ISSN: 0973-7510
E-ISSN: 2581-690X
Dry microalgal biomass was previously tested as prebiotic to enhance the growth of probiotic bacteria. However, the drying process could be ineffective for scaling up probiotic production. This study aimed to investigate the use of fresh, pretreated microalgal biomass to promote the growth of Lactobacillus plantarum JCM 1149. Chlorella sp. KLSc61 cells were pretreated by three different methods: physical treatment with microwave radiation at power levels of 300, 500, and 700 W; chemical treatment with 0.1 M citric acid and 0.5 M sodium hydroxide; and biological treatment with cellulase enzyme. The 2.5% pretreated Chlorella cells were then added to L. plantarum JCM 1149 culture, and the growth was observed at 37 °C for 24 h of incubation. The results showed that, during the log phase (6-10 h), Chlorella cells pretreated with microwave radiation at 700 W were the most effective in promoting L. plantarum JCM 1149 growth, which was 1.4- and 1.5-fold of L. plantarum JCM 1149 without adding Chlorella and with untreated cells, respectively. Extension of the pretreatment time by microwave radiation at 700 W from up to 2 min increased the growth of L. plantarum JCM 1149 up to 1.8-fold of pretreatment time by microwave radiation at 700 W 1 min, compared to the control groups. Additionally, increasing the amount of Chlorella biomass up to 5% (w/v) extended the log phase of L. plantarum JCM 1149 and increased cell accumulation during the stationary phase. Unlike dry microalgal biomass, the simplicity of fresh, pretreated Chlorella biomass shown in this study may facilitate large-scale, commercial production of L. plantarum strains as probiotics.
Chlorella sp. KLSc61, Cell Pretreatment, Lactobacillus plantarum, Microalgae Biomass
Nowadays, healthy and functional foods are widely popular, and their nutritional value and health benefits are considered by most consumers. Functional food is defined as food containing some components or bioactive substances that provide benefits beyond basic nutrition, e.g., probiotic bacteria, plant extracts, and algal extracts. Additionally, it plays a significant role in reducing health risks and preventing some illnesses caused by diseases. Probiotic bacteria are gaining more interest, because of their benefit in maintaining the microflora balance in the human digestive system.1 Probiotic strains that are popular in the functional food market, e.g., Lactobacillus plantarum, L. rhamnosus, L. acidophilus, L. casei, L. reuteria, Bifidobacterium bifidum, B. infantis, B. longum, and Streptococcus lactis, have been previously reported for their ability to inhibit pathogenic microbes, reduce inflammation and other gastrointestinal disorders, support various processes in the nervous system and synthesize vitamin K, riboflavin, and folate.2-4 Several studies showed that the benefits of probiotic bacteria could be further enhanced by the addition of prebiotics, growth-promoting and nondigestible components that increased the growth of beneficial bacteria.
Generally, prebiotics were obtained from carbohydrate-rich or high-dietary fiber plants, such as bananas, tomatoes, asparagus, garlic, and legumes.5,6 However, the quality control of prebiotic production from plants could be challenging because of various factors that may affect plant growth and the need for large cultivation areas. Alternatively, microalgae have become potential prebiotic resources. Dry microalgal biomass contains high carbohydrate contents compared to plants. Algae have higher carbohydrate content than terrestrial plants (20-50% dry weight) and possess simpler carbohydrate structures like starch and various polysaccharides, making them more suitable for bioenergy production. In contrast, plants have more complex structures due to lignocellulosic biomass, making their carbohydrates difficult to break down.7-9 Several reports revealed that Scenedesmus intermedius has carbohydrate for 271.3 mg/g of dry biomass, Klebsormidium flaccidum GN-2 has carbohydrate for 0.304 g/L of starch, and Chlorella spp. have carbohydrate 230-260 mg/L of dry weight, especially C. vulgaris, which contains total fiber up to 261.8 mg/g of dry biomass.10,11 Additionally, microalgae contain other basic nutrients essential to the human body, such as proteins, minerals, vitamins, and lipids. Unlike plants, microalgae grow rapidly. Growth requirements are minimal and can be easily modified for yield optimization. Furthermore, microalgae do not require agricultural land for upscale production. These benefits indicate that microalgae are potential resources for prebiotic production.
The use of microalgal biomass as a prebiotic is challenging because of the recalcitrance of their cell wall. Physical, biological and chemical pretreatment methods have been previously tested for microalgal cell wall disruption. A previous study showed that pulsed electric field (PEF) was the most efficient for extraction in Chlorella vulgaris, resulting to be an effective technique to release small ionic solutes and carbohydrates up to 75% and 39%, respectively.12 Previous studies demonstrated the benefits of pretreatment methods on dry microalgal biomass. Several drying methods have been used to reduce moisture content, including oven drying, sun drying, drum drying, freeze drying, spray drying, and microwave drying. Despite the benefits of drying methods in preserving valuable microalgal components and enhancing storage stability,13 the process could be time-consuming and non-economical for a large-scale production. To reduce the time and processes of drying microalgal biomass and to preserve all vital components of microalgae as is. In this study, we investigated the potential of fresh, pretreated Chlorella sp. KLSc61 as an effective and economically viable prebiotic for enhancing L. plantarum JCM 1149 growth. This advancement offered a significant improvement over traditional dry biomass methods, providing an alternative way for more efficient and scalable prebiotic production for the functional food industry.
