ISSN: 0973-7510
E-ISSN: 2581-690X
Carotenoids are a group of compounds that provide various health protective benefits. Several microbes, including yeasts, are known to produce such compounds as secondary metabolites and thus these yeasts can be utilized as microbial cell factories for mass production of such compounds. In this investigation, pigment making yeast Rhodotorula taiwanensis was collected from fermentative cake of Bodo community of Assam. The pigment was extracted through acetone-petroleum ether partition and was identified through ultra-high pressure liquid chromatography with tandem mass spectrometry (UHPLC-MS/MS) technique which reveals the presence of Resveratrol, Quercetin, Phytofluene and Xanthophyll. The crude extract showed antimicrobial properties towards Staphylococcus aureus, Escherichia coli, Candida albicans and Pseudomonas aeruginosa. Further, effect of nutrient and UV radiation was examined on pigment production, where glucose and yeast extract medium showed higher amount of pigment production compared to sucrose and tryptone, respectively. This study emphasizes upon yeast pigments and the production for their commercial use in various field of industries.
Yeast, Rhodotorula taiwanensis, Carotenoids, Spectroscopy, UHPLC-MS/MS, Assam
The northeastern region of India harbours several ethnic indigenous tribal communities that are different from each other by virtue of their distinct individual cultural heritage. The diverse ethnic groups in northeast India comprises of Aryan, Chinese, and Mongoloid ancestry. The extensive indigenous knowledge system of each community is inextricably tied to its unique socio-cultural and environmental contexts. These communities are known to produce household alcoholic beverages from starch materials such as rice and each of these fermentation processes is carried out by consortia of microorganisms including yeast.1-5 Since the microbial composition of fermentative cake of each community is diverse from each other, hence these cakes can serve as good source of beneficial yeasts that can be employed in production of secondary metabolites, which includes various pigment compounds such as carotenoids.6,7
Carotenoids are a key group of microbial metabolites that can be synthesised by certain microorganisms. These compounds are generally produced by plants where these molecules aid in light absorption and removal of excess solar energy.8-10 The primary function of carotenoids in microorganism is to protect cells from the damaging effects of radiation and reactive forms of oxygen.11 Over the past few years, a substantial amount of data has been collected. regarding their potential health-protective benefits of these pigmented compounds.12-14 Thus far, lutein astaxanthin, zeaxanthin, lycopene, and β-carotene have been the primary carotenoids investigated.15
Yeasts have been proposed as possible natural sources of pigment since they are known to synthesise carotenoids such as torulene, β-carotene, astaxanthin and torularhodin below a variety of stress conditions. Yeasts from certain genera of Rhodosporidium, Rhodotorula, Sporobolomyces and Sporidiobolus are referred to as “red yeasts” which have been stated to produce carotenoid compounds.7,16-19 Several studies have been showed to create carotenoids mixtures from inexpensive carbon sources.20 There is good promise for these yeasts as biocatalyst owing to their capability of biotransformation of wide range of carbon sources into useful metabolites and their resistance towards several inhibitory compounds that arises as byproducts in Agro-food industry.9,21,22 Coloured basidiomycetous yeasts belonging to the Rhodotorula species are easily recognised by their characteristic yellow, orange, or red colonies. The carotenoids produced by these yeasts can be employed as an antibacterial agent and as a substitute for synthetic food colouring.23 The genus Rhodotorula has a wide natural occurrence from milk and fruit juices to lakes, soil, and air.24 Being extremely aerobic, Rhodotorula species give rise to great amounts of lipids and pigments and because of their safety towards human health, these yeasts are widely used in the food industry.25
Keeping these beneficial traits of Rhodotorula, this investigation was aimed to isolate pigmented yeasts from fermentative cakes of ethnic communities and characterization of pigments produced by these isolated yeasts. Antimicrobial assessment and LC-MS characterization of the pigments were performed and finally, effect of nutrients and UV-radiation in production of pigment was studied.
Collection of samples
Fermentative cake samples were collected from Bodo community from Kamrup, Assam (26.07 °N & 91.60 °E). These fermentation cakes are used as substance for the initial culture of indigenous alcoholic fermentation by these communities and harbours various species of yeasts.26,27 Polypropylene bags were utilized to transport the collected fermentative starter cakes which were then stored in 4 °C prior to experiments in the lab.
