ISSN: 0973-7510
E-ISSN: 2581-690X
Pseudomonas aeruginosa is capable of producing biofilms on various surfaces, medical implants and burn wounds which inhibit the action of antimicrobial agents. This study was undertaken for the screening, identification and purification of a natural antimicrobial compound from the hospital waste sites against P. aeruginosa. The partial purification of the antimicrobial agent was performed with ethyl acetate from the culture supernatant of the isolate and further purified by HPLC. For the characterization of the active compound, we have used Fourier Transform Infrared Spectroscopy (FT-IR) and Electrospray Ionization mass spectrometry (ESI-MS) analysis. The purified compound was tested for anti-biofilm activity in vitro against P. aeruginosa and on various surfaces such as plastic, glass, and steel. The potent isolate was confirmed as Bacillus licheniformis strain RG1002. The HPLC purified compound was characterized as lichenysin using FTIR and ESI-MS analysis. This study identifies and characterize the potent antimicrobial agent against other important human pathogens such as Staphylococcus aureus, Salmonella typhi, Shigella flexneri and Escherichia coli. Purified lichenysin showed strong inhibitory effect against P. aeruginosa and other important pathogens and inhibits the biofilm formation of P. aeruginosa in vitro on various types of surfaces. The current study suggests the application of lichenysin as a protective coating on various indwelling medical devices and catheters to prevent the biofilm formation by P. aeruginosa.
Antimicrobial Agents, Biofilms, Pseudomonas aeruginosa, Lichenysin, Bacillus licheniformis RG1002
Pseudomonas aeruginosa is a versatile Gram-negative, rod shaped bacterium which can survive on variety of surfaces such as glass, plastic, steel and the medical devices made of these materials. The adhesion of this bacterium on various surfaces is attributed to its morphology and virulence factors, such as biofilms, exopolysaccharides and outer membrane proteins.1-3 Due to intricate network of virulence factors, it causes lethal infections such as pneumonia, meningitis, enteritis and septicemia. It is also the major cause of in-dwelling medical devices related infections in humans.4 During the COVID-19 pandemic ventilator-associated pneumonia (VAP) caused by P. aeruginosa was commonly seen in the patient with long stays in the hospital.5 P. aeruginosa biofilm is also associated with burn wound infection.6 World Health Organization (WHO) listed it as a critical pathogen due to emergence of carbapenem resistant strains.7 Biofilms formed by P. aeruginosa mainly consist extracellular molecules such as elastase, polysaccharide, rhamnolipids and siderophores and pyocyanin.8-10
Surfactants known as surface active agents are amphiphilic molecules which reduces the surface tension of two immiscible liquids and first discovered in 1968 from Bacillus subtilis.2 Since then many Bacillus species were identified which produces surfactin like lipopeptides and have enormous applications in pharmaceutical, petroleum, cosmetics, medical and food industries.2 Global market of natural surfactants is projected to increase by 5.5% annually from 2020 to 2026 due to adverse environmental impact of synthetic surfactants.11 Microbial biosurfactant’s chemical nature depends on the culture conditions, raw materials and used strain, it may consist varying length of fatty acids and peptide moiety that depends on the carbon and nitrogen source in the culture medium. Most of the biosurfactants produced by bacteria are synthesized by non-ribosomal peptide synthetases (NRPS) which consists multienzyme complex.12
Lichenysin is a cyclic lipopeptide produced by Bacillus licheniformis which is similar to surfactin in structure except difference in types of amino acids. It is stronger than surfactin in activity due to exposed carboxyl group present in amino acids.13 Lichenysins are also synthesized by NRPS and encoded by genes licA-TE in almost all Bacillus strains; however, its expression may be different in various strains depending on the raw material used in culture medium and growth conditions.14,15 Surfactins have broad spectrum of antifungal, antibacterial and antiviral activity and have been used in various industries.16 The antimicrobial property of surfactin are due to the disruption of cell membrane and inactivation of various cellular enzymes in target pathogens by chelating the metal ions required for the activity of enzymes and proper framework of lipid bilayer.17,18
Surfactins are mostly used for bioremediation of petroleum contaminated sites due to their excellent property for reducing the interfacial tension among the two immiscible fluids.19,20 They are also used as emulsifier, anti-foaming and anti-caking agents in food, pharmaceuticals and cosmetics.21 However, role of lichenysin as a biofilm inhibitor and its application as a protective coating on medical devices is not yet explored. Since, lichenysin are low molecular weight compound and have low toxicity they can be used as anti-biofilm compounds on various surfaces.