Microalga strain and microalgal biomass preparation
Chlorella sp. KLSc61 was isolated and obtained from a previous study.14 Chlorella stock cultures were grown and maintained on Tris Acetate Phosphate (TAP) agar,15 and all Chlorella growth experiments were performed in TAP broth under continuous light at 7,400 lux and 27 °C on a rotary shaker at 120 rpm.
To determine the harvesting stage of the Chlorella cell mass for pretreatment experiments, Chlorella cell culture was conducted as follows. Single colonies were inoculated into 100 mL of TAP broth in a 250 mL flask for 36 h to obtain a starter culture. Cell concentration was determined by hemocytometer (Boeco, Germany). The starter culture was subsequently transferred to fresh 100 mL of TAP broth in a 250 mL flask with an initial cell concentration of 1 × 105 cells/mL. Subsequently, cells were counted every 24 h for 10 days using the hemocytometer.
To prepare Chlorella biomass, a Chlorella colony was picked up on a stock plate and then inoculated in 100 mL of TAP broth. After 3 days of stock culture, Chlorella cells were inoculated in a new 500 mL of TAP broth in two 1-L flasks to make an initial cell concentration of 1 × 105 cells/mL. The culture was grown for 5 days under similar growth conditions. Cells were harvested by centrifugation at 3,500 × g for 5 min. The supernatant was discarded. The cell pellet was re-suspended and washed in 20 mL of distilled water and collected by centrifugation as described above. This was repeated three times. The collected pellet was kept and stored at -20 °C until the pretreatment experiment.
Preparation of bacterial cultures
L. plantarum JCM 1149 was purchased from the Japan Collection of Microorganisms (JCM), and the strain was grown and maintained on de Man, Rogosa, and Sharpe (MRS) agar (Difco™ & BBL™, USA) and stored at 4 °C until use. Before use, L. plantarum JCM 1149 from stock culture was reactivated by streaking on MRS agar and incubating at 37 °C for 24 h. To generate the L. plantarum JCM 1149 growth curve, a single colony was inoculated into 5 mL of MRS broth in a 15 mL test tube and incubated at 37 °C for 24 h. Subsequently, 200 µL of the culture, which was equal to 0.5 McFarland standard, was transferred into a new test tube containing 5 mL MRS broth. The culture was incubated at 37 °C, and the optical density (OD) at 600 nm was measured at 2 h intervals over the first 12 h of cultivation using a microplate reader (Biochrom EZ Read 2000). Additionally, the culture was collected at every hour from 4 to 6 h of cultivation and serially diluted from 10-1 to 10-10, using 0.85% (w/v) NaCl 20 µL of each dilution was dropped onto MRS agar using the drop-plating technique to determine the growth of L. plantarum JCM 1149 as CFU/mL.16 All plates were incubated at 37 °C for 24 h, counted, and reported as CFU/mL as determined by the formula below. All experiments were conducted in triplicate.
CFU/mL = Average number of counted colonies × calculate in milliliters Unit / Dilution factor
Pretreatment of microalgal biomass
To prepare 5% (w/v) of Chlorella biomass, 1 g of Chlorella biomass was resuspended in 20 mL of distilled water. The 5% (w/v) of cell suspension was pretreated, using the physical and chemical methods. Briefly, for the physical pretreatment, the suspension was heated, using microwave irradiation at power levels 300, 500 and 700 W for 1 min. For the chemical pretreatment, the suspension was mixed with 0.1 M citric acid or 0.5 M sodium hydroxide. The mixture was stirred for 3 h. Pretreated cells were collected by centrifugation at 3,000 × g for 5 min and subsequently washed with 20 mL of distilled water. These steps were repeated until the suspension reached pH 7. The biological pretreatment followed the protocol described in a previous study.17 0.5 g of microalgal biomass was resuspended in 10 mL of citrate buffer (pH 4.8) supplemented with 0.01 g of cellulase powder to obtain the initial enzyme activity at 100 units/g. The mixture was incubated at 50 °C for 2 h and subsequently at 100 °C for 3 min for enzyme deactivation. Pretreated cells were obtained by centrifugation and washed with distilled water as described for the chemical pretreatment.
In vitro study of Lactobacillus plantarum JCM 1149 growth measurement
5 mL of the 5% (w/v) of Chlorella pretreated biomass suspension (in Material and Methods above) was added to 5 mL of L. plantarum JCM 1149 culture (1 × 106 CFU/mL). The bacterial culture was incubated at 37 °C for 24 h. Bacterial growth (CFU/mL) was determined, using the drop-plating technique at 0, 2, 4, 6, 8, 12, and 24 h. All experiments were performed in triplicate.
Optimization of microalgal biomass pretreatment and bacterial growth enhancement
The microwave irradiation at 700 W varied between 1- and 2-min, suspension of the pretreated cells was prepared as described above. To determine the optimal pretreated cell concentration, the final concentration was adjusted to 2.5% and 5% (w/v). The effects of the pretreated cells on L. plantarum JCM 1149 was investigated as described in the previous experiment. The experiment was performed in three replicates.