Isolation, purification and maintenance of yeasts
To inoculate petri-plates with YPD agar media, 1.0 g of crudely powdered cake samples were liquified in 10 mL of distilled water and diluted 103 times (1% w/v yeast extract, 2% w/v mycological peptone, 2% w/v dextrose and 2% w/v agar) enhanced with 100 mg/L chloramphenicol (Sigma C-3175) and 50 mg. mg/L chlorotetracycline (Sigma 26430).26 The plates underwent incubation at 30 ± 2 °C for 24 hrs. In order to get pure colonies, isolates of yeast were sub-cultured several times from primary mother cultures, screened and observed with the support of a phase contrast microscope (Leica DM750). Pure colonies of yeasts were cultivated in YPD broth and incubated at 30 ± 2 °C for 24 hrs and kept at 4 °C until further use.
Scanning electron microscopy
Scanning Electron Microscopy (SEM) was executed by following the standard methods.28 In summary, the isolates underwent three washes in 0.1 M phosphate buffer with pH 7.2 after being fixed for 24 hrs at 4 °C with 2.5% glutaraldehyde. After two hours of post-fixation in osmium tetroxide (2%), the samples were dehydrated in gradients of 30%, 50%, 70%, 95%, and 100% ethanol. The dried cell forms were recovered in liquid CO2, covered with gold particles in a vacuum evaporator, and observed beneath a scanning electron microscope (JSM-6360, JEOL) after being cleaned with acetone.
Biochemical characteristics
Assimilation of Carbon sources
Yeast Nitrogen Base (YNB) liquid media were used to examine the yeast isolates’ digestion of carbon sources enhanced with 2% (w/v) carbon sources. 10 carbon sources viz., arabinose, cellobiose, trehalose, mannitol, raffinose, fructose, maltose, galactose, mannose and inositol were selected for this study.29 After inoculating carbon source containing 100 mL of YNB media with roughly 10 µL of precultured inoculum (OD600 ~1.0) of midlog phase cells, the cells were incubated for 24 hrs at 30 ± 2 °C in an orbital shaker incubator set at 100 rpm. The turbidity of the medium was used to record the yeasts growth pattern.29
Nitrogen sources assimilation
Yeasts were grown in different nitrogen sources and the relative growth of the isolates was measured through OD600 values of the liquid cultures. These nitrogen sources included ammonium sulphate (7.5 gm/L) and amino acids [ leucine (71.4 mg/L); glycine (84.2 mg/L); tryptophan (482.6 mg/L) and methionine (20.3 mg/L)].30 The isolates were cultivated under comparable conditions subsequently where each nitrogen source was introduced to 100 mL of Synthetic Medium (SM) that contained the aforementioned nitrogenous sources.29
Organic acid utilization
Organic acid assimilation by the yeast isolates were determined by using malic, tartaric, pyruvic, citric and propionic acids as substrate following the methodology of Fonseca.31 Briefly, the selected isolates were cultured in liquid Mineral Medium (MM, pH 4.5) supplemented with individual organic acids at concentrations of 0.5% (w/v) as sole carbon source.32 10 µL of pre cultured inoculums (OD600 ~1) of mid log phase was inoculated onto 100 mL of MM (pH 4.5) containing each of the mentioned organic acid. The cultures were incubated for 24 hrs at 30 ± 2 °C and the growth pattern of the isolates was recorded.29
Molecular identification of the yeast isolates
For determining molecular distinctiveness, genomic DNA yeasts was isolated following the standard method described by Parasar et al.26 Extracted DNA was exposed to PCR amplification by means of universal primers ITS1 and ITS4 for amplifying D1/D2 regions.33 Details of the DNA extraction and PCR amplification was followed from previously published methodology.34 The PCR amplicons were purified and sequenced in a 96-capillary sequencer (Shimadzu). Sequences found was analysed through a BLAST search in contrast to the non-redundant nucleotide (nt) database to ascertain identity of the isolate and were submitted to NCBI Genebank.
For phylogenetic investigation, numerous sequence alignment was made in MEGA11. Phylogenetic tree was created using the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) method in view of bootstrap value from 1000 re-samplings in MEGA11 software.35
Extraction of Pigment
Using acetone and petroleum ether the pigments were extracted as per previous methodology.7 Briefly, 100 mL of 1N HCL and 150 g of yeast cells were combined in a water bath set at 70 °C and incubated for 1.5 hrs. The combination was then centrifuged for 10 minutes at 7000 rpm at 4 °C in a refrigerated centrifuge. After discarding the supernatant, 100 mL of acetone were added to the cell pellets and vortexed vigorously. The cells were then detached from the supernatant by centrifuging them for 10 mins and the supernatant was filtered through Whatman No. 1 filter paper. 100 mL of petroleum ether (40-60 °C) was blend with 100 mL acetone-containing supernatant and gently mixed by turning the funnel upside down few times. The combination was then permitted to stand for few mins for separation of two phases. The bottom acetone layer was detached and the upper layer comprising petroleum ether along with the pigments was gathered. Lastly, petroleum ether was liquified in a lyophilizer (EBT10N-80) and until further use, the resultant dry crude extract was stored at -20 °C.
