ISSN: 0973-7510
E-ISSN: 2581-690X
The study provides a platform to make chicken antibodies (IgY) an alternate source of antibodies to treat rabies infection in developing countries. The study also attempts to provide an overview of the possibility of replacing IgG (antisera) by IgY antibodies. Producing antibodies in a large scale in egg laying chickens is commercially feasible in view of their low body weight and high rate of egg production. It is relatively easy, economical and safe to collect eggs from hens compared to the bleeding the horses, rabbits and other mammals for their serum. HRIG (Human Rabies Immunoglobulin) and ERIG (Equine Rabies Immunoglobulin) are highly expensive and less abundant. In this study, we have analyzed the titer of IgY antibodies and compared with IgG antibodies raised in rabbits.
Chicken antibodies, rabies antiserum, HRIG, ERIG.
Stray dog menace and the incidence of dog bites, hence the necessity to regulate the population of dogs in India have been recently a topic of much debate. The fall out of this kind of a situation has been the increasing incidence of dog bites in rural as well as urban areas and the need for the treatment with anti-rabies vaccine in such cases. Rabies is an acute viral encephalitis affecting carnivores and bats, although it can affect any mammal. It is invariably fatal once the clinical symptoms appear. It is a neuroinvasive disease of humans caused by the bite of rabid animals. It is treated by giving injection of anti-rabies vaccine.
Since the time of the reported existence of an immunoglobulin–G like molecule in chicken (Leslie and Clem; 1969), which is now known as IgY, it has evoked keen interest in various fields. Although it may be functionally similar to the mammalian Ig G, quite a few differences exist between the two. A few studies have impressed the use of immunoglobulin therapy broadening the arsenal available to combat pathogens in medicine and IgY is a promising candidate both as an alternative to antibiotics and as an useful tool in research and diagnostics (Michael et al., 2010).
In the present, when there is a need for anti-rabies vaccine the possibility of the use of IgY antibodies, in the place of the antisera is overwhelming. Hence this study is an attempt to provide an overview of the possibility of replacing IgG antisera by IgY antibodies for passive immunization. This present study is comparing the productivity of IgY in hens and the IgG in rabbit against the attenuated anti-rabies virus. The study focuses on the possibility of IgY antibodies as an alternative to mammalian IgG antibodies to treat rabies. The work compares the titers of both chicken and rabbit antibodies
Chicken
21 week old White Leghorn chickens (body weight 1kg) were obtained from L.K. Poultry farm, Ayyampalayam, Palladam, Coimbatore. Birds were maintained in standard steel cages. Water was made available ad libitum with 20 liter water reservoir and nipple piping to regulate water flow as and when required by the bird. A ball valve was provided to view the water level. The standard poultry feed was obtained from L.K. Poultry Farm, Palladam. The birds were fed ad libitum both in the morning and afternoon every day.
Immunization Schedule
The test chicken was immunized with RabipurTM (Rabies vaccine) obtained from the Pharmacy of SEESHA Hospital, Karunya Nagar, Coimbatore. In accordance with the manufacturers instruction the appropriate quantity of the vaccine powder was dissolved in the given solution. For the Primary immunization 500 microliter of the vaccine along with Freund’s Complete Adjuvant (FCA) was administered intramuscularly in the pectoral muscle at multiple points with the needle inclining at 450A. Similarly booster dosage was given in the same manner with Freund’s incomplete adjuvant (FIA). The immunization schedule for chicken has been presented in Table 1.
Table (1):
Chicken Immunization Schedule.
Test Chicken |
Control |
|
|---|---|---|
Primary Immunization |
0 Day |
Nil |
First Booster |
15th Day |
Nil |
Second Booster |
30th Day |
Nil |
Rabbit
Approximately 6 week old male New Zealand White Rabbits with a body weight of 4 kg were obtained from “Small Animal Breeding Station” Mannuthy, Thrissur, Kerala. The animals were maintained in standard Rabbit cages with wooden framing. The rabbit wet feed was made available for the rabbits ad libitum along with supplemented fresh cabbage leaves and carrot. Water was available ad libitum.
Immunization Schedule
Primary immunization was done using RabipurTM(Rabies vaccine) obtained from the Pharmacy of SEESHA Hospital . The dose of the antigen was similar to that of the chicken. The immunization schedule for Rabbit has been presented in Table 2.
Table (2):
Rabbit Immunization schedule.