Statistical analysis
All the experiments were designed in a completely randomized design (CRD), and the data variability was analyzed via one-way ANOVA. Statistically significant differences between means were evaluated using Duncan’s multiple range test with IBM SPSS Statistics version 29, with significance set at p < 0.05. Each experiment was performed in triplicate. The optimization with response surface methodology (RSM) was applied to identify optimal CFU using best Watt and time level using the Minitab® 19 software.
Chlorella sp. KLSc61 harvesting stage
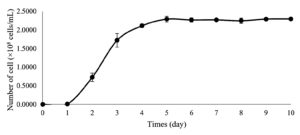
The growth curve of Chlorella sp. KLSc61 was generated (Figure 1) to determine the harvesting stage. Chlorella growth was relatively unchanged during the first 24 h of cultivation. This was followed by a rapid increase of microalgal cell numbers that reached 2.11 ± 0.05 × 108 cells/mL on day 4. Biomass accumulation was the highest (2.28 ± 0.08 × 108 cells/mL) on day 5 and remained relatively stable until day 11. As a result, the log phase was determined as between day 1 and day 5 of the cultivation, and day 5 was used as the harvesting stage to obtain the maximum number of Chlorella sp. KLSc61 cells in the following experiments.
Lactobacillus plantarum JCM 1149 growth enhancement by pretreated biomass of Chlorella sp. KLSc61
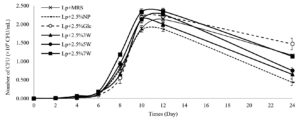
The effects of pretreated Chlorella sp. KLSc61 biomass on L. plantarum JCM 1149 growth are shown in Figure 2. For the physical pretreatment (Figure 2A), microwave irradiation at different levels resulted in distinct bacterial growth rates. At 8 h, the highest bacterial growth (1.18 ± 0.23 × 109 CFU/mL) was obtained with the addition of microalgal biomass that was pretreated at 700 watts (Lp + 2.5% 7W). This was followed by the pretreatment at 500 W (Lp + 2.5% 5W). A statistically insignificant difference was observed between the addition of microalgal biomass with 300 W (Lp + 2.5% 3W) pretreatment and the control group supplied with non-pretreated cells (Lp + 2.5% NP). At 24 h, supplementation with 2.5% glucose (Lp + 2.5% Glc) in the control group yielded 1.47 ± 0.15 × 109 CFU/mL, while addition of the 700-watts pretreated biomass resulted in the second highest level (1.13 ± 0.23 × 109 CFU/mL). This was followed by Lp + 2.5% 5W and Lp + 2.5% 3W at 0.75 ± 0.82 × 108 CFU/mL and 0.65 ± 0.11 × 109 CFU/mL, respectively. In contrast, Lp + 2.5% NP displayed the lowest level at 0.43 ± 0.88 × 109 CFU/mL.
0.1 M Citric acid and 0.5 M NaOH were used for the chemical pretreatment of the microalgal biomass. The effect of these pretreated biomass on L. plantarum JCM 1149 is shown in Figure 2B and 2C. Chemically pretreated Chlorella was able to slow the decline in bacterial growth upon entering the stationary phase, compared to non-pretreated biomass (Lp + 2.5% NP). At 10 and 12 h, Lp + 2.5% NP growth was comparable with those added with microalgal cells pretreated with 0.1 M citric acid (Lp + 2.5% 0.1A) and 0.5 M NaOH (Lp + 2.5% 0.5B). However, at 12 h, Lp + 2.5% NP growth (0.43 ± 0.08 × 109 CFU/mL) became approximately 0.5 and 0.3 folds of Lp + 2.5% 0.1A (0.87 ± 0.08 × 109 CFU/mL) and Lp + 2.5% 0.5B (1.57 ± 0.27 × 109 CFU/mL). A distinction was also observed between the effect of the two chemicals. The growth of Lp + 2.5% 0.1A was lower than that of Lp + 2.5% Glc from 8 h and thereafter. In contrast, Lp + 2.5% 0.5B exhibited growth comparable to Lp + 2.5% Glc from 8 to 24 h of the cultivation.
Cellulase was used as the biological pretreatment of Chlorella biomass. The growth of L. plantarum JCM 1149 added with cellulase-pretreated microalgal cells (Lp + 2.5% C) increased rapidly upon entering the log phase at 6 h and reached 2.18 + 0.08 × 109 CFU/mL at 10 h (Figure 2D). This was significantly higher than that of Lp + 2.5% NP (1.85 ± 0.20 × 109 CFU/mL). Additionally, at 24 h, the cell accumulation of Lp + 2.5% C (0.90 ± 0.14 × 109 CFU/mL) was 2.18-fold of Lp + 2.5% NP (0.43 ± 0.08 × 109 CFU/mL). However, the growth of Lp + 2.5% C had a lower level of cell accumulation when compared with Lp + 2.5% Glc at 10 h and thereafter. From these experiments, the finding suggested that microwave pretreatment, especially 700 W, of Chlorella sp. KLSc61 biomass showed the best result to promote L. plantarum growth JCM 1149, compared to other pretreatment methods.