Analysis using Liquid Chromatography-Mass Spectrometry (LC-MS)
For determination of the constituents, the crude pigments were exposed to Liquid Chromatography-Mass Spectrometry (LC-MS) using Electrospray ionisation (ESI) technique. Chromatographic separation was attained through a UHPLC system linked to an ESI-Orbitrap mass spectrometer, through minor adjustments. A gradient separation was performed for every sample using a mobile phase that hold solvents A (water containing 0.01% formic acid) and B (pure acetonitrile) at a constant flow rate of 0.5 ml/min. Initial two minutes, 95% solvent A was utilize to introduce a sample volume of 2 µl into a Hypersil Gold C18 column (150 × 3.00 mm, 3 µm particle size, Thermo, USA). Starting from the 2nd minute of injection until the 8th minute, linear gradients of solvent B were implemented, transitioning from 5% to 95%, then kept at solvent B (95%) for 1 minute. Finally, the system was reverted to the primary conditions (solvent B, 5%) for 1 minute. After calibrating the run period for 10 mins, the column was re-equilibrated in solvent B. The chromatogram was recorded using a PDA indicator with three different wavelengths-270, 340, and 600 nm and the chemicals were identified using complete mass spectrum analysis. Putative carotenoid compounds were recognized by linking the observed MS spectra with those from the Mass Bank Database and literature.7,36
Antimicrobial activity
Antimicrobial screening assessment was performed by using Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Candida albicans and Candida tropicalis microbial strains. In brief, 30 mg of crude pigment extracts was liquified in 1 mL of methanol and strain through 0.2 µm nylon membrane filter.37 The antibacterial activity was assessed using the agar disc diffusion method. Fungi were cultivated for 48 hours at 28 ± 2 °C in Potato Dextrose Broth (PDB, HiMedia) and the bacteria were cultivated at 37 ± 2 °C overnight in Mueller Hinton Broth (MHB, HiMedia) as an inoculum following methodology of Ekpo et al.38 Bacteria (108 CFV/mL) and fungi (104 spores/mL) that were distributed on Mueller Hinton Agar (MHA) and Potato Dextrose Agar (PDA) media, respectively, were used as the last inoculum. The disc (6 mm in diameter) was infused with 10 µL (300 µg/disc) and 50 µL (1500 µg/disc) of 30 mg/mL extracts and were positioned on seeded agar plates. Methanol deprived of the test compound was used as the negative control.39 Levofloxacin (5 mcg), Cefoxitin (30 mcg), Gentamicin (10 mcg), Tetracycline (30 mcg) and Ceftazidime (30 mcg) were employed as bacterial positive controls and Itraconazole (10 mcg) and Amphotericin-B (10 units) for fungi. For 24 hours, the test plates were incubated at 37 ± 2 °C for bacteria and for 72 hours at 28 ± 2 °C for fungi. The zone of inhibition was recorded after the conclusion of incubation period.
Pigment enhancement
With the view to assess any changes in pigment production, yeasts were grown in different carbon sources and subjected to UV radiation for specified time as mentioned below.
Nutritional alteration
Yeasts were grown separately in 2 carbon sources (glucose and fructose) then 2 nitrogen sources (yeast extract and tryptone). Each carbon source was separately added to the YNB media (with ammonium sulphate, w/o carbon source) at 2% w/v concentration. Similarly, nitrogen sources were distinctly added to the YNB medium (w/o ammonium sulphate, with glucose 1%w/v) at concentration of 1% w/v. Cells were incubated at 30 °C for 24 ± 2 hours. After completion of incubation, cells were accumulated through centrifugation at 12000 rpm for 10 min. 3 g of dry cell mass was supplementary processed for quantification of crude pigment.
UV mutagenesis
For UV mutagenesis, the isolate was exposed to UV radiation for 10 mins, 30 mins and 60 mins. Cells were dispersed in petri-dishes filled with distilled water at a density of OD600~1. Plates were exposed under UV and 1 mL of cell suspension was collected at definite time intervals as mentioned. 100 µL of collected cell suspension was infuse in YPD agar plates and incubated for 72 ± 2 hours at 30 °C. Individual colonies grown at different exposure level was isolated and again streaked in agar plates along with unexposed (control) isolates. After incubation at 30 °C for 24 ± 2 hours, 3 g dry cell mass was collected and further processed.