The purpose of the present study was to perform screening, identification and characterization of a natural antimicrobial agent producing bacteria against P. aeruginosa from the hospital waste sites in India. The most potent isolate RG1002 was identified as Bacillus licheniformis strain RG1002 by biochemical and molecular analysis. Purification of the active compound was performed by HPLC and elution fractions were checked for the antimicrobial activity against the test organism P. aeruginosa ATCC 27853. The active fraction was analyzed by FT-IR and ESI-MS techniques for the identification of the compound which is confirmed as lichenysin. It inhibits the biofilm formation of P. aeruginosa on various surfaces such as steel, glass and plastic which are typically used for the manufacturing of medical devices and catheters. Thus, lichenysin may be a safe, non-toxic and natural antimicrobial agent which can be used as a protective pre-coating compound on the medical devices.
Sample collection and bacterial strains
Soil samples were collected from different places of the hospital waste sites of Varanasi district of Uttar Pradesh, India (Coordinate: 25.3176° N, 82.9739° E). Soil was obtained from up to 20 cm deep by removing the top 2-3 cm upper layer. The samples were stored in a sterile zip lock bag and refrigerated until further use. Culture media and chemicals were procured from HiMedia Laboratories (India) and Lobachem (India). Soil extract agar (M455), nutrient broth (M002), Nutrient agar (M001), LB broth (M1245), and tryptic soy broth (M011) were procured from HiMedia laboratories. Vancomycin (SD155), ampicillin (SD002A), and ciprofloxacin (SD060) were procured from SRL (Sisco Research Laboratory) Pvt. Ltd., India. The test bacteria used in this study was P. aeruginosa ATCC 27853. The other important Gram-negative and Gram-positive bacteria used in this study were Shigella flexneri MTCC 9543, Escherichia coli MTCC 1304, Bacillus subtilis ATCC 6633, Salmonella typhi MTCC 581 and Staphylococcus aureus ATCC 25923 obtained from Microbial type culture collection (MTCC), Chandigarh, India or provided by Dr. Vineeta Singh from IET, Lucknow, India.
Primary and secondary screening of antimicrobial producing strains
1 gm of soil sample was homogenized in 10 ml of water. Serial dilutions were made and 0.1 ml of every dilution was plated on soil extract agar medium. Plates were incubated for 48 hrs at 30°C and observed for bacterial colonies. The replica plate was made on nutrient agar (NA) and overlaid with soft agar mixed with 10 µl of overnight grown P. aeruginosa culture. Plates were incubated at 37°C for 24 hr to check the zone of inhibition.22 Total eight isolates were found active against P. aeruginosa and were streaked for pure single colony on NA plates. Secondary screening was done for all the active isolates against Gram-positive Bacillus subtilis ATCC 6633 and Staphylococcus aureus ATCC 25923 and Gram-negative Escherichia coli MTCC 1304 and Salmonella typhi MTCC 581 and Shigella flexneri MTCC 9543. Since, most of the bacteria produce antimicrobial agents in culture supernatant as secondary metabolites, the active isolates were grown in LB broth and supernatant was filtered by 0.2 µm sterile syringe filter (Whatman, USA). The filtered supernatant was checked for antimicrobial activity against all the indicator bacteria by well diffusion method.23
Biochemical and molecular characterization of antimicrobial producing isolate
Morphological and phenotypic identification was done by Gram-staining and various biochemical assays such as citrate utilization, methyl red, urease, indole, catalase and Voges-Proskauer test. All the tests were performed in specific medium according to standard protocols in triplicate. The results were represented as average of the three replicates in comparison to control sample. For molecular identification of the potent isolate, we have performed 16S rRNA gene sequencing using universal primers23 (UNI-16-GT-F: 5′-AGAGTTTGATCCTGGCTCAG-3′ and UNI-16-GT-R: 5′-CGGTTACCTTGTTACGACTT-3′). The genomic DNA was extracted from 2ml of overnight grown bacterial culture using Xploregen DNA extraction Kit (Xploregen Discoveries, India) according to the manufacturer’s instructions. Genomic DNA was estimated qualitatively and quantitively using agarose gel electrophoresis and Nanodrop (Thermo Fisher Scientific, India) and stored at -20°C till further experiment. 10 ng of purified genomic DNA was used for the PCR amplification along with 0.5 µM of each primer in 50 µl PCR reaction with 1 unit of KOD Hot start DNA polymerase as per the manufacturer’s instruction (Merck, India). Initial denaturation was at 95°C for 2 min then 25 cycles of denaturation at 95°C for 30 sec, annealing at 56°C for 20 sec and extension at 70°C for 60 sec. The PCR product was purified using PCR purification kit (Xploregen Discoveries, India) and sequencing was performed and outsourced from Biokart India Pvt. Ltd. NCBI blast was used to identify the homologous bacterial 16S ribosomal sequences and phylogenetic tree was performed by maximum likelihood test method using MEGA-X-11 software.24 The 16S rRNA sequences were submitted to NCBI GenBank (Accession no: OM438151).
