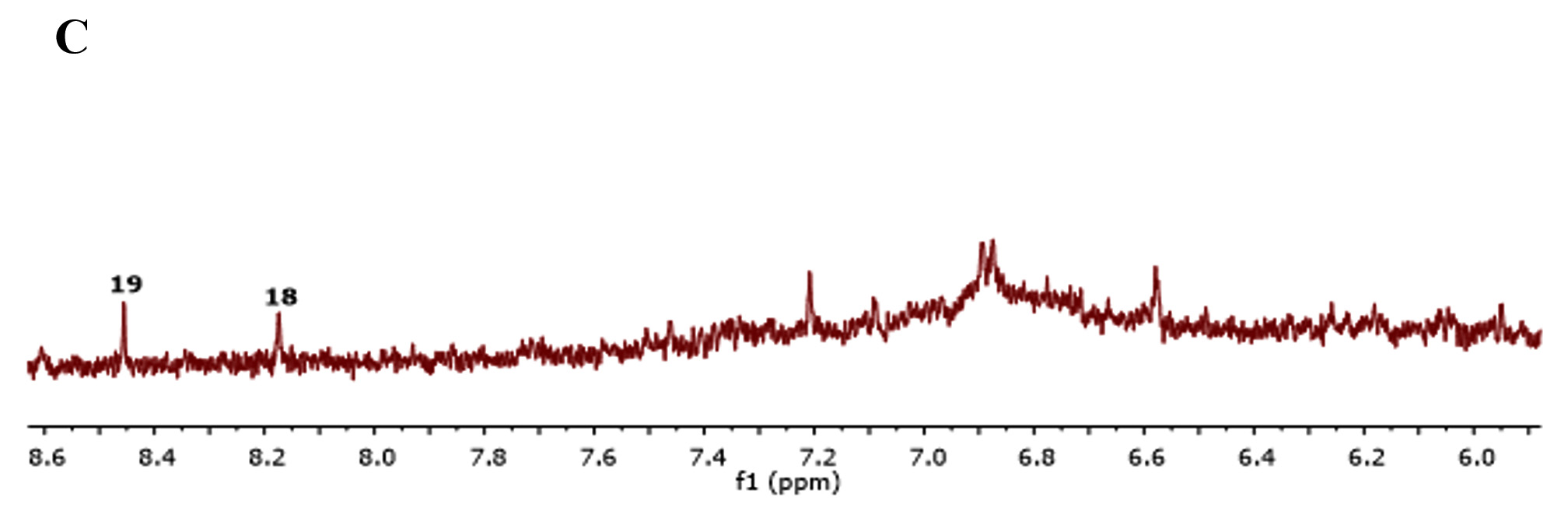 Fig. 4. 1H-NMR spectra of the fermented Cyperus rotundus extract. (A) Spectra of d 1.0 to 3.0 ppm. (B) Spectra of d 3.0 to 6.0 ppm. (C) Spectra of d 6.0 to 8.0 ppm. Identified 1H-NMR signals; (1) Lactic acid, (2) Alanine, (3) Acetic acid, (4) Succinic acid, (5) Pyruvic acid, (6) citric acid, (7) Choline, (8) Beta Glucose, (9) Betaine, (10) Acetoacetate, (11) Caffeine, (12) Lysine, (13) Ascorbic acid, (14) Fructose, (15) Xylose, (16) Alpha Glucose, (17) Sucrose, (18) Anserine and (19) Fumaric acid
Fig. 4. 1H-NMR spectra of the fermented Cyperus rotundus extract. (A) Spectra of d 1.0 to 3.0 ppm. (B) Spectra of d 3.0 to 6.0 ppm. (C) Spectra of d 6.0 to 8.0 ppm. Identified 1H-NMR signals; (1) Lactic acid, (2) Alanine, (3) Acetic acid, (4) Succinic acid, (5) Pyruvic acid, (6) citric acid, (7) Choline, (8) Beta Glucose, (9) Betaine, (10) Acetoacetate, (11) Caffeine, (12) Lysine, (13) Ascorbic acid, (14) Fructose, (15) Xylose, (16) Alpha Glucose, (17) Sucrose, (18) Anserine and (19) Fumaric acid In conclusion the fermented C. rotundus extract demonstrated high antioxidant and antibacterial activity in comparison to the non-fermented samples. 1H-NMR metabolomics profiling indicated the presence of 19 bioactive compounds in fermented C. rotundus extract and the metabolites responsible as for antioxidant and antimicrobial activity. The identified metabolites including sugar and amino acids, and their biological activity demonstrated high potential for commercial applications for fermented C. rotundus as natural remedy. The results indicated the fermentation using L. plantarum can enhance the antioxidant activity and antimicrobial activity of C. rotundus extract. Further study is recommended to determine the mechanism of the antimicrobial activity of the fermented extract.
Acknowledgements
The author would like to thank Dr. Belal J Muhialdin for all the support during lab work and for the design of this study.
Funding
None.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
Not applicable.
- Tepe B., Daferera D., Sצkmen M., Polissiou M., Sצkmen A. In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii M. Zohary et P. H. Davis. J Agric. Food Chem., 2004; 52: 1132–7. PubMed PMID: 14995110.
Crossref - Apostu, M. The effect of ergogenic substances over sports performance. Procedia -Social and Behavioral Sciences, 2014; 1179: 329–334.
Crossref - Volpe S.L. Magnesium, the metabolic syndrome, insulin resistance, and type 2 diabetes mellitus. Critical Reviews in Food Science and Nutrition, 2008a; 48(3): 293–300.