Test Rabbit |
Control |
|
|---|---|---|
Primary Immunization |
0 day |
Nil |
Booster |
15th day |
Nil |
Isolation and Purification of Immunoglobulins from egg yolk
The egg yolk antibodies were purified according to the method described by Polson et al., (1980). The egg yolks were separated and washed with distilled water to remove as much albumin as possible and rolled on a paper towel to remove adhering egg white. The yellow yolk without the adhering membrane was allowed to flow into the graduated measuring cylinder and mixed well with equal amount of buffer –”S” (with NaCl). To the resulting mixture 10.5 % PEG 8000 in buffer-”S” was added to a final concentration of 3.5%. The mixture was transferred to a 250 ml Erlenmeyer flask and homogenized in an orbital shaker at 100 strokes min-1for 30 minutes in room temperature. The homogenized mixture was centrifuged at 11,000 rpm for 20 minutes. The supernatant was filtered through double-layered cheese cloth and 42% PEG in buffer-”S” (with NaCl) was added to make a final concentration of 12% PEG. Then the mixture was homogenized for 30 minutes and centrifuged at 11,000 rpm for 20 minutes. The pellet was redissolved in buffer-”S” (with NaCl) to the original yolk volume.
An equal volume of 4M ammonium sulphate (pH7) was added with the redissolved pellet and incubated at 00C . The precipitate was centrifuged at 11,000 rpm for 20 minutes and the pellet obtained were again redissolvedin buffer-”S” (without NaCl).
Purification of Egg yolk anti-bodies by dialysis
The egg yolk antibodies were desalted by dialysis against buffer S (without NaCl) to remove ammonium sulphate. The cellulose membrane tubing (pore size – 0.25µm) was cut into a piece of required and convenient length. It was allowed to boil for 10 minutes in 2% (W/V) sodium bicarbonate and 1mM of EDTA (pH 8.0). After activating the tubing, it was handled with gloves. The tubing was cooled and rinsed thoroughly with water. Before use, the bag was opened and washed inside and outside with distilled water. A quantity of 4 ml of pooled IgY fraction was transferred to the dialysis membrane bag. The bag was twisted and tied at both the ends after including some air. The bag was carefully immersed in a beaker containing buffer S (without NaCl) and the apparatus was maintained at 50C in refrigerator. At every 6 hour of interval the buffer was replaced with fresh buffer. Buffer was frequently changed until the ammonium sulphate got eluted completely. The elution was confirmed with Nessler’s reagent which forms brown precipitate in the presence of ammonium sulphate. The content was stored in vial after dialysis at 40C.
Determination of protein content in IgY fraction (Lowry et al (1951)
The total protein content was estimated according to the method described by Lowry et al (1951).
Gel electrophoresis for antibodies
The protein profiles of the antibodies were carried out by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using 12% gel according to the method of Laemmli (1970).
Estimation of antibody titre by ELISA
The antibody titrations were carried out using Nunc polyvinyl microtitre plates by the method of Volleret al. (1976). Indirect antibody capture assay (IACA) showed that the antibodies are generated in chickens against injected rabies viral antigens. The antibody level in column purified fraction of was checked in ELISA by diluting fractions with different dilutions with PBS (1:10, 1:100, 1:1000 and 1:10,000 dilutions).
