ISSN: 0973-7510
E-ISSN: 2581-690X
In the current study, fungi from the mangrove ecosystem of Mumbai were isolated and their metabolites were screened for antibacterial potential. Two weeks old broth and mycelium were extracted using chloroform and methanol. Antibacterial property of solvent extracts was evaluated at various concentrations (2 – 10 µg/ml) against Staphylococcus aureus, Bacillus subtilis, Enterococcus faecium, Enterococcus faecalis, Klebsiella pneumonia and Escherichia coli, by well diffusion method. Fungi isolated were identified as Aspergillus niger, Aspergillus flavus, Trichoderma harzianum, Cylindrocladium scoparium and Colletotrichum wuxiense. Results revealed that broth solvent extracts of isolates inhibited the growth of all gram-positive test bacteria, chloroform broth extract of Cylindrocladium scoparium, Colletotrichum wuxiense and ethanolic broth extract of Aspergillus flavus, Trichoderma harzianum exhibited antibacterial potential against gram negative test organisms. Chloroform and ethanol mycelium extracts of Trichoderma harzianum and Aspergillus flavus, respectively, exhibited 100% growth inhibition potential against all test organisms. The current investigation endorses the potent secondary metabolism of the identified isolates and their potential to synthesise antibacterial compounds.
Mangrove Ecosystem, Fungi, Secondary Metabolites, Antibacterial Activity
As per the WHO, the rising resistance of microbes against available antibiotics has left majority of the existing drugs obsolete.1 Since last two decades, the surge in the mortality rate due to infectious diseases is attributed to the emerging drug resistance of the microbes against available antibiotics.2 Since the emergence of drug resistance, the scientific community and the pharmaceutical firms have looked up to natural resources to find novel medications to address this lethal threat.3 The biotic community of the marine ecosystem is presumed to be a rich source of biochemicals with wide therapeutic applications. In earlier studies, many compounds like nigrospoxydons A – C, epoxydon, dehydroxychlorofusarielin B, penicipyrone, 7-oxobrefeldin A, spirodioxynaphthalene etc., have been isolated and characterised from marine fungi and bacteria with potential to treat various human ailments.4,5 Recent technological developments and methodologies have made it possible to prospect the marine environment more effectively and to quantify compounds derived from marine sources chemically and biologically.6,7 Thus, it has led to the discovery of new ways as well as a focus on these topics, with several marine microorganisms currently being used in medicine.8 Secondary metabolites from marine fungi are an extremely important source of anti-infective compounds.9,10 From the marine environment, mangrove is an important ecological, economic and social ecosystem. Mangrove fungi are also called manglicolous fungi, which include mostly marine fungi along with a few terrestrial species which can be found in mangrove areas.11 There is a great deal of biodiversity in mangrove forests and they constitute a transitional ecosystem which contains organisms that are either terrestrial or aquatic.12 Due to their adaptation to extreme environmental conditions, mangrove fungi have a unique source of genetic code and of great scientific interest because they account for the second largest proportion of fungi on the planet.13,14 Mangrove fungi are an important source of genetic data because they have distinctive metabolism.15,16 Studies are being conducted to better understand their genetic transformation and metabolism of the numerous unique antimicrobial metabolites that have been discovered from mangrove fungi in order to use them in the development of drugs.17,18 As a biodiversity hotspot, the mangrove ecosystem offers unique opportunities to uncover bioactive and chemical compounds of medicinal nature.19,20 In the recent past, a number of studies have been carried out on metabolites of mangrove-associated bacteria and fungi which has resulted in the discovery of some novel bioactive compounds.21,22 In the present study, fungi have been isolated and identified from the Mangrove ecosystem of the Mumbai coastal region and their metabolites have been evaluated for antibacterial potential.
Sample collection and Identification of Fungi
Water sample was collected from the Gorai Mangrove area, in Mumbai, India and stored in a sterile vial till further use. Within 24 hours of collection, the diluted sample was spread on Potato Dextrose agar medium (PDA) and incubated at room temperature for 3 – 4 days. PDA was supplemented with streptomycin to prevent bacterial contamination.23,24 Freshly cultured purified colony isolates were identified as described in earlier studies.25
Metabolite Extraction and Fermentation
Broth fermentation was carried out in a 250 ml Erlenmeyer Conical flask. A small block of mycelium (2 cm2) was taken from a pure culture plate and inoculated in 50 ml Potato Dextrose broth and cultured for 2 weeks at room temperature on a rotary shaker at rpm 150.26-28. After two weeks of incubation, broth of each isolate was treated with 50 ml Ethanol and 50 ml chloroform separately. The flasks were left on a rotating shaker set at 250 rpm for a day to allow metabolites to dissolve in the organic solvent.26 After 24 hrs, broth was filtered using double Whatmans filter paper (qualitative paper with grade 597).29 The filtrate was dried on a rotary evaporator and the left thick paste-like substance was stored at 4°C in glass vials for further studies. Further, mycelium collected was washed with warm water to ensure no residue of solvent and nutrient medium is left. The washed mycelium was oven-dried and coarse powdered using mortar and pestle. The dry mycelium coarse powder was extracted for its metabolites using ethanol and chloroform separately.30,31 In 50ml of solvent 10g of mycelium powder were added and left on a rotary shaker at 150 rpm for 48 hrs.32 The extracts were filtered after 48 hrs and the solvent was allowed to evaporate thus leaving behind a thick paste which was stored in glass vials at 4°C until further use.