(A)
(B)
(C)
(D)
Figure 2. Growth measurement of Lactobacillus plantarum JCM 1149 after supplementation with 2.5% (w/v) pretreated biomass of Chlorella sp. KLSc61 via four types of pretreatment methods: (A) supplementation with Chlorella sp. KLSc61 biomass pretreated by power levels of a microwave; (B) supplementation with Chlorella sp. KLSc61 biomass pretreated by 0.1 M citric acid; (C) supplementation with pretreated Chlorella sp. KLSc61 biomass pretreated by 0.5 M sodium hydroxide; (D) supplementation with Chlorella sp. KLSc61 biomass pretreated by cellulase enzyme. All experiments were done in triplicate and statistical analysis using ANOVA and DUNCAN at p-value < 0.05
Effect of timing and biomass concentration of Chlorella sp. KLSC61-pretreated cells on the growth of Lactobacillus plantarum JCM 1149
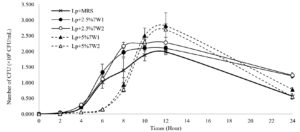
Among the pretreatment methods used in this study, Lp + 2.5% 7W showed the highest growth during the log phase from 8 h to 12 h of the cultivation. Bacterial growth enhancement was further optimized by adjusting the irradiation timing from 1 min to 2 min and the pretreated biomass concentration from 2.5% to 5%. At 8 h, the highest bacterial cell accumulation level (2.17 ± 0.12 × 109 CFU/mL) was observed with the addition of 2.5% Chlorella irradiated for 2 min (Lp + 2.5% 7W2). This was followed by Lp + 2.5% 7W1 (1.97 ± 0.13 × 109 CFU/mL) which was supplemented with 2.5% Chlorella biomass pretreated for 1 min. In contrast, the use of 5% biomass pretreated for 1 min (Lp + 5% 7W1) and 2 min (Lp + 5% 7W2) resulted in significantly lower growth. However, at 10 h, the growth of Lp + 5% 7W1 and Lp + 5% 7W2 rapidly increased and became comparable to that of Lp + 2.5% 7W1 and Lp + 2.5% 7W2. Additionally, at 12 h, the highest level of bacterial growth was observed in Lp + 5% 7W1 (2.82 ± 0.06 × 109 CFU/mL) and followed by Lp + 5% 7W2 (2.70 ± 0.53 × 109 CFU/mL). These levels were relatively higher than that of Lp + 2.5% 7W1 (2.10 ± 0.07 × 109 CFU/mL) and Lp + 2.5% 7W2 (2.28 ± 0.16 × 109 CFU/mL). At 24 h, Lp + 2.5% 7W2 was able to maintain the viable cell number at 1.23 ± 0.08 × 109 CFU/mL, followed by Lp + 2.5% 7W1, Lp + 2.5% 7W1 and Lp + 2.5% 7W2, respectively (Figure 3). These results indicated the benefits of increasing pretreating time to 2 min and microalgal concentration to 5% on cell accumulation of L. plantarum JCM 1149.
Figure 3. Growth measurement of Lactobacillus plantarum JCM 1149 after supplementation with Chlorella sp. KLSc61 biomass at concentrations of 2.5% and 5% (w/v) conditioned with microwaves for different durations
Optimization of colony forming unit with a microwave watt and time
The optimization of colony forming unit (CFU) was used to estimate the concentration of L. plantarum JCM 1149 in the tested sample. A Box-Behnken design was used for a response surface methodology with a total of 72 runs in triplicate. The two variables, level of the SI unit of the power in an electric circuit or watt of a microwave (Watt, 300 – 700W) and time for growing L. plantarum JCM 1149 (Time, 0-24 h) were tested along with colony forming unit (CFU) used to estimate the concentration of L. plantarum JCM 1149 in the tested sample as responses. The maximizing variability of the concentration of bacterium could be explained by the relationship can be modeled with second-order polynomial equation with watt and time variable as the following Equation:
CFU = -472 + 285.3 Time – 0.50 Watt – 10.36 Time*Time + 0.00055 Watt*Watt + 0.0564 Time*Watt
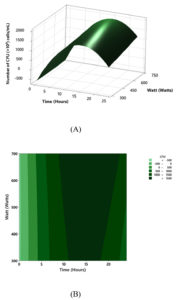
The goal of optimization in this study was to obtain the maximum CFU value using adjusting watt and time. The optimization process was done by modeling and supporting visually by surface plot and contour plot. CFU was designated as the dependent variable, while watt of microwave (W) and time for growing bacteria (Times) were independent ones. How changes in watt and time affected CFU was visualized by the 3D surface plot. The plot in Figure 4A showed the increase of watt and time caused an increase of bacterial CFU. The increase of Watt caused a surface tension to increase. In contrast, the increase of time caused the surface increase up to the optimal time (more than 12 h), and then a decrease in the CFU.
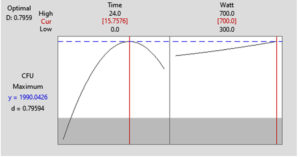
According to the graphical perspective of the watt-time plane with the top-down view (Figure 4B), the contour plot revealed a higher CFU area (>1,500), which may be part of an elliptic region at the top of a concave 3D surface plot. The result indicated that 700 W with 15.75 h to produce the maximized CFU with 1.99 × 109 CFU/mL. Figure 5 showed that both the middle level of the time and highest level of watt were the best to achieve the maximum CFU and also defined the optimal CFU level is defined as 700 W and 15.7576 h.