Growth kinetics and time dependent antimicrobial activity
We have explored the growth pattern, biomass production and antimicrobial properties of the potent isolate Bacillus licheniformis RG1002 for 10 days as described previously.25,26 The seed culture of the strain RG1002 was grown in 5 ml nutrient broth (NB) medium for overnight and diluted 100x in fresh 100 ml NB medium next day in a 500 ml flask. The flask was kept in shaker incubator at 150 rpm for 10 days at 37°C. Each day, 1 ml culture was taken out from the culture flask aseptically and absorbance was measured at 600 nm in spectrophotometer using medium only as a blank. For biomass calculation and antimicrobial activity against the test organism, 1 ml broth culture of potent antimicrobial producing isolate was taken out every 24 hr, centrifuged at 8000 rpm for 5 min and the supernatant was filtered with 0.45 µM sterile syringe filter (Whatman, USA). The pellet was dried in air and weighed to calculate the biomass per ml. Filtered supernatant of Bacillus licheniformis RG1002 was checked for the antimicrobial properties against P. aeruginosa by well diffusion method as described previously.
Extraction and partial purification of antimicrobial compound
The active component of Bacillus licheniformis RG1002 cell free supernatant was partially purified using a variety of dual-phase solvent methods including polar and non-polar solvents like methanol, ethyl acetate, chloroform, n-hexane, and n-butanol. The strain was cultivated in 30 ml of NB medium for 72 hours at 37°C and supernatant was collected by centrifugation for 5 min at 8000 rpm. Each of the aforementioned solvents was combined with 25 ml of the supernatant. To separate the two phases, the mixture was shaken for six hours at 250 rpm and then centrifuged for five minutes at 6000 rpm. After being collected in a different tube, the organic phase was vacuum-extracted. The dried crude extract was tested for antibacterial activity against the test organism and kept in storage at 4°C.
Purification and identification of active compound
The antibacterial component was purified by HPLC; the dried crude extract which was partially purified was dissolved in autoclaved distilled water and filtered by 0.2 µm syringe filter (Whatman, USA). Mobile phase was filtered by 0.45 µm nylon syringe filter. A mobile phase was loaded onto a C18 column of a high-performance liquid chromatography apparatus (LC-20AP, Shimadzu, Japan) with a flow rate of 1 ml/min with UV absorbance at 280 nm wavelength.27 The composition of mobile phase was acetonitrile and HPLC grade water with 0.1% TFA in an 80:20 (v/v) ratio for each run. Every elution fraction associated with a particular peak was collected and subjected to an antimicrobial activity test against P. aeruginosa. The vacuum dried active elution fraction from HPLC was dissolved in acetonitrile and analyzed using electrospray ionization mass spectrometry in E2695/HPLC-TQD Mass Spectrophotometer (Water Alliance, USA). The range of the mass/charge ratio was set between 100 and 2000 Da, the mass/charge (m/z) ratio of the [M+H]+ ion or its adduct was measured. Using Fourier Transform Infrared Spectroscopy (FTIR) analysis, the structural groups of the purified molecule were determined. In summary, 10 µl of the dissolved purified compound was employed for the FTIR measurement in the Spectrum Two FT-IR spectrometer (PerkinElmer). The frequencies used to get the FTIR spectra ranged from 4000 to 500 cm-1.27,28
MIC and oil displacement assay of purified lichenysin
The minimum inhibitory concentration (MIC) was calculated against the various pathogens via 96-well microtiter plate technique described earlier with some minor modifications.26,27
S. flexneri, S. typhi, E. coli and P. aeruginosa were grown for in LB (Luria Bertani broth) and S. aureus and B. subtilis were grown in TSB (tryptic soya broth) for 24 hr. Next day, each bacterial culture was diluted 1000 times in a fresh culture medium and 150 µl broth culture was inoculated in every well of a 96-well plate in triplicate. The lichenysin was mixed with starting concentration of 100 µg/ml and serially diluted 2-fold in successive wells. The 96-well plate was kept at 37°C for 24 hr and absorbance was measured at 540 nm with a Multiskan FC Microplate Photometer (Thermo Fisher Scientific, India). The oil displacement method, as described by Walter et al.,29 was modified to determine whether lichenysin produced from Bacillus licheniformis strain RG1002 has biosurfactant property. Briefly, 5 ml of Mustard oil (obtained from the seeds of Brassica juncea) was placed on the top of 15 ml of distilled water in a 70 mm petri dish. On the oil layer, 25 µl of purified lichenysin of 100 µg/ml concentration was placed and for control 25 µl of distilled water was placed on the oil layer in another petridish. The diameter of displaced oil was measured and compared with control.