Antibacterial Activity
Antibacterial activity of extracts from mycelium and broth fermentaion was carried out by the well diffusion method.33,34 Test organism used in the study were Staphylococcus aureus MTCC 96, Bacillus subtilis MTCC 441, Enterococcus faecalis MTCC 439, Enterococcus faecium MTCC 9728, Klebsiella pneumonia MTCC 432 and Escherichia coli MTCC 64. The bacterial cultures was collected from MTCC Chandigarh, Punjab. Cultures were further maintained in laboratory as described earlier by Patankar et al.35 Metabolites at various concentrations were evaluated for their bioactivity against test organisms. Extracts were dissolved in dimethyl sulfoxide (DMSO) and 100 µl from each concentration was added to each well (6 mm) in nutrient agar plates seeded with test bacteria. The plates were kept in the refrigerator for 20-30 minutes at 4°C for diffusion of in the medium followed by incubation of plates for 24 hrs at room temparature. Streptomycin and DMSO were used as positive and negative control, respectively. After incubation, inhibitory activity was assessed by measuring the zone of inhibition in mm around metabolite-loaded wells.
pH of the water sample collected from the mangrove site was found to be 7.6. The spread plate followed by a microscopic and staining study revealed the presence of 5 fungi in a water sample. The identified fungi were Aspergillus niger, Aspergillus flavus, Trichoderma harzianum, Cylindrocladium scoparium and Colletotrichum wuxiense (Table 1 & 2).
Table (1):
Cultural and Morphological Characteristics of Isolates.
| Isolate Name | Media | Colony size (7 Days) | Surface texture | Reverse (Pigment + color of sulcation) | Color (center) | Zonation | Margin (Colour) | Elevation | Growth phase | Shape | Microscope examination |
|---|---|---|---|---|---|---|---|---|---|---|---|
| J | PDA | 10 mm | Granular | White with black spores and sulcation | Black with base white | Off White | Round, White | Erose | Zonate | Circular | Hypha with vesicle and phialides covered with conidia |
| K | 16 mm | Granular | Green colour with sulcation | Green | Green | Filamentous, Green | Raised | Zonate | Circular | Tubular thin walled hyphae with vesicle bearing cylindrical phialides with conidia | |
| L | 11 mm | Granular | Dark green, No sulcation | Dark olive green | – | Lobate | Cuteriform | Powdery | Punctiform | Branched conidiophores cluster into fascicles | |
| M | 3 mm | Granular | Dark green, No sulcation | Dark olive green | – | Lobate | Cuteriform | Powdery | Punctiform | Macroconidia with branched conidiophore | |