Figure 4. (A), Three-dimensional surface plot of contour maps of the effect of watt and time on CFU surface; (B), contour maps of the effect of watt and time on CFU
Harvesting time is an important factor that affects the production of microalgal biomass. Generally, the late log phase is considered optimal for harvesting microalgal cells because of the relatively high cell number and protein content which consequently lowers the production cost.18 In contrast, early or late harvesting may result in insufficient cell numbers or protein degradation, respectively.19 In the present study, the growth curve analysis indicated that the late log phase of Chlorella sp. KLSc61 occurred on day 5 with the maximum cell number (2.28 ± 0.08 × 108 cells/mL). As a result, day 5 was designated as the optimal harvesting time of this microalga. Growth conditions play an important role in determining the harvesting time. This was relatively similar in a previous study where the starting point of the stationary phase was day 6 for Chlorella vulgaris cultivated in Detmer’s medium.20 There are various factors that determine the optimal harvesting time. Macronutrient types and contents are generally tested for their effects on microalgal growth. For example, nitrate stimulated the growth of C. vulgaris CV 211-11b, while replacing nitrate with nitrite resulted in a reduction of cell growth. Supplementation of medium with nitrate resulted in the growth rate that was approximately 1.6 folds of the use of nitrite.21 Light/dark cycling also affects biomass accumulation of microalgae. Chlamydomonas sp. JSC4 exhibited a higher growth rate when grown under the continuous light condition, compared to the 12 h:12 h light/dark condition.22 Thus, the relatively early harvesting time (day 5) of Chlorella sp. KLSc61 may be contributed by the use of continuous light during the cultivation in the present study.
Microalgal cells accumulate various types of intracellular primary and secondary metabolites while being cultivated. These metabolites may be beneficial for the growth of probiotic bacteria, enabling microalgae as alternative prebiotic resources. However, the bacterial access to the intracellular microalgal metabolites is considered a challenging factor that affects the use of microalgae as prebiotics. Pretreatments of microalgal cells are often necessary for facilitating the breakdown of the cell wall and promote the release of valuable intracellular compounds. The present study demonstrated the effects of physical, chemical and biological pretreatments of Chlorella sp. KLSc61 on the growth of L. plantarum JCM 1149. Microwave irradiation was found the most effective method for the pretreatment of microalgal cells. The effect of the physical pretreatment also depended on the power levels, as indicated by the direct relationship between the microwave power levels (300, 500 and 700 W) and the bacterial growth levels (Figure 2A). High-frequency electromagnetic waves in the microwave cause water and other liquid molecules inside algal cells to vibrate rapidly, generating the heat.23 The heat may contribute to the breakdown of glycosidic bonds found in cellulose and hemicellulose of the cell wall, possibly leading to the formation of smaller polysaccharide molecules, i.e. oligosaccharides, disaccharides and monosaccharides. Moreover, the breakdown of Chlorella cell wall could release intracellular substances to outside of algal cells. These smaller molecules of polysaccharide and intracellular substances could promote the growth of L. plantarum JCM 1149. Our result was consistent with a previous study where microwave irradiation of C. vulgaris cells with a power level of 700 W for 50 s increased the yield of the lipid extraction from 28.9% to 31.7% of dry biomass.24 Additionally, pretreatment with microwave irradiation generated pores on Chlorella cell wall, which consequently led to the rupture of the cell membrane. For the chemical pretreatment, the relatively lower bacterial growth in Lp + 2.5% 0.1A, Lp + 2.5% 0.5B and Lp + 2.5% C was likely due to the low chemical concentrations used in the present study. Previously, Prasetia et al. demonstrated that sodium hydroxide at 0.5 M, 1 M and 1.5 M was necessary for the separation of lignin from cellulose.25 Utilization of cellulase was previously successfully used in combination with ultrasound waves for the pretreatment of Gracilaria verrucosa.26 The reducing sugar yield increased from 0.5% to 2% (w/v). In contrast, our study indicated that the use of cellulase as the only biological pretreatment was likely insufficient to promote the breakdown of the cell wall of Chlorella sp. KLSc61. However, all pretreated microalgal cells were able to enhance the bacterial growth when compared to the use of non-pretreated cells. This was likely because live Chlorella sp. KLSc61 cells may produce and secrete extracellular substances that negatively affected the growth of bacterial cells.27 Additionally, we found that the addition of glucose resulted in a slower increase of bacterial growth. This was indicated by the lower bacterial growth of Lp + 2.5% Glc, compared to other groups supplied with pretreated cells at 8 h of the cultivation. This was likely because L. plantarum is a bacterium that thrives in environments with moderate glucose levels.28,29 Although glucose is a primary carbon source for many Lactobacillus species, an excessive amount may cause osmotic stress, leading to reduced cell viability or altered metabolic pathways.30
Optimization of microwave irradiation time and biomass concentration was able to further enhance L. plantarum JCM1149 growth. We found that both parameters interconnectedly affected bacterial growth. Microwave irradiation at 2 min was better than 1 min for bacterial growth when the biomass concentration was 2.5%. The increase of microwave irradiation time was previously demonstrated to promote the extraction of lutein from Chlorella sorokiniana (NIOT-2). The increase of the microwave exposure time from 15 s to 180 s significantly maximizes the lutein content at 7.10 + 0.32 mg/g. However, longer exposure durations lowered the extracted lutein content.31 This supports the better growth promotion of microalgal cells pretreated with microwave for 2 min in the present study. For biomass concentration, a previous experiment showed that growth of Lactobacillus brevis strains was elevated when supplied with C. vulgaris at 1.5% (w/v), compared to 0.1% (w/v).32 This was in contrast to our study, where the 5% microalgal biomass concentration delayed the entering to log phase of the bacterium to day 8, as opposed to day 6 found in 2.5% microalgal biomass concentration. This was likely due to an excessive amount of microalgal metabolic compounds which required a longer period for bacterial adjustment. The results obtained in our study showed that the optimized pretreatment of fresh Chlorella sp. KLSc61 cells significantly enhanced the growth of L. plantarum JCM 1149. This suggests its application in other marketable Lactobacillus strains. Chlorella cells could become an alternative resource of prebiotic for probiotic bacteria production in pharmaceutical, nutraceutical and function food aspects.