Quantitative biofilm assay
Biofilm inhibition formed by P. aeruginosa with or without purified lichenysin was quantitatively measured by crystal violet dye as described earlier with some minor modifications.26 Briefly, P. aeruginosa was grown in LB at 37°C and 150 rpm for 24 hr. Next day, culture was diluted One hundred times in fresh LB with 0.25% glucose (Biofilm medium) and 150 µl was aliquoted in 96 well plate in triplicate with 25 µg/ml (MIC) and 12.5 µg/ml (1/2 of the MIC) lichenysin except negative controls which have only biofilm medium and positive controls have P. aeruginosa in biofilm medium. The 96 well plate was incubated at 37°C for 48 hr. Culture was discarded and wells were washed three times with PBS (pH 7.4). To fix the biofilm 150 µl of 95% ethanol was aliquoted in each well for 2 min and discarded. Each well was stained with 150 µl of 1% crystal violet (w/v) dye for 5 min. Wells were again washed three times with 150 µl PBS (pH 7.4). The absorbance was measured at 540 nm in microtiter plate reader (Multiskan™ FC Microplate Photometer, Thermo Fisher Scientific, India).
Biofilm inhibition assay on various surfaces
To assess the role of lichenysin as an anti-biofilm and protective compound on various surfaces we have used plastic, steel and glass thin sterile hollow tubes of 1.0 cm in length. Few tubes were soaked in 25 µg/ml lichenysin for 60 min to make a protective layer on the outer and inner surfaces of the tubes. As described above in the methodology for biofilm assay, P. aeruginosa was grown in LB for 24 hr and diluted 100x in fresh biofilm medium. Each well of 12 well microtiter plate received 300 µl of P. aeruginosa culture. Pre-soaked tubes of plastic, steel and glass were incubated in duplicate in 12-well microtiter plate and for control, tubes without soaked in lichenysin were also incubated. The microtiter plate was incubated at 37°C for 48 hr. The tubes were transferred in a new sterile 12-well plate and cleaned 3 times with PBS (pH 7.4) to remove planktonic cells. Every well received 300 µl of 1% crystal violet (w/v) dye for 5 min to stain the biofilm. Wells were washed three times with 300 µl PBS (pH 7.4) and 300 µl of 30% acetic acid was aliquoted in each well for 15 min at room temperature to dissolve the biofilm. 150 µl of dissolved crystal violet solution was transferred from each well to new 96-well plate and the absorbance was measured at 540 nm in microtiter plate reader (Multiskan™ FC Microplate Photometer, Thermo Fisher Scientific, India).
Statistical analysis
The experimental data of zone of inhibition, minimum inhibitory concentration, growth rate, biomass and biofilm assay were analyzed by student t-test from triplicate experiments. The data represents the mean±SD. p-value less than 0.05 is considered significant (*) and less than 0.01 as highly significant (**). 16S rRNA gene sequence accession number of Bacillus licheniformis RG1002 submitted to NCBI GenBank database is OM438151.
Identification of antimicrobial agent producing bacterial strain
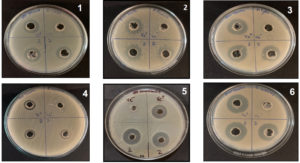
We have screened more than 500 bacterial isolates from different soil samples against test pathogen P. aeruginosa ATCC 27853 by soft agar method. Out of which eight isolates were showing activity against the test organism. We have purified these isolates for single colony on NB agar plate by streak plate method and perform the secondary screening against all the pathogens mentioned in the methodology. The isolate RG1002 was showing maximum antimicrobial activity against all the used pathogens in this study and used for further characterization (Figure 1).
Figure 1. Antibacterial activity of cell free supernatant of isolate RG1002 against:
- E. coli MTCC1304 [culture supernatant; 2,2], positive control (Ampicillin; ve+), (negative control; medium only; ve-)
- S. flexneri MTCC 9543 [culture supernatant;2,2], (positive control; Ampicillin; ve+), (negative control; medium only; ve-)
- S. Typhi MTCC 581 [culture supernatant;2,2], (positive control;Ciprofloxacin); ve+, (negative control; medium only; ve-)
- S. aureus ATCC 25923 [culture supernatant;2,2], (positive control;Vancomycin; ve+), (negative control; medium only; ve-)
- P. aeruginosa ATCC27853 [culture supernatant;2,2], (positive control; Gentamicin; ve+), (negative control; medium only; ve-)
- B. subtilis ATCC 6633 [culture supernatant;2,2], (positive control; Vancomycin; ve+), (negative control; medium only; ve-)
The morphology of RG1002 isolate was rod shaped under the compound microscope after Gram staining which suggest as Gram-positive bacillus sp. Biochemical tests were performed as per standard protocol for RG1002 isolate and was found positive for catalase, citrate and Voges Proskauer tests and negative for Urease, methyl red and Indole tests which further confirm the genus Bacillus for RG1002 isolate (Table S1).