| N | 19 mm | Cottony | Off White, No sulcation | Pure white | – | Ciliate, White | Raised | Cottony | Myceloid | conidia |
Table (2):
Isolated Fungi and Microscopy.
| Isolate | Growth on Plate | Microsopic Examination | Identified fungi | |
|---|---|---|---|---|
| J | 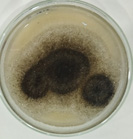 |
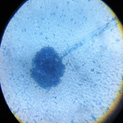 |
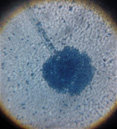 |
Aspergillus niger |
| K |  |
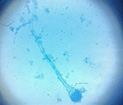 |
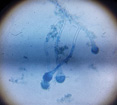 |
Aspergillus Flavus |
| L | 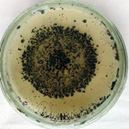 |
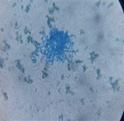 |
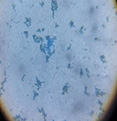 |
Trichoderma Harzianum |
| M | 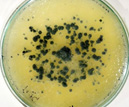 |
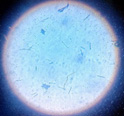 |
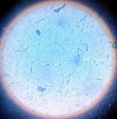 |
Cylindrocladium Scoparium |
| N | 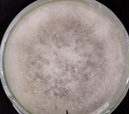 |
 |
 |
Colletotrichum Wuxiense |
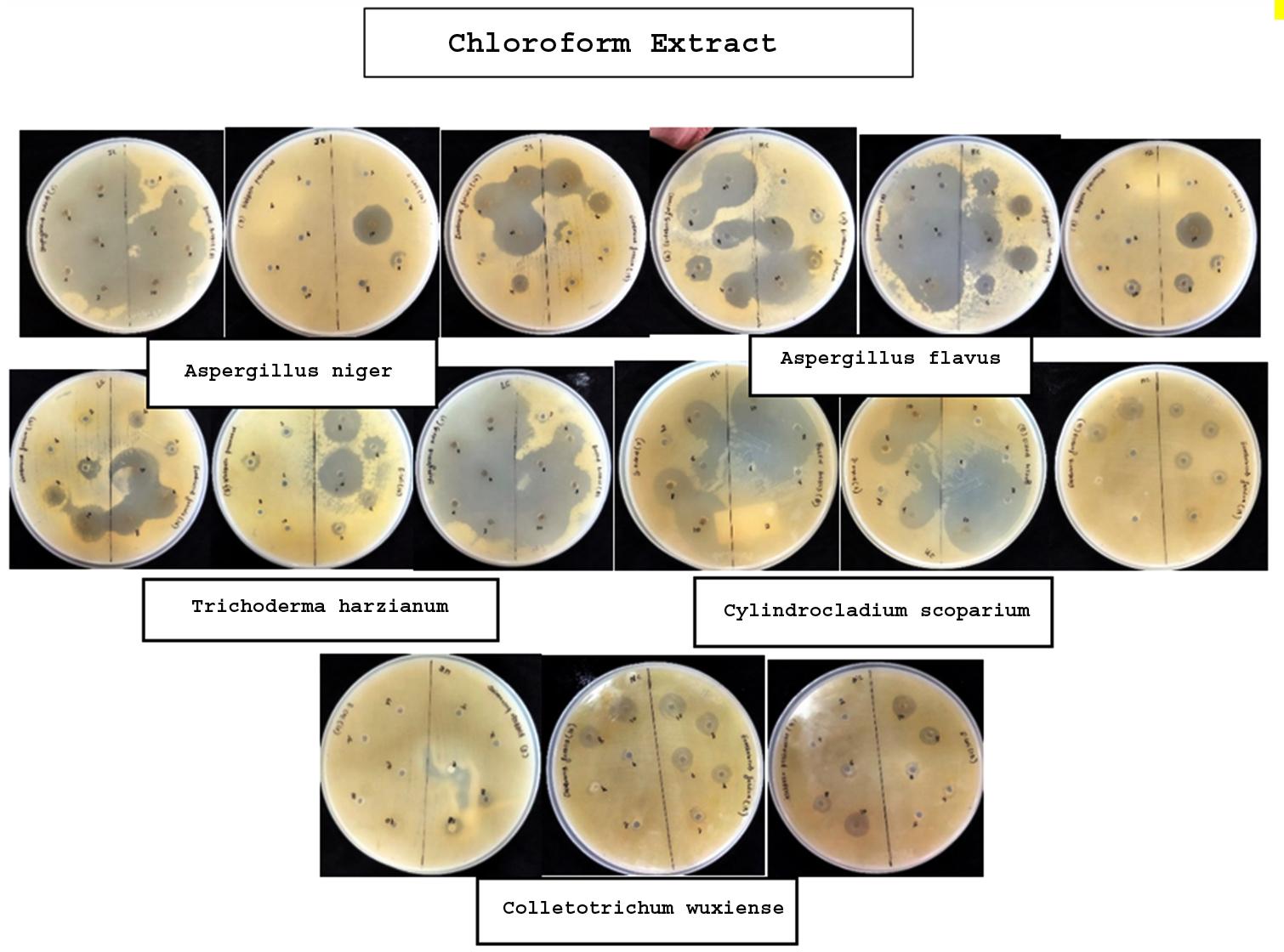
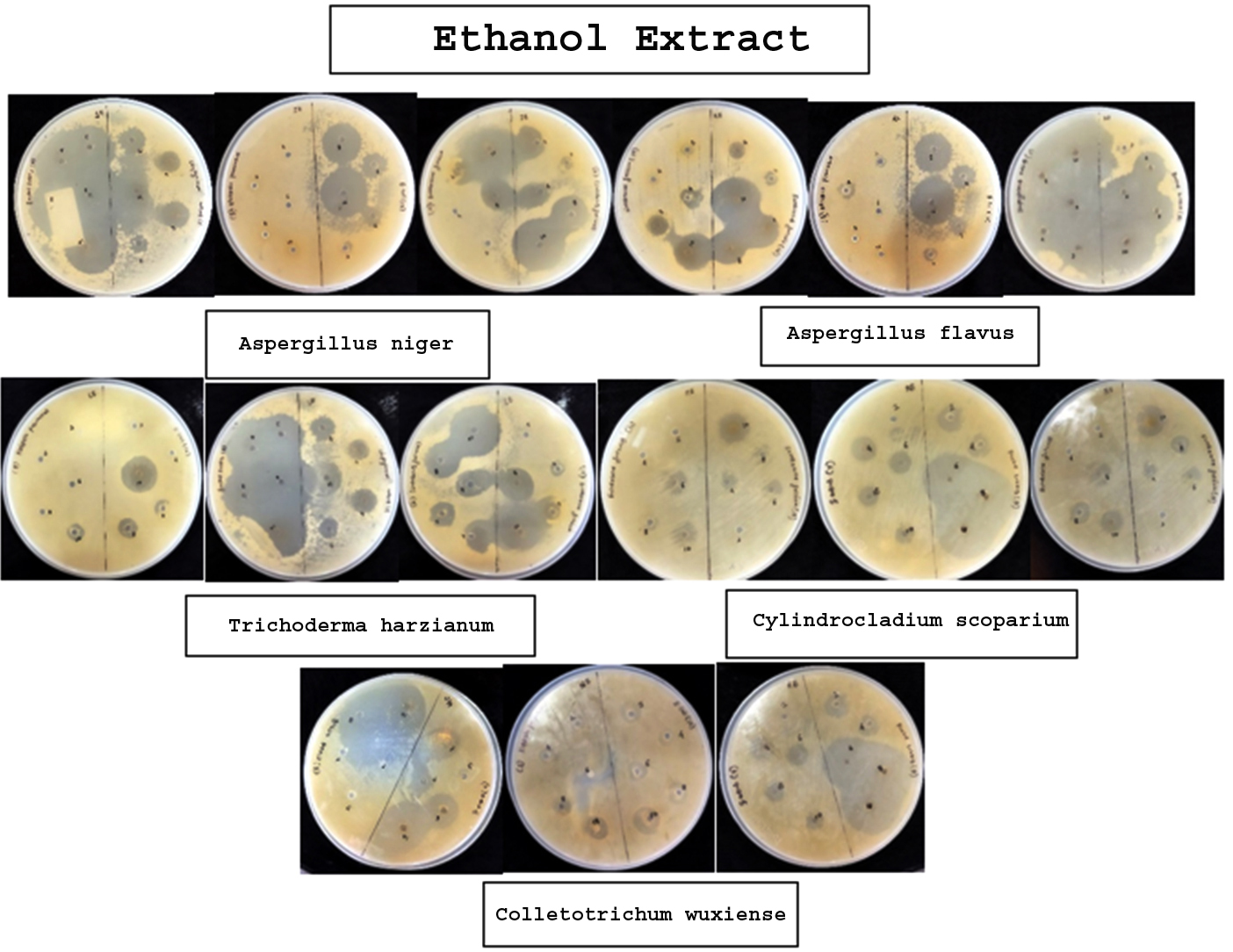
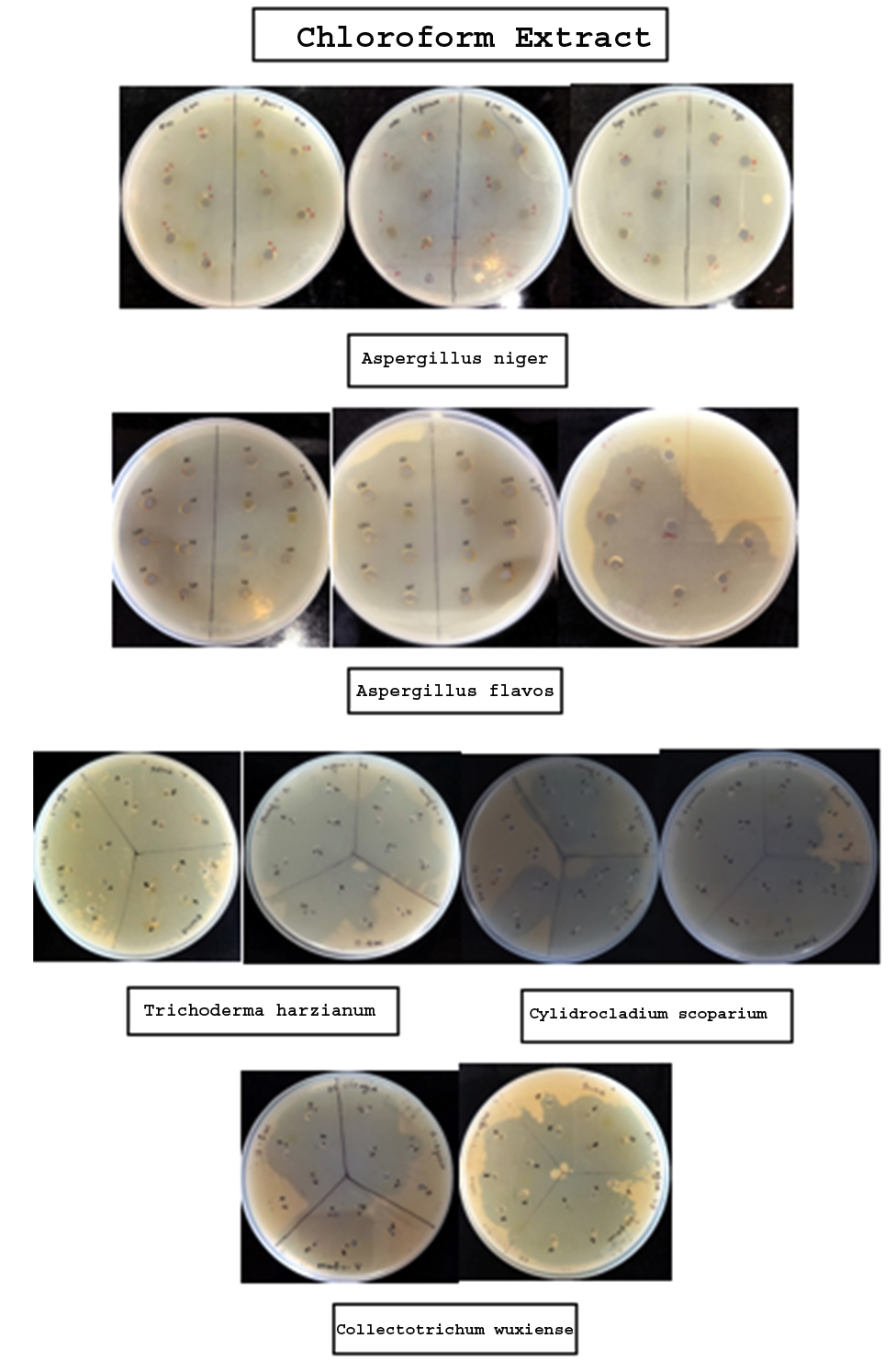
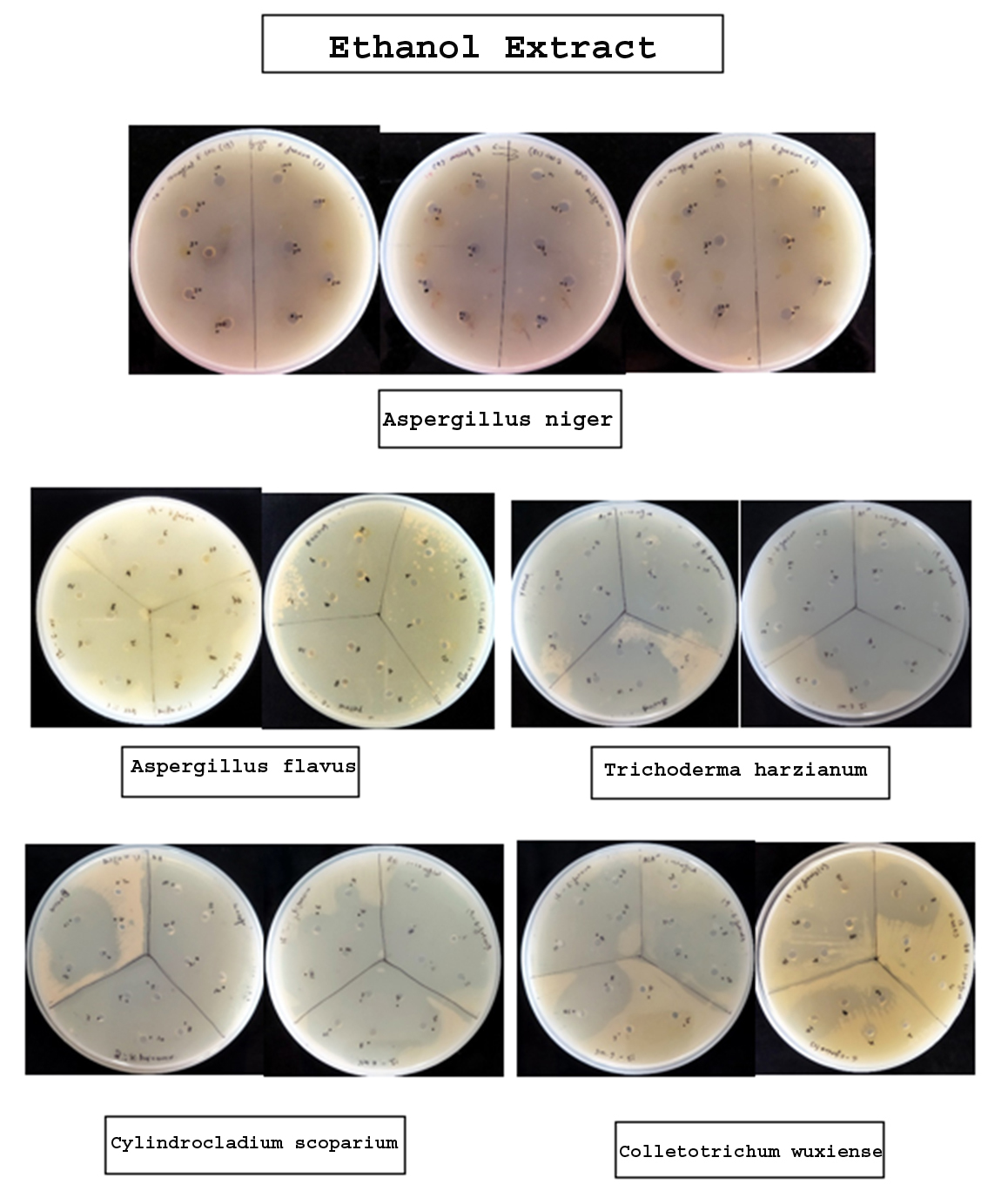
The broth of identified fungi was extracted by ethanol and chloroform separately. Solvent extracts after drying were stored in vials. The yield (metabolites) of each broth and mycelium extract of individual solvent and fungi is given in Table 3 and 4, respectively. Antibacterial