Table:
Comparison of the pretreatment methods and their benefits in the current study and previous studies
No. |
Microalgae |
Probiotic strains |
Optimum pretreatments |
Benefits |
Ref. |
|---|---|---|---|---|---|
1 |
Chlorella sp. KLSc61 |
Lactobacillus plantarum JCM 1149 |
– Microwave 700 W – Fresh microalgal cells |
Increase of L. plantarum JCM 1149 growth |
This study |
2 |
Arthrospira (Spirulina) platensis mixed with Chlorella vulgaris |
Bifidobacterium animalis and Lactobacillus casei |
– Phosphoric acid hydrolysis – Lyophilized microalgal/ cyanobacterial cells |
Increase of bacterial biomass, lactic acid and acetic acid |
33 |
3 |
Arthrospira platensis and C. vulgaris |
Lactobacillus rhamnosus |
– Pulsed electric fields (PEF) (3 kV/cm, 100 kJ/kg) – Chlorella and Spirulina extracts |
Increase of L. rhamnosus growth, short chain fatty acid and 3-phenyl lactic acid |
34 |
4 |
C. vulgaris, Desmodesmus maximus, Chlorococcum sp. cf hypnosporum and Spirulina platensis |
Lactobacillus sp. Enterococcus sp. Bifidobacterium spp. |
– Enzymatic digestion by in vitro gastrointestinal digestion – Freeze-dried digested microalgae biomass |
Increase the relative abundance of probiotic bacterial cells, promote some phenolics, propionic and acetic acids |
35, 36 |
5 |
A. platensis F&M-C256 |
Lactobacillus plantarum ATCC 8014 |
– non-pretreatment – Lyophilized A. platensis biomass |
Increase of L. plantarum ATCC 8014 growth |
37 |
7 |
C. pyrenoidosa |
Prevotella sp. Butyrivibrio sp. Alistipes sp. |
– non-pretreatment – Freeze-dried microalgae biomass |
Promoting the growth of some gut microbial community |
38 |
8 |
Isochrysis galbana |
Lactobacillus reuteri |
– non-pretreatment – Freeze-dried microalgae biomass |
Represent a reliable culture medium for growing L. reuteri |
39 |
In previous studies, various types of research attempted to develop the optimum pretreatment technology to utilize microalgae biomass or extracts for a benefit of growing probiotic bacteria (Table). For example, using mild phosphoric acid thermopressurization under pH 2.0 and 4.5 atm (156 °C) to treat lyophilized microalgae integrated with cyanobacteria biomass could enhance Bifidobacterium animalis and Lactobacillus casei growth.33 Moreover, other methods such as Pulse electric field (PEF) and enzymatic digestion were proposed to pretreat microalgae cell mass and resulted in promoting probiotic bacterial growth and biomass including some bioactive compounds and organic acids as summarized in Table. From all previous works, we found that no one has used fresh microalgae biomass pretreated with physical pretreatment conditions such as microwave which was a shorten step to prepare microalgae cell to feed and promote the growth of probiotic bacteria. Our results (Figure 2A) showed that using microwave treatment for a short period of time promoted the growth of L. plantarum JCM 1149, which was likely due to the preservation of bioactive compounds. This was consistent with the objective of other previous studies to reduce energy consumption and process time for pretreatment. Additionally, integrated approaches, such as combining chemical and biological methods or using ultrasound in conjunction with microwaves, were possibly developed to enhance yields and make the process more suitable for commercially industrial applications. These studies reflect modern efforts to develop highly efficient technologies for microalgae processing, aiming to establish a cost-effective biorefinery process that is practical for industrial-scale implementations.