Between day 7 and day 21, an increase of 70% in the titre value of IgY antibodies has been observed in the test chicken immunized with anti-rabies antigen. The titer values were high in both 1:10 and 1:100 dilutions. The titre values of IgY antibodies from hen and IgG from rabbit against attenuated rabies antigen is given in
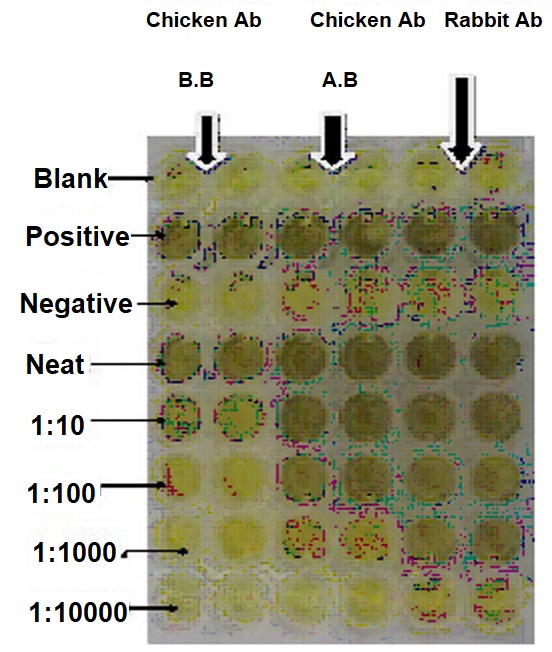 Figure: 1. and the Titre plate of Rabies attenuated antigen against IgY from chicken and IgG from Rabbit is given in
Figure: 1. and the Titre plate of Rabies attenuated antigen against IgY from chicken and IgG from Rabbit is given in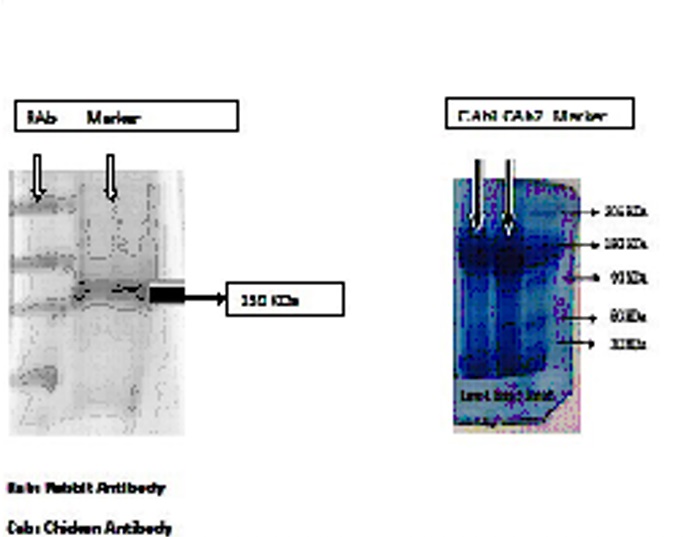 Figure: 2. The electrophoretic separation of IgY antibodies of hen is given in
Figure: 2. The electrophoretic separation of IgY antibodies of hen is given in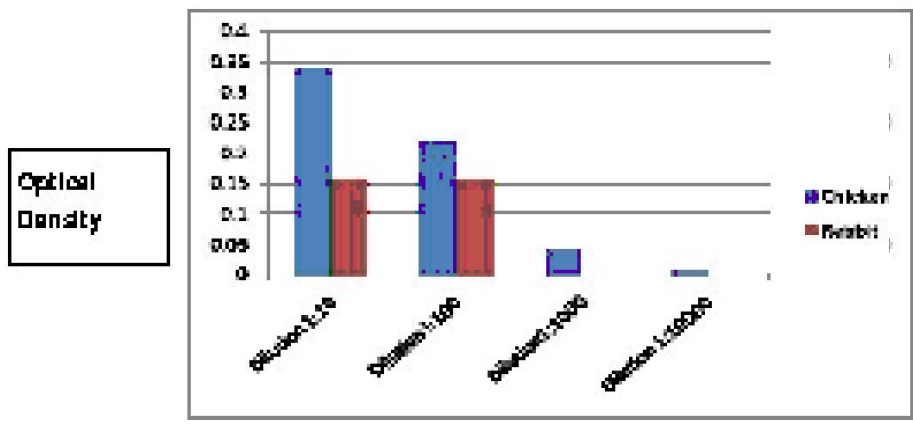 Figure: 3. The Ig Y anti-rabies antibody has KDa value of 180.
Figure: 3. The Ig Y anti-rabies antibody has KDa value of 180.The titer value, in hen is reportedly influenced by a number of factors, such as the strain and age of hens as well as the route of immunization. Compared to the younger hens, the older ones, not only tolerate severe immunization, but also have higher IgY titers. (Trott et al.,2009). The route of immunization that has been used in the present study, that is, intramuscular immunization, has reportedly yielded higher levels of specific IgY the subcutaneous route. (Chang et al., 1999)
Although in the present study, the attenuated rabies virus has been used as the antigen for immunization, the use of viral proteins (as antigen) expressed by bacterial cells may provide a good amount and cheap antigen for immunization. Further studies in this line are worth pursuing.
The total protein was estimated in the dialysed solution of IgY from the egg yolk and was found to have a protein value of 96 mg per egg. This value is comparable with that of Hatta et al., (1990) who reported a value of 70 to 100mg per egg whereas Hatta et al (1988) had recorded a value mg 40 mg per egg. The difference in the value among different studies may be attributed to the strain and age of hens used for the study. (Trott et al., 2009). According to him the total egg antibody output was directly correlated with the total egg mass productivity.
20 ml of blood sample was collected and the serum was used for the anti-body IgG titres. The titre of rabbit anti-rabies IgG was considerable. And the result is shown in figures 1 and 2. Earlier studies indicated certain advantages of rabbit IgG, such as lower immunogenicity (Redwan et al., 2009) higher affinity and recognition of different epitopes and safe (Kamar et al., 2005; Klam, 2009)
A study similar to the present one was carried out on the productivity of anti-human Rotovirus (HRV) hen yolk antibody (IgY) and comparing it with that of anti-HRV rabbit serum antibody (IgG). The hens laid eggs continuously without any change in the egg laying rate and the yolk of the egg laid over a year showed high level of neutralizing titer against HRV. It was reported that the productivity of anti-HRV IgY by a hen (one year) was at least 15 times more than those by an immunized rabbit in the neutralization titres of the antibodies. (Hatta et al.,1993).An yet another advantage of IgY antibodies is the phylogenetic distance. Many authors have reported that chickens often produce antibodies against phylogenetically highly conserved mammalian protein more efficiently than do rabbits. (Scharde et al 2005).