property of metabolites was assessed by measuring inhibitory zone around the 6 mm wells loaded with extracts of different concentrations. The results revealed the bacterial growth inhibition property of all the broth and mycelium extracts (Table 5 & 6; Figure 1 & 2). Extracts obtained from broth exhibited the growth inhibition property against all gram-positive test bacteria. Further, chloroform broth extract of Cylindrocladium scoparium, Colletotrichum wuxiense and ethanolic broth extract of Aspergillus flavus, Trichoderma harzianum also inhibited the growth of gram-negative bacteria. Mycelial extracts exhibited less bacterial growth inhibition as compared to broth extracts. Out of mycelium extracts, chloroform extract of Trichoderma harzianum and ethanolic extract of Aspergillus flavus exhibited 100% growth inhibition of all test microbes (Table 6).
Table (3):
Weight of extracted metabolites from Broth fermentation (250 ml).
Name of the isolate |
Chloroform Extract Yield (mg) |
Ethanol Extract Yield (mg) |
|---|---|---|
Aspergillus niger |
80.3 |
75.2 |
Aspergillus flavus |
111.00 |
80.6 |
Trichoderma harzianum |
98.90 |
168.6 |
Cylindrocladium scoparium |
34.00 |
90.4 |
Colletotrichum wuxiense |
82.90 |
67.50 |
Table (4):
Weight of Extracted metabolites from dry mycelium biomass (per 10 gm).