This study investigated the effects of physical, chemical and biological pretreatment of Chlorella sp. KLSc61 biomass on the enhancement of L. plantarum JCM 1149 growth. The result showed the physical pretreatment with microwave irradiation at 700 W for 1 min was better than other methods. The increase of the irradiation time to 2 min also increased the bacterial growth promoting effect of the microalgal biomass. The higher biomass concentration at 5% may result in a higher bacterial growth but delayed the entering of the log phase. The result shown here indicated the potential application of pretreated Chlorella sp. KLSc61 as a prebiotic resource to enhance the growth of other probiotic Lactobacillus strains. Additionally, this study investigated the best strategy of Chlorella sp. KLSc61-pretreated biomass, and different pretreatment methods were applied, including microwave irradiation at power levels of 300, 500, and 700 W; chemical pretreatment with 0.1 M citric acid (acidic compound) and 0.5 M sodium hydroxide (basic compound); and biological pretreatment with cellulase enzymes. The results showed that the most suitable condition was the addition of Chlorella sp. KLSc61 biomass pretreated with microwave irradiation at 700 W and a concentration of 2.5% (w/v) to L. plantarum JCM 1149 growth medium (MRS), which significantly promoted the growth of L. plantarum JCM 1149. This could increase L. plantarum JCM 1149 growth up to 1.1 and 1.4 times greater than that of untreated Chlorella biomass and L. plantarum in MRS media without added algae biomass, respectively. Furthermore, extending the time of microwave pretreatment combined with increasing the biomass concentration significantly improved L. plantarum JCM 1149 growth either by enhancing L. plantarum JCM 1149 cell accumulation and/or expanding L. plantarum JCM 1149 exponential phase. Based on this finding, we interestingly investigated the substances that influenced increasing L. plantarum JCM 1149 growth. Polysaccharides from the algal cell wall or some bioactive compounds inside the algal cell can also exist. This would be interesting for the further use of algal cells as an alternative source of prebiotics for probiotic supplementation in pharmaceutical and nutraceutical use.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Biology, School of Science, King Mongkut’s Institute of Technology, Ladkrabang, for laboratory support, and all students in Wipawee’s laboratory.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
WD conceptualized the study, performed data curation, project administration and collected resources. LK applied methodology. PB and LK performed formal analysis. KL performed statistical analysis and data optimization. WD validated the study. LK wrote original draft. KW, CK, KL, CM and WD reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
The authors gratefully acknowledge the funding support from W.D. and School of Science, KMITL.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92.
Crossref - Seddik HA, Bendali F, Gancel F, Fliss I, Spano G, Drider D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics and Antimicro Prot. 2017;9(2):111-122.
Crossref - Reid G. The growth potential for dairy probiotics. Int Dairy J. 2015;49:16-22.
Crossref - Hamad G, Ombarak RA, Eskander M, et al. Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. Lebenson Wiss Technol. 2022;163(113603):113603.
Crossref - Chung WSF, Walker AW, Louis P, et al. Modulation of the human gut microbiota by dietary fiber occurs at the species level. BMC Biol. 2016;14:1-13.
Crossref - Can SS, Seyhaneyldiz E, Dorucu M. The Effects of Probiotic-Prebiotic on the Biomass and Protein Content of Spirulina platensis in Different Temperatures and Illuminations. Turk J Fish Aquat Sci. 2022;22(10):TRJFAS20844.
Crossref - Babich O, Sukhikh S, Larina V, et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties, and Applications. Plants. 2022;11(6):780.
Crossref - Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew Sustain Energy Rev. 2018;92:394-404.
Crossref - Smith AM, Zeeman SC. Starch: A Flexible, Adaptable Carbon Store Coupled to Plant Growth. Annu Rev Plant Biol. 2020;71:217-245.
Crossref - Gao L, Qin Y, Zhou X, et al. Microalgae as future food: Rich nutrients, safety, production costs and environmental effects. Sci Total Environ. 2024;927:172167.
Crossref - Bernaerts TMM, Gheysen L, Kyomugasho C, et al. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018;32:150-161.
Crossref - Postma PR, Pataro G, Capitoli M, et al. Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field-temperature treatment. Bioresour Technol. 2016; 203:80-88.
Crossref - Hernandez A, Gonzalez-Moya M, Marquez A, Acevedo L. Review microalgae drying: A comprehensive exploration from conventional air drying to microwave drying methods. Future Foods. 2024;10:100420.
Crossref - Laokua N, Rittiyan N, Kornrawudaphikasama Y, et al. Optimal conditions for maximized H2 yield from a new green algal strain Chlorella sp. KLSc61. J Appl Phycol. 2022;34(4):1909-1919.
Crossref - TAP and Tris-minimal. Chlamydomonas Resource Center. 2015. Accessed January 27, 2025. http://www.chlamycollection.org/TAP.html
- Kong C, Cheng L, Krenning G, et al. Human milk oligosaccharides mediate the crosstalk between intestinal epithelial Caco-2 cells and Lactobacillus plantarum WCFS1 in an in vitro model with intestinal peristaltic shear force. J Nutr. 2020;150(8):2077-2088.
Crossref - Jonjaroen V, Ummartyotin S, Chittapun S. Algal cellulose as a reinforcement in rigid polyurethane foam. Algal Res. 2020;51(102057):102057.