Quantification of crude pigment
With the aim to quantify crude pigments, 3 g dry cell mass from above experiments were mixed with 10 mL of acetone and vortexed thoroughly. Occasionally, cells were crushed with micro-pestle and again vortexed for 15 minutes. Cells were pelleted at 6000 rpm for 20 mins, and the supernatant was collected. 10 mL acetone was partitioned with 10 mL petroleum ether and gently mixed. After phase separation, the upper petroleum ether layer comprising the pigments were collected. After that the volume was evaporated under reduced pressure. In 1 mL methanol, the remaining crude extract was dissolved and absorbance was measured at 450 nm in order to enumerate the crude pigments as an equivalent of β-carotene standard. Standard curve of β-carotene was prepared in methanol for the quantification of the obtained crude extract.
External morphology and micrographs
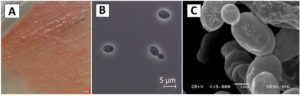
1 (One) pigmented pure yeast colony was obtained from the fermentative cake. The isolate appeared brick red in colour having smooth and elevated colony texture. The morphological feature, phase contrast and SEM images of the isolate is shown in Figure 1.
Figure 1. Morphology of Rhodotorula taiwanensis in (A) YPD agar media, (B) Phase contrast micrograph at 100x magnification and (C) Scanning electron micrograph
Biochemical characteristics
Different carbon, nitrogen and organic acid sources were evaluated by measuring their OD values at 600 nm after 24 hours of incubation in order to observed the growth behaviour of the yeast isolate. Relative growth was measured in terms of no growth (OD600~0.0), low growth (OD600~0.0-0.5), moderate growth (OD600~0.5-1.0) and thriving growth (OD600>1.0) as shown in Table 1.
Table (1):
Comparative growth of the yeast Rhodotorula taiwanensis in different sources
| Carbon source | Arabinose | Cellobiose | Trehalose | Mannitol | Raffinose | Fructose | Maltose | Galactose | Mannose | Inositol |
|---|---|---|---|---|---|---|---|---|---|---|
| +++ | ++ | +++ | ++ | ++ | + | +++ | +++ | ++ | ++ | |
| Nitrogen source | Ammonium | Glycine | Leucine | Methionine | Tryptophan | |||||
| + | +++ | − | + | +++ | ||||||
| Organic acid | Tartaric acid | Malic acid | Citric acid | Pyruvic acid | Propionic acid | |||||
| +++ | + | +++ | +++ | + | ||||||
−: No growth, +: low growth, ++: Moderate growth, +++: luxuriant growth
Phylogenetic analysis and molecular identification
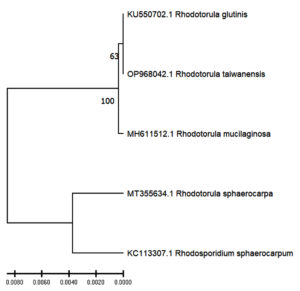
To check the identity of the yeast isolate, the PCR amplicons were sequenced and the retrieved sequence was subjected to BLAST search in NCBI database. Depending on the 100% similarity with the existing record, the isolate was identified as Rhodotorula taiwanensis. The sequence was submitted to NCBI and Accession number was obtained (OP968042). Phylogenetic tree was created using UPGMA method, which is shown in Figure 2. In this study, Phylogenetic study shows that the tested isolate, i.e. Rhodotorula taiwanensis shows closest resemblances with Rhodotorula glutinis, whereas it exhibits least phylogenetic relation Rhodotorula sphaerocarpa and Rhodosporidium sphaerocarpum.
Figure 2. The UPGMA phylogenetic tree showing relation of tested isolate Rhodotorula taiwanensis (OP968042) with other Rhodotorula species. Rhodotorula glutinis (KU550702), Rhodotorula mucilaginosa (MH611512), Rhodotorula sphaerocarpa (MT355634) and Rhodosporidium sphaerocarpum (KC113307) were taken as reference samples from NCBI database for establishing the identity of the newly identified strains
LC-MS analysis of extracted pigments
Crude pigment extract was exposed to LC-MS analysis and characteristic mass spectra transitions were obtained for the identification of each carotenoid by Electrospray ionization (ESI) technique (Table 2). Carotenoid ionized by ESI showed the protonated molecular ions: 226.1 for Resveratrol, 304.2 for Quercetin, 542.2 for phytofluene and 679.4 for Xanthophyll (Figure 3).