Name of the isolate |
Chloroform Extract Yield (mg)) |
Ethanol Extract Yield (mg) |
|---|---|---|
Aspergillus niger |
101.6 |
56.70 |
Aspergillus flavus |
45.3 |
60 |
Trichoderma harzianum |
9.4 |
80.9 |
Cylindrocladium scoparium |
30.9 |
81.5 |
Colletotrichum wuxiense |
74.3 |
12.7 |
Table (5):
Antibacterial activity of Broth extracts.
| Fungal Isolates | Zone of Inhibition in mm | ||||||
|---|---|---|---|---|---|---|---|
| Concentration in µg/ml | E. coli | E. feacalis | E. faecium | K. pneumonia | B. subtilis | S. aureus | |
| Chloroform Extract | |||||||
| Aspergillus niger | 2 4 6 8 10 |
– – – – 29 mm |
– 14 mm 24 mm 27 mm 41 mm |
– – – 15 mm 30 mm |
– – – – – |
– 30 mm TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Aspergillus flavus | 2 4 6 8 10 |
9 mm 12 mm 10 mm 15 mm 19 mm |
– – – 12 mm 19 mm |
– 14 mm 32 mm 32 mm – |
– – – – – |
– 13 mm – 23 mm 23 mm |
9 mm – – 29 mm 29 mm |
| Trichoderma harzianum | 2 4 6 8 10 |
9 mm 11 mm 15 mm 26 mm 32 mm |
4 mm 6 mm 18 mm 21 mm 29 mm |
– – 8 mm 11 mm 18 mm |
– – – – – |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Cylindrocladium scoparium | 2 4 6 8 10 |
– – – – 7 mm |
– – – 14 mm 19 mm |
7 mm 8 mm 11 mm 12 mm 10 mm |
– – – – 9 mm |
– – – TBTC TBTC |
– 21 mm 15 mm 25 mm 36 mm |
| Colletotrichum wuxiense | 2 4 6 8 10 |
– – – – 7 mm |
– – 8 mm 9 mm 11 mm |
7 mm 8 mm 8 mm 8 mm 11 mm |
– – – 7 mm 10 mm |
– – – TBTC TBTC |
– 20 mm 17 mm 25 mm 36 mm |
| Ethanol Extract | |||||||
| Aspergillus niger | 2 4 6 8 10 |
– 12 mm 17 mm 29 mm 37 mm |
4 mm 26 mm 20 mm 30 mm 41 mm |
– – – 26 mm 40 mm |
– – – – – |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Aspergillus flavus | 2 4 6 8 10 |
– – – 15 mm 32 mm |
– 21 mm 21 mm 21 mm 21 mm |
19 mm 30 mm 30 mm 30 mm 30 mm |
– – – – 28 mm |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Trichoderma harzianum | 2 4 6 8 10 |
– – – 12 mm 26 mm |
16 mm 18 mm 18 mm 21 mm 27 mm |
– 7 mm 7 mm 18 mm 21 mm |
– – – – 11mm |
TBTC TBTC TBTC TBTC TBTC |
16 mm 17 mm 15 mm 16 mm 21 mm |
| Cylindrocladium scoparium | 2 4 6 8 10 |
– – – – – |
– – – – 10 mm |
– 7 mm 7 mm – 20 mm |
– – – – – |
16 mm 17 mm 40 mm 41 mm 41 mm |
– 16 mm 15 mm 14 mm 14 mm |
| Colletotrichum wuxiense | 2 4 6 8 10 |
– – – – – |
– – – – 7 mm |
– – – – 10 mm |
– – – – – |
7 mm 8 mm 28 mm 28 mm 28 mm |
– 8 mm 11 mm 17 mm 19 mm |
TBTC – zone too big to count means 100% inhibition
Table (6):
Antibacterial activity of Mycelial Extracts.