Crossref - Yixing S, Yu J, Moretti M, Siegfried EV. Harvesting time and biomass composition affect the economics of microalgae production. J Clea Prod. 2020;259:120782.
Crossref - Jothibasu K, Muniraj I, Jayakumar T, et al. Impact of microalgal cell wall biology on downstream processing and nutrient removal for fuels and value-added products. Biochem Eng J. 2022;187(108642):108642.
Crossref - Tavares L, Nudi MH, Arroyo PA, Godoy RFB, Trevisan E. Effect of different concentrations of phosphorus and nitrogen on the growth of the microalgae Chlorella vulgaris. Int J Energy Environ Eng. 2023;14:563-572.
Crossref - Pozzobon V, Cui N, Moreaud A, Michiels E, Levasseur W. Nitrate and nitrite as mixed source of nitrogen for Chlorella vulgaris: Growth, nitrogen uptake and pigment contents. Bioresour Technol. 2021;330:124995.
Crossref - Kato Y, Fujihara Y, Vavricka CJ, Chang JS, Hasunuma T, Kondo A. Light/dark cycling causes delayed lipid accumulation and increased photoperiod-based biomass yield by altering metabolic flux in oleaginous Chlamydomonas sp. Biotechnol Biofuels. 2019;12(1):39.
Crossref - Thiangthong N, Inthajak P, Jaichakan P, Panpa W, Pattarapisitporn A, Klangpetch W. Effects of Microwave-pretreatment of Rice Hull on Arabinoxylan Extraction for Xylooligosaccharides Production by Commercial Xylanases. Agricultural Sci J. 2019;50(1):526-532.
- Garoma T, Janda D. Investigation of the effects of microalgal cell concentration and electroporation, microwave and ultrasonication on lipid extraction efficiency. Renew Energy. 2016; 86:117-123.
Crossref - Prasetia IGNJA, Deviana S, Damayanti T, Cahyadi A, Wirasuta IMAG. The effect of NaOH concentration in delignification proceeds on microcrystalline cellulose from green algae (Cladophora sp.) as the renewable marine product. J Pharm Sci Community. 2018;15(2):68-71.
Crossref - Park MR, Jeong GT. Production of reducing sugar in Gracilaria verrucosa using physio-chemical pretreatment and subsequent enzymatic hydrolysis. Algal Res. 2021;60(102531):102531.
Crossref - Li Y, Xu Y, Song R, et al. Flocculation characteristics of a bioflocculant produced by the actinomycete Streptomyces sp. hsn06 on microalgae biomass. BMC Biotechnol. 2018;18(1):58.
Crossref - Esivan SMM, Rashid R, Jati A, Zaharudin NA. Growth of Lactobacillus casei and Propionibacterium jensenii in Different Glucose Concentration and Incubation Temperature. In: Proceedings of the 6th International Conference on Fundamental and Applied Sciences. Springer Nature Singapore. 2021:97-105.
Crossref - Noonin C, Putpim A, Thongboonkerd V. The direct inhibitory effects of Lactobacillus acidophilus, a commensal urinary bacterium, on calcium oxalate stone development. Microbiome. 2024;12(1):175.
Crossref - Senz M, Keil C, Schmacht M, Palinski S, Cammerer B, Hageback M. Influence of media heat sterilization process on growth performance of representative strains of the genus Lactobacillus. Fermentation. 2019;5(1):20.
Crossref - Mary LJT, Persia JT, Dharani G. Rapid green microwave assisted extraction of lutein from Chlorella sorokiniana (NIOT-2) – Process optimization. Food Chem. 2022;372:131151.
Crossref - Scieszka S, Klewicka E. Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria. Foods. 2020;9(7):959.
Crossref - Leal BES, Prado MR, Grzybowski A, et al. Potential prebiotic oligosaccharides from aqueous thermopressurized phosphoric acid hydrolysates of microalgae used in treatment of gaseous steakhouse waste. Algal Res. 2017;24(part A):138-147.
Crossref - Ricos-Munoz N, Soler AR, Castagnini JM, Moral R, Barba FJ, Pina-Perez MC. Improvement of the probiotic growth-stimulating capacity of microalgae extracts by pulsed electric fields treatment. Innov Food Sci Emerg Technol. 2023;83:103256.
Crossref - Minekus M, Alminger M, Alvito P, et al. A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct. 2014;5(6):1113-1124.
Crossref - de Medeiros VPB, de Souza EL, de Albuquerque TMR, et al. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021;60(102547):102547.
Crossref - Niccolai A, Shannon E, Abu-Ghannam N, Biondi N, Rodolfi L, Tredici MR. Lactic acid fermentation of Arthrospira platensis (spirulina) biomass for probiotic-based products. J Appl Phycol. 2019;31(2):1077-1083.
Crossref - Wan X, Li T, Liu D, et al. Effect of marine microalga Chlorella pyrenoidosa ethanol extract on lipid metabolism and gut Microbiota composition in high-fat diet-fed rats. Mar Drugs. 2018;16(12):498.
Crossref - Colantoni E, Palone F, Cesi V, et al. Innovative method to grow the probiotic Lactobacillus reuteri in the omega3-rich microalga Isochrysis galbana. Sci Rep. 2022;12(1):3127.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.