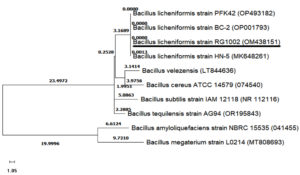
Molecular characterization of isolate RG1002 was done by 16S rRNA gene sequencing and phylogenetic tree was constructed. The homology search in NCBI database and phylogenetic tree suggest that isolate RG1002 showed 100% similarity with Bacillus licheniformis. 16S rRNA sequencing data of strain Bacillus licheniformis RG1002 was submitted to NCBI GenBank database with accession number OM438151 (Figure 2).
Figure 2. Phylogenetic tree of Bacillus licheniformis RG1002 made by maximum likelihood test using MEGA-X-11 software. Bacillus licheniformis RG1002 (OM438151) is showing 100% similarity to Bacillus licheniformis BC-2(OP001793)
Growth kinetics and antimicrobial property
To assess the growth rate, biomass production and antimicrobial properties of the strain Bacillus licheniformis RG1002, we performed the growth kinetics study as described in methods. The results suggest that maximum growth of this strain was achieved in 3 days post inoculation which also correspond to the biomass production in our used culture conditions (Figure S1, S2). The antimicrobial compound produced in the cell free supernatant of Bacillus licheniformis RG1002 strain was highly potent and stable since it showed antimicrobial activity from day 1 to day 10 as measured by zone of inhibition assay against the test pathogen (Figure S3).
Purification and characterization of antimicrobial compound
Mostly antimicrobial compounds are produced into culture supernatant by bacteria as a secondary metabolite. We have used bi-phasic solvent system to partially purify the bioactive compound present in the cell free supernatant of Bacillus licheniformis RG1002 strain. Among various combinations of polar and non-polar solvents, we found ethyl acetate in a ratio of 1:2 (v/v) was the best for extracting the active compound, indicating its polar nature. For further purification, we have used HPLC, reverse phase C18 column as described in method. HPLC analysis reveals one major peak at retention time 8.257 and two small peaks at 7.463 and 9.483 retention time. We have collected the elution fractions and tested for antimicrobial properties of every elution fraction against the test organism P. aeruginosa. The results suggested stronger antimicrobial activity in peak-3 (retention time 8.257) compared to peak- 4 (retention time 9.483) (Figure 3).
Figure 3. HPLC purification of partially purified crude extract from RG1002 isolate. Antibacterial activity of elution fractions were checked against P. aeruginosa on NA plate (A) indicates zone of inhibition from elution fraction with retention time 9.483 and (B) indicates elution fraction 8.257
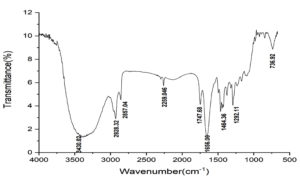
FTIR spectral analysis of purified lichenysin showed nine major peaks and suggest the aliphatic nature of the compound (Figure 4A). The important region of FTIR spectra suggest the intense frequency peaks between 3500 cm-1 – 3000 cm-1 which suggest the aliphatic C-H functional groups.29 The frequency range between 2857 cm-1 to 1747 cm-1 suggest the presence of -CH3 alkyl groups in the bioactive compound which could belong to long chain fatty acid attached to the peptide ring of lichenysin. Peaks at 1656 cm-1 suggest the presence of C-N functional groups in the amino acids and peaks at 1292 cm-1 suggest the alkyl ketone functional groups in the peptide ring of the compound.30,31 Soft electrospray ionization mass spectrometry was performed with 10 mg of purified compound for the determination of molecular weight of the bioactive compound. The m/z ratio of the highest intensity peak for [M+H]+ was 1035.40 Da (Figure 4B) which has been previously reported as lichenysin.32
Figure 4A. FTIR analysis of the lichenysin obtained from Bacillus licheniformis RG1002 to predict the functional group presents in the purified compound. Values represents percent transmission vs wavenumber
Oil displacement assay and MIC determination
It has been previously reported that biosurfactant reduce the surface tension of two immiscible liquids and shows the oil repelling activity.29,32 To confirm this, we performed oil displacement assay as described in methods. The results suggested that lichenysin at 100 µg/ml concentration made the clear zone of 25 mm diameter on the oil surface compared to control (Figure S4). This suggest the biosurfactant property of lichenysin produced by strain Bacillus licheniformis RG1002.
We have calculated the minimum inhibitory concentration of purified lichenysin against all the pathogens as mentioned in the methods using 96-well microtiter plate assay. The results suggest that lichenysin MIC against S. flexneri, B. subtilis and P. aeruginosa were 25 µg/ml and against S. aureus and E. coli were 12.5 µg/ml. The maximum MIC was against S. typhi at 50 µg/ml (Table S2).