The increasing incidence of dog bites has necessitated the need for a greater amount of anti-rabies anti-bodies. The production of human or equine anti-rabies anti-bodies ia very expensive and the production has its limitations. Therefore, a large quantity but at the same time a cheaper production technology is needed. Hence, the IgY antibodies may be considered favorably to fulfil the requirement. It will be socially relevant and appropriate to carry out further studies in this direction to produce hassle free IgY anti-rabies antibody. From the titer values it is clear that high sensitivity values are observed in IgY samples compared to IgG samples establishing that antirabies IgY antibody is an abundant and an alternative source of antibodies. Further studies will be performed making IgY antibodies a “gold standard” in commercializing antibody production.
- Leslie ,G.A., Clem, L.W .Phylogen of immunoglobulin structure and function. 3. Immunoglobulins of the chicken J. Exp. Med.., 1969; Dec 1; 130(6) : 1337-52.
- Michael,A.,Meenatchisundarm,S., ParameshwariG.,Subburaj,T., Selvakumaran,R.andRamalingam,S .Chicken egg yolk antibodies (IgY) as an alternative to mammalian antibodies., I.JST., 2010; 3(4): 468-472
- Polson,A., Von Wechmar, M.B and Regenmortel,M.H.V.V. Isolation of viral IgY antibodies from yolks of immunized hens. Immunolcommun. 1980; 9: 475-493.
- Lowry, Olive, H. Nira ,J .Rosebrough, A., Lewis Farr and Rose J. Randall. Protein measurement with Folin Phenol Reagent; JBC. 1951,265-275.
- Laemmeli,U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature., 1970. 227: 680 – 685.
- Voller,A., Bartlett., Ann. and Bidwell. The Detection of viruses by Enzyme-Linked Immunosorbent Assay (ELISA). J. Gen. Virol..,1976; 33: 165 -167.
- Trott,D.L., Yang,M., Utterback, P.L., Utterback, C.W., Koelkeback, K.W., and Cook, M.F. Utility of spent single comb white leghorn hens for production of polyclonal egg yolk antibody. J. Appl. Poultry Res.; 2009; 18: 679-89.
- Chang, H.M., Ou-Yang, R.F., Chen, Y.T., Chen, C.C., Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype C in chicken egg yolk (IgY) , ýJ. Agric. Food Chem. 1999;47:61-66
- Hatta, Hajime, Mujo Kim and Takehiko Yamamoto. A novel isolation method for hen yolk antibody “IgY”. J. Biol. Chem.. 1990; 54 (10), 2531-2535
- Redwanel,R.M., Fahmy,A., Eihanafy,A., Abd El-Baky.N and Sallan,S.M. Ovine anti-rabies anti-body production and evaluation.Comp Immunol Microbiol Infect Dis. 2009, 32: 9-19.
- Kamar, N., Borde,J.S., Sandres-Saune,K., Suc,B., Barange,K., Cointadut,O., Lavayssiere,L., Durand, Izopet,J. and Rostaing,L. Inducting therapy with either anti-CD25 monoclonal antibodies or rabbit antitshymocyte globulins in liver transplantation for hepatitis C. Clinical Transplantation. 2005, 19:83-89.
- Klem,P., Cooper, J.E., Weiss,A.S., Grall,J., Owen,P., Chan, and Wiseman,A.C. Reduced dose rabbit antithymocyte globulin induction for prevention of acute rejection in high-risk kidney transplant recipients. Transplantation. 2009,88: 891-896.
- Hatta H., Tsuda, K., Akachi, S., Kim,M., and Yamamoto, T. Productivity and some properties of egg yolk anti-body (IgY) against human rotovirus compared with rabbit IgG. Bioscience, Biotechnology and Biochemistry; 1993,57: 450-454.
- Hatta.H, Sim J. S. and Nakai S., J. Food Sci., 1988,53,425
- Scharde, R., Calzado, E.G., Samiento, R.,Chacana, P.A., Porankiewicz-asplund, J. Terzolo, H.R. Chicken egg yolk antibodies (IgY-Technology) a review of progress in production and use in research and human and veterinary medicine. Alternatives to Laboratory Animals. 2005, 33: 129-154.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