Figure 3. LCMS spectra of the identified compound (A) Resveratrol, (B) Quercetin, (C) phytofluene (D) Xanthophyll
Table (2):
ESI characteristic carotenoid transitions
No. |
Transition (m/z) |
Abundance |
Mol. Formula |
Mol. Weight |
Name of the Compound |
|---|---|---|---|---|---|
1 |
226.1 |
269766.2 |
C14H12O3 |
228.2 |
Resveratrol |
2 |
304.2 |
716082.5 |
C15H10O7 |
302.23 |
Quercetin |
3 |
542.2 |
582317.8 |
C40H62 |
543.5 |
Phytofluene |
4 |
679.4 |
1944234.8 |
C42H58O6 |
658.9 |
Xanthophyll |
Antimicrobial activity
Antimicrobial action of crude pigment extract was examined in order to discover the zones of inhibition through disc diffusion method. Table 3 stated the zone of inhibition of crude pigment extract along with other standard antibiotics. Here, 0.3 mg (T1) showed less antimicrobial activity, whereas higher microbial inhibition was detected in 1.5 mg (T2) amount. Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus were amongst the test organisms used which showed zone of inhibition 14 ± 1.0, 14 ± 1.0 and 11.5 ± 1.5 respectively at the pigment extract concentration 0.3 mg and at pigment concentration 1.5 mg these test organisms showed zone of inhibition 11.5 ± 0.5, 16 ± 1.0 and 15 ± 2.0 respectively. Similarly, Candida albicans showed zone of inhibition 14 ± 1.0 and 23.5 ± 1.5 for the pigment concentration 0.3 mg and 1.5 mg respectively. However, the crude extracts were found to show no effect on Klebsiella pneumoniae and Candida tropicalis.
Table (3):
Antimicrobial activity of pigment extracted from Rhodotorula taiwanensis against human bacterial pathogens
| Microorganism | Zone of inhibition (% v/v) | Test Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blank | Control | |||||||||
| Methanol extract | Cefoxitin (CX) (30 mcg) | Levofloxacin (LE) (5 mcg) | Gentamicin (GEN) (10 mcg) | Tetracycline (TE) (30 mcg) | Ceftazidime (CAZ) (30 mcg) | Itraconazole (IT) (10 mcg) | Amphotericin-B (AP) (100 units) | Pigment extract (0.3 mg) | Pigment extract (1.5 mg) | |
| Staphylococcus aureus | 0 | 32.5 ± 2.5 | 14 ± 2.0 | – | – | – | – | – | 11.5 ± 1.5 | 15 ± 2.0 |
| Escherichia coli | 0 | – | – | 19.5 ± 1.5 | 0 | – | – | – | 14 ± 1.0 | 16 ± 1.0 |
| Klebsiella pneumoniae | 0 | – | – | 19.5 ± 1.5 | 0 | – | – | – | 0 | 0 |
| Pseudomonas aeruginosa | 0 | – | – | 28.5 ± 1.5 | – | 21.5 ± 1.5 | – | – | 14 ± 1.0 | 11.5 ± 0.5 |
| Candida albicans | 0 | – | – | – | – | – | 0 | 14 ± 1.0 | 14 ± 1.0 | 23.5 ± 1.5 |
| Candida tropicalis | 0 | – | – | – | – | – | 0 | 0 | 0 | 0 |
Pigment enhancement
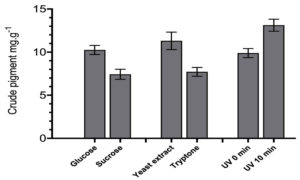
R. taiwanensis was grown in distinct carbon and nitrogen sources and were exposed to UV radiation to observe differences in pigment production. In case of carbon sources, glucose media produce ~1.3-fold amount of crude extract compared to sucrose medium. Similarly, ~1.4-fold increase in pigment production was detected in yeast extract compared to tryptone medium. Similarly, the quantity of crude extract was observed to be ~1.3-fold higher in UV exposed (10 min) isolates compared to unexposed (0 min) isolates. The results are shown in Figure 4.