Anti-biofilm activity on various surfaces
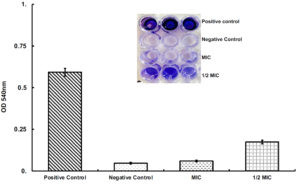
Anti-biofilm activity of lichenysin was tested against P. aeruginosa at MIC (25 µg/ml) and half of the MIC (12.5 µg/ml) as mentioned in the method. The results suggested that lichenysin at 25 µg/ml and 12.5 µg/ml concentration significantly inhibit the biofilm formation of P. aeruginosa. However anti-biofilm activity at 25 µg/ml concentration was more than 12.5 µg/ml (Figure 5).
Figure 5. Quantitative measurement of biofilm inhibition in the presence of lichenysin at 25 μg/ml (MIC) and 12.5 μg/ml (1/2 of the MIC). Data represents the mean±SD from triplicate experiments
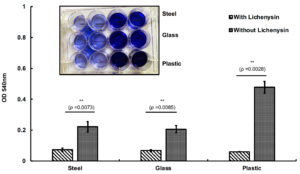
To check the anti-biofilm activity of lichenysin on various surfaces we used steel, glass and plastic hollow tubes to mimic the catheters used as indwelling medical devices in patients. The results suggested that pre-coating of lichenysin with 25 µg/ml significantly reduced the biofilm formation on plastic, steel and glass surfaces as compared to the control tubes without lichenysin coated (Figure 6). The inhibition of biofilm was seen in decreasing order in glass, steel and plastic tubes that suggest that plastic material was more prone to develop biofilm formation by P. aeruginosa (Figure 6).
Bacillus licheniformis is a ubiquitous facultative anaerobic bacterium which is present in diverse environmental samples and known to produce surfactant termed as lichenysin.32 Biosurfactants have excellent surface tension reducing properties due to the presence of hydrophilic and lipophilic moiety in its structure. It has low-toxicity, biodegradable and eco-friendly hence have various applications such as sanitizers, cleaners, bioremediation of petroleum waste, pharmaceuticals, food industry and cosmetics.33,34 Lichenysin is amphipathic molecule which has hydrophilic peptide ring and varying length of hydrophobic fatty acid chain.35 They have distinct amino acid moiety at different positions which altered their physiological and surfactant properties.36
In the current study, we have purified antimicrobial compound from Bacillus licheniformis RG1002 which has broad spectrum activity against important human pathogens and possess anti-biofilm activity against P. aeruginosa on various surfaces such as plastic, steel and glass that suggest its role as a protective agent on medical devices and catheters. We envisioned that hospital waste sites are prone to contaminate by various drugs and infective wastes that may create the selection pressure on the soil microbes to survive in that environment. Hence, microbes present in the vicinity of hospital waste soil may possess the broad-spectrum antimicrobial activity. In the primary screening we have identified eight isolates which were showing activity against test organism P. aeruginosa, however secondary screening of the cell free culture supernatant of isolates suggested that isolate RG1002 was highly active against all the tested pathogens. The partial purification of the antimicrobial compound was done by ethyl acetate which suggested the polar nature of the bioactive compound. HPLC results suggested the highest peak at 8.257 retention time which corroborates with the results of antimicrobial activity in the well diffusion assay (Figure 3). Fourier transform infrared spectroscopy (FTIR) analysis of the purified compound confirm the presence of methyl groups in fatty acid chain and amino acid residues in the peptide ring of lichenysin. The previously reported C-15 lichenysin has molecular formula C53H94N8O12 and molecular weight as 1035.4 g/mol. It has peptide ring composed of aspartyl-D-leucyl-L-isoleucyl-beta-alanyl-L-glutaminyl-D-leucyl-L-valyl.32 Our ESI-MS results of purified compound have highest intensity peak at m/z ratio of 1035.4 which confirm the compound as lichenysin. The zone of inhibition results suggested that lichenysin was highly stable at 37°C as we did not find any decrease in antimicrobial activity from day 2 to day 10 against the test organism P. aeruginosa (Figure S3). To confirm the biosurfactant property of lichenysin we performed the oil displacement test, results suggested that lichenysin showed higher zone of oil displacement as compared to control (Figure S4) which confirm the surface tension reduction property of lichenysin.