Crossref - Pirzada A. M., Ali H.H., Naeem M., Latif M., Bukhari A.H., Tanveer A. Cyperus rotundus L. : traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol., 2015; 174: 540–560.
Crossref - Kim H.K., Choi Y.H., Verpoorte R. NMR-Based Metabolomic Analysis of Plants. Nat. Protoc., 2010; 5(3): 536–549. DOI: 10. 1038/nprot. 2009. 149.
Crossref - Gallo V., Mastrorilli P., Cafagna I., Nitti G.I., Latronico M., Longobardi F., Minoja A.P., Napoli C., Romito V.A., Schהfer H., et al. Effects of Agronomical Practices on Chemical Composition of Table Grapes Evaluated by NMR Spectroscopy. J. Food Compos. Anal., 2014; 35(1): 44–52.
Crossref - Pereira A.L.F., Maciel T.C. Rodrigues S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res. Int., 2011; 44: 1276–1283.
Crossref - Meng R.G., Tian Y.C., Y., Y., S., J. Evaluation of DPPH Free Radical Scavenging Activity of Various Extracts of Ligularia Fischeri in Vitro: A Case Study of Shaanxi Region. Indian J. Pharm. Sci., 2016; 78(4): 436–442.
Crossref - Nadjet G., Zaouia K., Laboratoire V. P. R. S., Ouargla U.D., Ouargla D.G. Comparison of Antioxidant Activity and Phenolic Content of Three Varieties of Algerian Dates. Ajae., 2012; 2(1): 42–48.
- Benzie I.F.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Anal. Biochem., 1996; 76: 70 –76.
Crossref - Aween M.M., Hassan Z., Muhialdin B.J., Eljamel Y.A., Al Mabrok A.S., Lani M.N. Antibacterial Activity of Lactobacillus acidophilus Strains Isolated from Honey Marketed in Malaysia against Selected Multiple Antibiotic Resistant (MAR) Gram Positive Bacteria. J. Food Sci., 2012; 77(7).
Crossref - Abas F., Shitan M., Shaari K. & Lajis N.H. Comparison of Partial Least Squares and Arti fi cial Neural Network for the prediction of antioxidant activity in extract of Pegaga (Centella) varieties from 1 H Nuclear Magnetic Resonance spectroscopy, 2013; 54: 852–860.
Crossref - Mediani A., Abas F., Ping T.C., Khatib A., Lajis N.H. Influence of Growth Stage and Season on the Antioxidant Constituents of Cosmos Caudatus. Plant Foods Hum. Nutr., 2012; 67(4): 344–350.
Crossref - Chen C.K., Muhamad A.S. & Ooi F.K. Herbs in exercise and sports. Journal of Physiological Anthropology, 31(1): (2012) 1–7.
Crossref - Safriani N., Arpi N., and Erfiza N.M., “Potency of curry (Murayya koeniigi) and salam (Eugenia polyantha) leaves as natural antioxidant sources”, Pakistan Journal of Nutrition, 2015; 14(3): 131-135.
Crossref - Safriani N., Erfiza N.M. & Arpi N. Antioxidant Activities of Cyperus rotundus L. Rhizome and Areca catechu L. Seed. International Journal on Advanced Science, Engineering and Information Technology, 2016; 6(3): 285-288.
Crossref - Benzie I.F. & Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 1996; 239(1): 70-76.
Crossref - Halvorsen B.L., Holte K., Myhrstad M.C.W., Barikmo I., Hvattum E., Remberg S.F., … Blomhoff R. A systematic screening of total antioxidants in dietary plants. The Journal of nutrition, 2018; 132(3): 461-471.
Crossref - Albaayit S.F.A., Abba Y., Abdullah R. & Abdullah N. Evaluation of antioxidant activity and acute toxicity of clausena excavata leaves extract. Evidence-Based Complementary and Alternative Medicine, 2014(December).
Crossref - Sabeena Farvin K.H., Jacobsen C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem., 2013; 138(2–3): 1670–1681. DOI: 10. 1016/j. foodchem. 2012. 10. 078.
Crossref - Schaffner D.W. & Beuchat L.R. Fermentation of aqueous plant seed extracts by lactic acid bacteria. Appl. Environ. Microbiol., 1986; 51(5): 1072-1076.
- 24. Pyo Y.H., Lee T.C. & Lee Y.C. Enrichment of bioactive isoflavones in soymilk fermented with b-glucosidase-producing lactic acid bacteria. Food Research International, 2005; 38(5): 551-559.
Crossref - Yang S.O., Kim S.H., Cho S., Lee J., Kim Y.S., Yun S.S. & Choi H.K. Classification of fermented soymilk during fermentation by 1H NMR coupled with principal component analysis and elucidation of free-radical scavenging activities. Bioscience, Biotechnology, and Biochemistry, 2009; 73(5): 1184-1188.
Crossref - Khatoon S., Rai V., Kumar A., Rawat S., Mehrotra S. Comparative Pharmacognostic Studies of Three Phyllanthus Species. J. Ethnopharmacol., 2006; 104: 79 –86
Crossref - Shuib N.H., Shaari K., Khatib A., Maulidiani Kneer R., Zareen S., Raof S.M., Lajis N.H., Neto V. Discrimination of Young and Mature Leaves of Melicope Ptelefolia Using 1H-NMR and Multivariate Data Analysis. Food Chem., 2011; 126(2): 640–645. DOI: 10. 1016/j. foodchem. 2010. 10. 043.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