| Fungal Isolates | Zone of Inhibition in mm | ||||||
|---|---|---|---|---|---|---|---|
| Concentration in µg/ml | E. coli | E. feacalis | E. faecium | K. pneumonia | B. subtilis | S. aureus | |
| Chloroform Extract | |||||||
| Aspergillus niger | 2 4 6 8 10 |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
| Aspergillus flavus | 2 4 6 8 10 |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
18 mm 27 mm 29 mm 30 mm 31 mm |
– -12 mm 22 mm 25 mm |
| Trichoderma harzianum | 2 4 6 8 10 |
30 mm 31 mm 33 mm 38 mm 40 mm |
27 mm 29 mm 32 mm 36 mm 40 mm |
23 mm 27 mm 29 mm 30 mm 33 mm |
18 mm 19 mm 20 mm 20 mm 20 mm |
24 mm 29 mm 29 mm 30 mm 34 mm |
29 mm 30 mm 32 mm 33 mm 33 mm |
| Cylindrocladium scoparium | 2 4 6 8 10 |
– – – 22 mm 24 mm |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
– – – – 13 mm |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Colletotrichum wuxiense | 2 4 6 8 10 |
9 mm TBTC TBTC TBTC TBTC |
– – – 11 mm 14 mm |
– 20 mm 24 mm 30 mmTBTC |
– – – – 11 mm |
23 mm TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Ethanol Extract | |||||||
| Aspergillus niger | 2 4 6 8 10 |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
– – – – – |
| Aspergillus flavus | 2 4 6 8 10 |
14 mm 17 mm 18 mm 18 mm 18 mm |
15 mm 16 mm 19 mm 23 mm 27 mm |
17 mm 18 mm 19 mm 24 mm 30 mm |
– 9 mm 17 mm 19 mm 25 mm |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Trichoderma harzianum | 2 4 6 8 10 |
– 31 mm TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
– – – 28 mm TBTC |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Cylindrocladium scoparium | 2 4 6 8 10 |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
– – – – 10 mm |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
| Colletotrichum wuxiense | 2 4 6 8 10 |
– – – 7 mm 13 mm |
– – – 15 mm 17 mm |
– – – 29 mm 34 mm |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
TBTC TBTC TBTC TBTC TBTC |
TBTC – zone too big to count means 100% inhibition
Mangroves comprise almost 181,000 Km2 and dominate the World’s Forth
coastline.6 Considering the kind of continuous changing environment the mangrove ecosystem has, fungi associated with mangroves help plants to sustain under turbulent environmental conditions.11 As a result of such interactions, mangrove fungi are believed to be the source of novel and unique natural compounds.36 In the last few decades, mangrove fungi have attracted researchers across the globe to investigate their metabolism which may lead to the discovery of natural compounds with therapeutic applications like antitumor, antiviral, antidiabetic and antimicrobial compounds.37,38 In the current study, results revealed the strong antibacterial potential of fungal metabolites against all test organisms. The antimicrobial potential of mangrove fungi has been reported in earlier studies.21,6,20 The extraction of broth and the nature of the solvent used plays an important role in the metabolite extraction. In the present study, non-polar and polar solvents were used for extraction purposes. Similar inferences have been drawn earlier by Prasannan et al. and Zhang et al.,22,23 wherein they used different organic solvents with varied polarity. However, our results are contradictory to the studies earlier carried out by Reshi et al.,24 wherein the authors concluded that in addition to polar and non–polar organic solvents, ethyl acetate, being the mid-polar solvent must be used for metabolite extraction from plants, bacteria and fungi. Many novel antimicrobial metabolites have been isolated from mangrove fungi and more and more studies are taken up to unravel the biologically active compounds.39 With increasing drug resistance, most of the available antibiotics have become obsolete, therefore the current circumstances demand natural and novel compounds to cater for the increasing drug resistance menace.40
The research validates that various gram-positive and gram-negative bacteria are susceptible to the antibacterial effects of fungal metabolites. Further characterisation of bioactive metabolites is needed to identify the active compounds which may strengthen the existing antibiotics and also may pave the way for the discovery of new compounds of medicinal nature.
ACKNOWLEDGMENTS
The authors would like to thank Department of Microbiology, Sandip University Nashik for providing all the necessary support to carry out this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RSP and NAR conceptualized the study. RSP performed experiments and data analysis. RSP and NAR wrote the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Uddin TM, Chakraborty AJ, Khusro A, et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14(12):1750-1766.
Crossref - Meh C, Sharma A, Ram U, et al. Trends in maternal mortality in India over two decades in nationally representative surveys. BJOG An Int J Obstet Gynaecol. 2022;129(4):550-561.
Crossref - Elmaidomy AH, Shady NH, Abdeljawad KM, et al. Antimicrobial potentials of natural products against multidrug resistance pathogens: a comprehensive review. RSC Adv. 2022;12:29078-29102.
Crossref - Lesley-Ann G, Newman DJ. Extremophilic Fungi from Marine Environments : Mar. Drugs. 2022;20(1):62.
Crossref - Debbab A, Aly AH, Lin WH, Proksch P. Bioactive compounds from marine bacteria and fungi: Minireview. Microb Biotechnol. 2010;3(5):544-563.
Crossref - Vladkova T, Georgieva N, Staneva A, Gospodinova D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants. 2022;11(3):439.
Crossref - Srinivasan R, Kannappan A, Shi C, Lin X. Marine bacterial secondary metabolites: A treasure house for structurally unique and effective antimicrobial compounds. Mar Drugs. 2021;19(10):530.
Crossref - Duraes F, Szemeredi N, Kumla D, et al. Metabolites from marine-derived fungi as potential antimicrobial adjuvants. Mar Drugs. 2021;19(9):475.
Crossref - Andryukov B, Mikhailov V, Besednova N. The biotechnological potential of secondary metabolites from marine bacteria. J Mar Sci Eng. 2019;7(6):176.
Crossref - Sun W, Wu W, Liu X, Zaleta-Pinet DA, Clark BR. Bioactive compounds isolated from marine-derived microbes in China: 2009-2018. Mar Drugs. 2019;17(6):339.
Crossref - Thatoi H, Behera BC, Mishra R. R. Ecological role and biotechnological potential of mangrove fungi: A review. Mycology. 2013;4(1):54-71.
- Maria GL, Sridhar KR, Raviraja NS. Antimicrobial and Enzyme Activity of Mangrove Endophytic Fungi of Southwest Coast of India. Journal of Agricultural Technology. 2005;1(1):67-80.
- Chen Y, Wang G, Yuan Y, et al. Metabolites With Cytotoxic Activities From the Mangrove Endophytic Fungus Fusarium sp. 2ST2. Front Chem. 2022;10:1-8.