The placement of foreign metal in our body poses the first site of infection by bacteria. The heterogeneous population of P. aeruginosa present within the biofilm matrix creates an intrinsic resistance to the antibiotic treatment. Thus, the conventional antibiotic therapy fails and surgical interventions are needed which causes more medical expenses and trauma to the patients. Therefore, we thought to make a protective layer of lichenysin on steel, glass and plastic material tubes to mimic the catheters and indwelling medical devices used in patients. Typically, bacteria interact with the host tissues in the vicinity of metal surfaces with adhesion molecules or outer membrane glycolipids and form the biofilm around the metal surfaces. Once the biofilm is formed bacterial cell density increases and causes chronic infection that inhibits antimicrobial therapy. Our results suggested that pre-coating with lichenysin at 25 µg/ml inhibited the colonization of P. aeruginosa on the various surfaces that eventually prevent the biofilm formation (Figure 6). Results also suggested that biofilm formation was more on plastic tubes than steel and glass tubes and coating of lichenysin significantly inhibit the biofilm formation by P. aeruginosa on all the materials used in the experiment. It could be possible that lichenysin coated on the hollow tubes may interact with the P. aeruginosa by its lipophilic tail with lipid-layer of bacterial outer membrane and disrupt the structural organization of bacterial cell wall as reported with antiviral activity of rhamnolipids with SARS-CoV-2 virus.37 In summary, our study defines the potential role of lichenysin biosurfactant as an antimicrobial agent against important human pathogens and its application as a pre-coating agent on various surfaces used in several indwelling medical devices and catheters in vitro conditions. We have demonstrated the significant anti-biofilm activity of lichenysin against P. aeruginosa. However, in vivo study remains to be investigated to check the efficacy of lichenysin in actual host environment. Anti-biofilm activity of lichenysin could be evaluated using animal models such as heart infective endocarditis and bone implant infection models as previously reported by our research group.37,38
In summary, our study defines the potential role of lichenysin biosurfactant as an antimicrobial agent against important human pathogens and its application as a pre-coating agent on various surfaces used in several indwelling medical devices and catheters in in-vitro conditions. We have demonstrated the significant anti-biofilm activity of lichenysin against P. aeruginosa. However, in vivo study remains to be investigated to check the efficacy of lichenysin in actual host environment. Anti-biofilm activity of lichenysin could be evaluated using animal models such as heart infective endocarditis and bone implant infection models as previously reported by our research group.
Additional file: Additional Table S1-S2 and Figure S1-S4.
ACKNOWLEDGMENTS
The authors would like to acknowledge the research and infrastructure support provided by the Head of the Department and Babasaheb Bhimrao Ambedkar University. Authors also acknowledge the help in the analysis of ESI-MS data by Sophisticated Analytical Instrument Facility (SAIF) of Central Drug Research Institute, Lucknow.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RKG conceptualized and supervised the study. AK and RS performed data curation. AK applied methodology. AK, RKG and SY performed results analysis. RS performed results validation. SY wrote and edited the first draft. RKG reviewed, edited and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Khelissa SO. Characterization of physiological properties associated with biofilm-detached cells and study of interactions between bacteria and materials: case of Staphylococcus aureus and Pseudomonas aeruginosa (Doctoral dissertation, Universitי des Sciences et Technologie de Lille-Lille I). 2017.
- Arima K, Kakinuma A, Tamura G. Surfactin, a crystalline peptide lipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Commun. 1968;31(3):488-494.
Crossref - Ghilini F, Pissinis D. E, Minan A, Schilardi PL, Diaz C. How functionalized surfaces can inhibit bacterial adhesion and viability. ACS Biomater Sci Eng. 2019;5(10):4920-4936.
Crossref - Jurado-Martin I, Sainz-Mejias M, McClean S, et al. Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int J Mol Sci 2021;22(6):1-35.
Crossref - Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig Transduct Target Ther. 2022;7(1):199.
Crossref - El-Khashaab TH, Erfan DM, Kamal A, El-Moussely LM, Ismail DK. Pseudomonas aeruginosa biofilm formation and quorum sensing lasR gene in patients with wound infection. Egypt J Med Microbiol. 2016;25(1):101-108.
Crossref - Nguyen M, Joshi SG. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital acquired infections: a scientific review. J Appl Microbiol. 2021;131(6):2715-2738.
Crossref - Brindhadevi K, LewisOscar F, Mylonakis E, Shanmugam S, Verma TN, Pugazhendhi A. Biofilm and quorum sensing mediated pathogenicity in Pseudomonas aeruginosa. Process Bio-chem. 2020;96:49-57.
Crossref - Das T, Manoharan A, Whiteley G, Glasbey T, Manos J. Pseudomonas aeruginosa biofilms and infections: roles of extracellular molecules. In Yadav MK, Singh BP (Eds.). New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms: Current Research and Future Trends. 2020:29-46.
Crossref - Davey ME, O’Toole GA, et al. Microbial biofilms: From ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64(4):847-867.
Crossref - Ahuja K, and Singh, S. Biosurfactants Market size by Products. Glob Mark Insight. 2023. https://www.gminsights.com/industry- analysis/ biosurfactants-market-report.
- Shaligram NS, Singhal RS, et al. Surfactin- A review on biosynthesis, fermentation, purification and applications. Food Technol Biotechnol. 2010;48(2):119-134.
- Anuradha SN. Structural and Molecular Characteristics of Lichenysin and Its Relationship with Surface Activity. In: Sen, R. (eds) Biosurfactants. Adv Exp Med Biol. 2010;672:304-315.