This investigation was aimed at finding pigmented yeasts from fermentative cakes of ethnic communities and to evaluate the effect of nutrient and UV-radiation stress in pigment production. The isolated yeast was identified as Rhodotorula taiwanensis which corresponds with our earlier findings where occurrence of the species was reported in fermentative cake of Mishing community.7 Being a naturally occurring yeasts, presence of Rhodotorula in the fermentative cake can be explained through the fact that several plant condiments are used in preparation of the fermentative cakes in ethnic communities and these microbial consortia are perpetuated from generation after generation, which are associated in fermentation process.6,40
The crude pigment shows antimicrobial action against E. coli, S. aureus, C. albicans and P. aeruginosa. These findings are similar to the previous reports of antimicrobial properties of carotenoids. Pigment from R. glutinis showed activity against S. aureus,41 that from Rhodosporidium showed antimicrobial action against P. aeruginosa, E. coli, C. albicans and S. aureus along with other microbes.42
This examination was aimed to study the carotenoid pigments production, trails for improvement the volumetric production and cellular carotenoids by using diverse carbon sources and nitrogen sources and to measure the effect of UV radiation in yeasts isolates. In case of carbon sources, the highest volumetric carotenoid production was observed in glucose by sucrose (Figure 4). This result corresponds with the previous findings, where glucose gave the highest cell density, volumetric production and cellular carotenoid accumulation in Rhodotorula species.43 Sugars such as glucose, encourage fast cell growth, which is directly correlated with the production of pigment and, most likely, results in a higher production of carotenoids.44 Similarly, for nitrogen sources, yeast extract yielded higher volumetric carotenoid production compared to tryptone (Figure 4). Positive effect of yeast extract on carotenoid production was reported, where yeast Phaffia rhodozyma showed improved carotenoid production when yeast extract was used in growth medium.45 Finally, the UV radiation enhanced carotenoid production in this study. This could be attributed to the fact that carotenoids have protective effect towards microbes from UV radiation damage.11 In addition, carotenoid production was observed to be significantly increased upon prolonged exposure to UV radiation in Bixa orellana L.46 Similarly, such enhancements were also seen in terrestrial micro-algae.47 Since β-carotene and other pigments are known to exhibit defensive mechanism against singlet oxygen and other ROS,48 hence increase in the pigmentation concentration in the tested yeast in this study could be explained where the cells produced higher amount of pigments to combat damaging effect of radiation. The naturally derive pigments have a broad range of applications in the food, pharmaceutical, textile, cosmetic, and other industries. As a result, there is a growing need for these colorants to be produced commercially to replace the labour-intensive and costly chemical syntheses.
Microbial pigments can be easily generated through simple fermentation processes, resulting in cost-effective production. This aspect is particularly beneficial for smaller manufacturers or businesses operating within budget constraints. These pigments, typically sourced from natural origin and are eco-friendly, biodegradable and contrasting with certain synthetic alternatives. This resonates with the current trend towards sustainability and environmental consciousness in the textile industry. Substituting chemical dyes with natural pigments could be a viable ecological strategy. This transition not only serves as a risk and pollution mitigation measure but also presents opportunities to explore new markets and business ventures by integrating ecological considerations into trade policies.
Carotenoids provides several health benefits because of its properties. This study showed that the yeasts Rhodotorula taiwanensis produced pigments which could be further enhanced using proper combination of raw materials and inducible condition which could be beneficial for industrial applications like food industries, pharmaceutical and Nutraceutical industries, cosmetic industries and also in textile industries as a fabric dye. Since the yeast is isolated from food material, it can be considered safe for application in food purpose. However, detailed investigation is required to be performed on characterization and purification of pigments from this yeasts which could not be performed in this investigation due to constraints.
ACKNOWLEDGMENTS
The authors acknowledge the Assam down town University for providing infrastructure and laboratory facilities to carry out the research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All data sets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Ethics Committee, Assam down town University, Guwahati, India, with the memo number AdtU/DRA/02021/093.
- Ghosh C, Das AP. Preparation of rice beer by the tribal inhabitants of tea gardens in Terai of West Bengal. Indian J Tradit Knowl. 2004;3(4):373-372.
- Jeyaram K, Singh WM, Capece A, Romano P. Molecular identification of yeast species associated with ‘Hamei’ – A traditional starter used for rice wine production in Manipur, India. Int J Food Microbiol. 2008;124(2):115-125.
Crossref - Sharma T, Mazumdar D (eds.). Eastern Himalayas: a study on anthropology and tribalism. New Delhi: Cosmo. 1980.
- Ghosh G (eds.). Tribals and their culture in Northeast India: Assam, Meghalaya and Mizoram. New Delhi.Ashish. 1992.
- Demuyter C, Lollier M, Legras JL, Le Jeune C. Predominance of Saccharomyces uvarum during spontaneous alcoholic fermentation, for three consecutive years, in an Alsatian winery. J Appl Microbiol. 2004;97(6):1140-1148.
Crossref - Nath BJ, Parasar DP, Sarma HK. Linking the Diversity of Yeasts Inherent in Starter Cultures to Quorum Sensing Mechanism in Ethnic Fermented Alcoholic Beverages of Northeast India. Front Sustain Food Syst. 2021;5:678045.