Crossref - Hoeksma J, Misset T, Wever C, et al. A new perspective on fungal metabolites: identification of bioactive compounds from fungi using zebrafish embryogenesis as read-out. Sci Rep. 2019;9(1):1-16.
Crossref - Rajasekar T, Balaji S, Kumaran S, Deivasigamani B, Pugzhavendhan SR. Isolation and characterization of Marine fungal metabolites against clinical pathogens. Asian Pacific J Trop Dis. 2012;2(Suppl 1):S387-S392.
Crossref - Deshmukh SK, Gupta MK, Prakash V, Reddy MS. Mangrove-associated fungi: A novel source of potential anticancer compounds. J Fungi. 2018;4(3):101.
Crossref - Wang X, Mao Z-G, Song B-B, et al. Advances in the study of the structures and bioactivities of metabolites isolated from mangrove-derived fungi in the South China Sea. Mar Drugs. 2013;11(10):3601-3616.
Crossref - Song R, Wang J, Sun L, et al. The study of metabolites from fermentation culture of Alternaria oxytropis. BMC Microbiol. 2019;19:4-11.
Crossref - Nayak BK, Janaki T, Ganesan T. Antimicrobial activity of Avicennia marina (Forsk) Vierh from Back water area of Puducherry, India. Int J ChemTech Res. 2014;6(11):4667-4670.
- Gerona ME, Salmo S. A systematic review of mangrove restoration studies in Southeast Asia: Challenges and opportunities for the United Nation’s Decade on Ecosystem Restoration. Front Mar Sci. 2022;9.
Crossref - Cadamuro RD, Bastos IMAdS, Silva IT, et al. Bioactive Compounds from Mangrove Endophytic Fungus and Their Uses for Microorganism Control. J Fungi. 2021;7(6):455.
Crossref - Chen S, Cai R, Liu Z, Cui H, She Z. Secondary metabolites from mangrove-associated fungi: source, chemistry and bioactivities. Nat Prod Rep. 2021;39(3):560-595.
Crossref - Keller NP. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol. 2019;17(3):167-180.
Crossref - Shi XX, Qiu H-P, Wang J-Y, et al. A handy method to remove bacterial contamination from fungal cultures. PLoS One. 2019;14(11):e0224635.
Crossref - Rafiq A, Khan SA, Akbar A, et al. Isolation and Identification of Antibiotic Producing Microorganisms From Soil. Int J Pharm Sci Res. 2018;9(3):1002-1011.
Crossref - VanderMolen KM, Raja HA, El-Elimat T, Oberlies N. H. Evaluation of culture media for the production of secondary metabolites in a natural products screening program. AMB Express. 2013;3(1):1-7.
Crossref - Wei L, Zhang Q, Xie A, et al. Isolation of Bioactive Compounds, Antibacterial Activity, and Action Mechanism of Spore Powder From Aspergillus niger xj. Front Microbiol. 2022;13:1-13.
Crossref - Surup F, Hennicke F, Sella N, et al. New terpenoids from the fermentation broth of the edible mushroom Cyclocybe aegerita. Beilstein J Org Chem. 2019;15:1000-1007.
Crossref - Ngene AC, Aguiyi J, Chibuike CJ, et al. Antibacterial Activity of Psidium guajava Leaf Extract against Selected Pathogenic Bacteria. Adv. Microbiol. 2019;09(12):1012-1022.
Crossref - Brazkova M, Angelova G, Mihaylova D, et al. Bioactive Metabolites from the Fruiting Body and Mycelia of Newly-Isolated Oyster Mushroom and Their Effect on Smooth Muscle Contractile Activity. Foods. 2022;11(24):3983.
Crossref - Zhai F-H, Han J-R. Mycelial biomass and intracellular polysaccharides yield of edible mushroom Agaricus blazei produced in wheat extract medium. LWT – Food Sci Technol. 2016;66:15-19.
Crossref - Awad NE, Kassem HA, Hamed MA, et al. Isolation and characterization of the bioactive metabolites from the soil derived fungus Trichoderma viride. An Int. J Fungal Biol. 2018;9(1):70-80.
Crossref - Gonelimali FD, Lin J, Miao W, et al. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol. 2018;9:1-9.
Crossref - Masri A, Brown DM, Smith DGE, Stone V, Johnston HJ. Comparison of In Vitro Approaches to Assess the Antibacterial Effects of Nanomaterials. J Funct Biomater. 2022;13(4):255.
Crossref - Patankar RS, Sankpal NY, Sonwane CG, Reshi NA. Assessment of antibacterial potential of metabolites of marine fungi isolated from coastal region of Mumbai. J Appl Pharm Sci. 2022;12(9):162-172.
Crossref - Lee NLY, Huang D, Quek ZBR, Lee JN, Wainwright BJ. Mangrove-associated fungal communities are differentiated by geographic location and host structure. Front Microbiol. 2019;10:1-9.
Crossref - Prasannan CB, Jaiswal D, Davis R, Wangikar PP. An improved method for extraction of polar and charged metabolites from cyanobacteria. PLoS One. 2018;13(10):1-16.
Crossref - Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Med. 2018;13(1):1-26.
Crossref - Shahid H Cai T, Wang Y, et al. Duclauxin Derivatives From Fungi and Their Biological Activities. Front Microbiol. 2021;12:766440.
Crossref - Saha M, Sarkar A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J Xenobiotics. 2021;11(4):197-214.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.