Crossref - Konz D, Doekel S, Marahiel MA. Molecular and biochemical characterization of the protein template controlling biosynthesis of the lipopeptide lichenysin. J Bacteriol. 2010;181(1):133-140.
Crossref - Harwood CR, Mouillon J-M, Pohl S, Arnau J. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. 2018;42(6):721-738.
Crossref - Meena KR, Kanwar SS. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Res Int. 2015;2015:473050.
Crossref - Chen X, Lu Y, Shan M, Zhao H, Lu Z, Lu Y. A mini-review: mechanism of antimicrobial action and application of surfactin. World J Microbiol Biotechnol. 2022;38(8):143.
Crossref - Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238-250.
Crossref - Kamal MS. A Review of Gemini Surfactants: Potential Application in Enhanced Oil Recovery. J. Surfactants Deterg. 2016;19(2):223-236.
Crossref - Negin C, Ali S, Xie Q, Xie Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum. 2017;3(2):197-211.
Crossref - Sar P, Ghosh A, Scarso A, Saha B. Surfactant for better tomorrow: Applied aspect of surfactant aggregates from laboratory to industry. Res Chem Intermed. 2019;45(12):6021-6041.
Crossref - Guo Y, Huang E, Yuan C, Zhang L, Yousef AE. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl Environ Microbiol. 2012;78(9):3156-3165.
Crossref - Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697-703.
Crossref - Jheel WA, Salim RM, Bneed QZ, Klaif SF. Phylogenetic Analysis of Proteus SPP from Cave-Dwelling Bats That are Risk to Human in Iraq. Ann Rom Soc Cell Biol. 2021;25(1):6936-6943.
- Yi PJ, Pai CK, Liu JR. Isolation and characterization of a Bacillus licheniformis strain capable of degrading zearalenone. World J Microbiol Biotechnol. 2011;27(5):1035-1043.
Crossref - Sankhwar R, Kumar A, Yadav S, Singh V, Gupta RK. Emycin-E purified from Streptomyces sp. RG1011 from Himalayan soil has antibiofilm activity against Staphylococcus aureus. Microb Pathog. 2023;182:106256.
Crossref - Yang H, Li X, Li X, Yu H, Shen Z. Identification of lipopeptide isoforms by MALDI-TOF-MS/MS based on the simultaneous purification of iturin, fengycin, and surfactin by RP-HPLC. Anal Bioanal Chem. 2015;407(9):2529-2542.
Crossref - Zanganeh MN, Kam SII, LaForce TCC, Rossen WRR. The method of characteristics applied to oil displacement by foam. SPE Journal. 2011;16(01):8-23.
Crossref - Walter V, Syldatk C, Hausmann R. Screening Concepts for the Isolation of Biosurfactant Producing Microorganisms. Landes Bioscience. 2013. https://www.ncbi.nlm.nih.gov/books/NBK6189.
- Ambarwati A, Wahyuono S, Moeljopawiro S, Yuwono T. Antimicrobial activity of ethyl acetate extracts of Streptomyces sp. CRB46 and the prediction of their bioactive compounds chemical structure. Biodiversitas Journal of Biological Diversity 2020;21(7).
Crossref - Deepashree CL, Kumar JKK, Prasad AGD, Mahsa Z, Gopal S. FTIR spectroscopic studies on cleome gynandra – comparative analysis of functional group before and after extraction. Rom J Biophys. 2013;22:137-143.
- Yeak KYC, Perko M, Staring G, et al. Lichenysin Production by Bacillus licheniformis Food Isolates and Toxicity to Human Cells. Front Microbiol. 2022;13:831033.
Crossref - Smith ML, Gandolfi S, Coshall PM, Rahman PKSM. Biosurfactants: a Covid-19 Perspective. Front Microbiol. 2020;11:1341.
Crossref - Khubaib MA, Raza ZA, Abid S, Nazir A, Tariq MR. Cell-free culture broth of Pseudomonas aeruginosa-an alternative source of biodis-persant to synthetic surfactants for dyeing the polyester fabric. J Surfactants Deterg 2021;24(2):343-355.
Crossref - Coronel-Leon J, Marques AM, Bastida J, Manresa A. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J Appl Microbiol. 2016;120:99-111.
Crossref - Santos VSV, Silveira E, Pereira BB. Toxicity and applications of surfactin for health and environmental biotechnology. J Toxicol Environ Health Part B. 2018;21:382-399.
Crossref - Raza ZA, Shahzad Q, Rehman A, Taqi M, Ayub A. Biosurfactants in the sustainable eradication of SARS COV-2 from the environmental surfaces. 3 Biotech. 2022;12(10):273.
Crossref - Gupta RK, Alba J, Xiong YQ, Bayer A S, Lee C Y. MgrA Activates Expression of Capsule Genes, but Not the a-Toxin Gene in Experimental Staphylococcus aureus Endocarditis, J Infect Dis. 2013;208(11):1841-1848.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.