Crossref - Parasar DP, Ramakrishnan E, Kabilan S, Kotoky J, Sarma HK. Characterization of b-Cryptoxanthin and Other Carotenoid Derivatives from Rhodotorula taiwanensis, A Novel Yeast Isolated from Traditional Starter Culture of Assam. Chem Biodivers. 2020;17(12):e2000198.
Crossref - Dufosse L, Galaup P, Yaron A, et al. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci Technol. 2005;16(9):389-406.
Crossref - Mata-Gomez LC, Montanez JC, Mendez-Zavala A, Aguilar CN. Biotechnological production of carotenoids by yeasts: An overview. Microbial Cell Factories. 2014;13:12.
Crossref - Riso P, Brusamolino A, Scalfi L, Porrini M. Bioavailability of carotenoids from spinach and tomatoes. Nutr Metab Cardiovasc Dis. 2004;14(3):150-156.
Crossref - Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53(1):1945-2005.
Crossref - Rodriguez-Concepcion M, Avalos J, Bonet ML, et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res. 2018;70:62-93.
Crossref - Melendez-Martinez AJ. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol Nutr Food Res. 2019;63(15):1801045.
Crossref - Melendez-Martinez AJ, Stinco CM, Mapelli-Brahm P. Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients. 2019;11(5):1093.
Crossref - Fernandes AS, do Nascimento TC, Jacob-Lopes E, De Rosso VV, Zepka LQ. Introductory Chapter: Carotenoids – A Brief Overview on Its Structure, Biosynthesis, Synthesis, and Applications. Progress in Carotenoid Research. 2018.
Crossref - Xie ZT, Mi BQ, Lu YJ, Chen MT, Ye ZW. Research progress on carotenoid production by Rhodosporidium toruloides. Appl Microbiol Biotechnol. 2024;108(1):7.
Crossref - Moline M, Libkind D, Van Broock M. Production of Torularhodin, Torulene, and β-Carotene by Rhodotorula Yeasts. In: Barredo, JL. (eds) Microbial Carotenoids From Fungi. Methods in Molecular Biology, vol 898. Humana Press, Totowa, NJ. 2012;898:275-283.
Crossref - Davoli P, Weber RWS. Carotenoid pigments from the red mirror yeast, Sporobolomyces roseus. Mycologist. 2002;16(3):102-108.
Crossref - Kot AM, Kieliszek M, Piwowarek K, Blazejak S, Mussagy CU. Sporobolomyces and Sporidiobolus – non-conventional yeasts for use in industries. Fungal Biol Rev. 2021;37:41-58.
Crossref - Mannazzu I, Landolfo S, da Silva TL, Buzzini P. Red yeasts and carotenoid production: outlining a future for non-conventional yeasts of biotechnological interest. World J Microbiol Biotechnol. 2015;31(11):1665-1673.
Crossref - Johnson EA. Biotechnology of non-Saccharomyces yeasts-the basidiomycetes. Appl Microbiol Biotechnol. 2013;97(17):7563-7577.
Crossref - Hu C, Zhao X, Zhao J, Wu S, Zhao ZK. Effects of biomass hydrolysis by-products on oleaginous yeast Rhodosporidium toruloides. Bioresour Technol. 2009;100(20):4843-4847.
Crossref - Karanjgaokar DR, Tarfe KS. Isolation of Pigmented Yeasts, Extraction of Pigment and Study of Antimicrobial Property of its Pigment. Int J Curr Microbiol Appl Sci. 2017;6(7):664-672.
Crossref - Wirth F, Goldani LZ. Epidemiology of Rhodotorula: An emerging pathogen. Interdiscip Perspect Infect Dis. 2012;2012(1):465717.
Crossref - Tkaeova J, Furdikova K, Klempova T, Dֿurcenska K, Certik M. Screening of carotenoid-producing Rhodotorula strains isolated from natural sources . Acta Chim Slovaca. 2015;8(1):7.
Crossref - Parasar DP, Sarma HK, Kotoky J. Exploring the Genealogy and Phenomic Divergences of Indigenous Domesticated Yeasts Cultivated by Six Ethnic Communities of Assam, India. J Biol Sci. 2017;17(2):91-105.
Crossref - Nath BJ, Parasar DP, Verma E, Sarma HK, Mishra AK. Assessing the Stimulatory Effect of Indole-3-Acetic Acid on Growth and Sustenance of Yeasts Isolated from Traditional Fermentative Sources Maintained by Six Ethnic Communities of Asssam, North-East India. J Pure Appl Microbiol. 2019;13(2):905-914.
Crossref - Oliveira MT, Specian AFL, Andrade CGTJ, Franca EJG, Furlaneto-Maia L, Furlaneto MC. Interaction of Candida parapsilosis isolates with human hair and nail surfaces revealed by scanning electron microscopy analysis. Micron. 2010;41(6):604-608.
Crossref - Das CP, Pandey A. Fermentation of traditional beverages prepared by Bhotiya community of Uttaranchal Himalaya. Indian J Tradit Knowl. 2007;6(1):136-140.
- Albers E, Larsson C, Liden G, Niklasson C, Gustafsson L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol. 1996;62(9):3187-3795.
Crossref - Fonseca A. Utilization of tartaric acid and related compounds by yeasts: Taxonomic implications. Can J Microbiol. 1992;38(12):1242-1251.
Crossref - Gimenez-Jurado G, Van Uden N. Leucosporidium fellii sp. nov., a basidiomycetous yeast that degrades L(+)-tartaric acid. Antonie Van Leeuwenhoek. 1989;55(2):133-141.
Crossref - Naumova ES, Serpova EV, Naumov G. I. Molecular systematics of Lachancea yeasts. Biochemistry. (Mosc). 2007;72(12):1356-1362.
Crossref - Nath BJ, Verma E, Sarma HK, Mishra AK, Tanti B, Jha DK. Evaluation of Basic Fermentation Parameters and Effective Combinations of Predominant Yeasts from Traditional Starter Materials of Indigenous Communities from Northeast India. J Am Soc Brew Chem. 2020;78(3):219-230.
Crossref - Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021;38(7):3022-3027.
Crossref - Rivera S, Vilaro F, Canela R. Determination of carotenoids by liquid chromatography/mass spectrometry: Effect of several dopants. Anal Bio Chem. 2011;400(5):1339-1346.
Crossref - Biswas D, Yoganandam GP, Dey A, Deb L. Evaluation of antimicrobial and wound healing potentials of ethanol extract of Wedelia biflora linn D.C. leaves. Indian J Pharm Sci. 2013;75(2):156-161.
- Ekpo, Mbagwu M, Herbert & Jackson, Clement & Eno, Michael. Antimicrobial and wound healing activities of Centrosema pubescens (Leguminosae). J Pharm Clin Sci. 2011;(1): 1-6.
- Manimala MRA, Murugesan R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J Appl Nat Sci. 2014;6(2):jans.v6i2.511.
Crossref - Tanti B, Gurung L, Sarma HK, Buragohain AK. Ethnobotany of starter cultures used in alcohol fermentation by a few ethnic tribes of Northeast India. Indian J Tradit Knowl. 2010;9(3):463-466.
- Naisi S, Bayat M, Salehi TZ, Zarif BR, Yahyaraeyat R. Antimicrobial and anti-biofilm effects of carotenoid pigment extracted from Rhodotorula glutinis strain on food-borne bacteria. Iran J Microbiol. 2023;15(1):79-88.
Crossref - Sinha S, Das S, Saha B, Paul D, Basu B. Anti-microbial, anti-oxidant, and anti-breast cancer properties unraveled in yeast carotenoids produced via cost-effective fermentation technique utilizing waste hydrolysate. Front Microbiol. 2023;13:1088477.
Crossref - Perrier V, Dubreucq E, Galzy P. Fatty acid and carotenoid composition of Rhodotorula strains. Arch Microbiol. 1995;164(3):173-179.
Crossref - Bhosale P, Gadre RV. Optimization of carotenoid production from hyper-producing Rhodotorula glutinis mutant 32 by a factorial approach. Lett Appl Microbiol. 2001;33(1):12-16.
Crossref - Fang TJ, Cheng YS. Improvement of astaxanthin production by Phaffia rhodozyma through mutation and optimization of culture conditions. J Ferment Bioeng. 1993;75(6):466-469.
Crossref - Sankari M, Hridya H, Sneha P, Doss CGP, Ramamoorthy S. Effect of UV radiation and its implications on carotenoid pathway in Bixa orellana L. J Photochem Photobiol B Biol. 2017;176:136-144.
Crossref - Mutschlechner M, Walter A, Colleselli L, Griesbeck C, Schobel H. Enhancing carotenogenesis in terrestrial microalgae by UV-A light stress. J Appl Phycol. 2022;34(4):1943-1955.
Crossref - Reis-Mansur MCPP, Cardoso-Rurr JS, Silva JVMA, et al. Carotenoids from UV-resistant Antarctic Microbacterium sp. LEMMJ01. Sci Rep. 2019;9(1):9554.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.