Sheetal Rana1, Ranjna Sharma2, Y P Sharma2 and Mohinder Kaur3
1Department of Microbiology, Dr. YS Parmar University of Horticulture &Forestry,Nauni, Solan – 173 230 H.P., India.
2Department of Forest Products, College of Forestry, Dr. YS Parmar University of Horticulture & Forestry, Nauni, Solan – 173 230 H.P., India.
3Department of Basic Sciences, College of Forestry, Dr. YS Parmar University of
Horticulture & Forestry, Nauni, Solan – 173 230 H.P., India.
ABSTRACT
Strain An-F was isolated from the rhizosphere soil of replant site of apple orchard at Shimla district H.P. and identiûed as Pseudomonas aeruginosa on the basis of biochemical tests and by 16S rRNA sequences. This bacterium exhibits a broad-spectrum antifungal activity towards phytopathogenic fungi. The antifungal metabolite by An-F was extracted and characterized using TLC and HPLC (High pressure liquid chromatography). Production of siderophores, phosphatase, HCN, ammonia, indole-3-acetic acid (IAA), gibberellins and cytokinins in An-F were determined. The antifungal metabolite produced by An-F has been identiûed as 2, 4-diacetylphloroglucinol on the basis of TLC and HPLC data. Present study reports the production of 2,4-diacetylphloroglucinol as well as IAA, gibberellins and cytokinins for the ûrst time by a saprophytic P. aeruginosa strain An-F isolated from the replant site of apple. Because of the production of siderophore, growth hormone, protease and phosphatase and its innate fungicidal potential, this strain can be used as biofertilizer and antagonist against a range of phytopathogenic fungi.
Keywords: Replant site, Pseudomonas aeruginosa, 2, 4-Diacetylphloroglucinol, Biocontrol.
INTRODUCTION
Apple (Malus domestica) replant disease (ARD) is distributed worldwide and is often encountered in establishing new orchards on old sites (Yao et al., 2006). Symptoms include death of fine feeder roots, stunted growth above- and below-ground, and reduced fruit yields (Rumberger et al., 2007). Causes of replant disease are fungi, bacteria, actinomycetes, nematodes, and their interactions. Other causes cited by researchers include fungi from the following genera: Pythium, Thielaviopsis, Rosellinia, Phytophthora, Cylindrocarpon, Fusarium, Rhizoctoni, Penicillium and Alternaria (Rutkowski et al., 2000b; Benizri et al., 2005). Certain fluorescent pseudomonads from soil have been shown to promote plant growth by inhibiting bacteria and fungi that are deleterious to plants. The production of antibiotic substances by some strains has been recognized as a major factor in the suppression of many root pathogens. In this study, we report on the isolation of a fluorescent Pseudomonas strain that can inhibit a number of plant root pathogens by producing DAPG. DAPG is a phenolic compound displaying a remarkably broad spectrum of toxic activity against bacteria, fungi and nematodes. It is one of the three phloroglucinols that have been isolated from fluorescent pseudomonads. We describe the isolation of this compound and also the development of an HPLC assay for its quantitative detection in culture media.
MaterialS and methods
Bacterial strain
Pseudomonas aeruginosa strain An-F (NCBI GeneBank accession no.KJ522924) isolated from the rhizosphere of apple of Shimla district (H.P.). The Pseudomonas culture was maintained in 20% glycerol at -20ºC was revived on nutrient agar and employed for the present study. Antifungal activity was checked by well plate assay method (Vincent, 1947) using dual culture technique against Dematophora necatrix, Fusarium oxysporum, Phytophthora cactorum and Pythium ultimum phytopathogens isolated from the replant site of apple rhizosphere. On one side of prepoured sterilized malt extract agar (MEA) plates, 72 h old culture bit of indicator fungi was placed. On the other side of plate, 100µl of 72 h old cell free supernatant of bacterial strain was added to well. Plates were incubated at 28±2ºC for 4 days and observed for inhibition of mycelial growth produced around the well. For control, culture bit of indicator fungus was kept in the centre of MEA plate and incubated at 28±2ºC for 4 days.
Detection of siderophore, phosphatase, hydrogen cyanide and ammonia production
Quantitative detection of siderophore production in liquid medium of Chrome-azurol-S (CAS) was carried out and change in the colour of reaction mixture was observed from dark blue to orange or pink (Schwyn and Neilands, 1987). Estimation of phosphorous (Bray and Kurtz, 1945 and Olsen et al., 1954) was done quantitatively in PVK broth supplemented with 5.0 g/l tri-calcium phosphate (TCP) and solubilization of phosphorous was calculated using standard curve of KH2PO4 (100-1000 µg/ml). For estimation of hydrocyanic acid (HCN) production by Pseudomonas sp., a color change in the sodium picrate containing filter paper strip was observed from yellow to orange brown to dark brown (Bakker and Schippers 1987). For the detection of ammonia production, the method of Lata & Saxena (2003) was used. Pseudomonas isolate was grown in 5ml of peptone water in tubes at 28±2ºC for 4 days. After 4 days, 1ml of Nessler’s reagent was added to each tube. Presence of very light brown color (+) indicates small amount of ammonia production and light brown (++) to orange brown color (++++) indicates large amount of ammonia production.
Detection and estimation of plant growth promoting phytohormone production
Quantitative measurement of auxins was done by colorimetric method (Gorden and Paleg, 1957) with slight modification. The gibberellin was estimated colorimetrically by the method of Holbrook et al., (1961). For bioassay of cytokinin, the radish cotyledon expansion test was employed (Letham, 1971). The bioassay response (final weight – initial weight) was expressed as increase in weight of cotyledon.
Effect of media on the production of antifungal activity
Six media i.e. King’s broth, PPM broth, YM broth, Syncase broth, Succinate broth and nutrient broth were used for growth and production of antifungal metabolites for antifungal activity. Antifungal activity was checked by well plate assay method (Vincent, 1947) using dual culture technique against Dematophora necatrix, Fusarium oxysporum, Phytophthora cactorum and Pythium ultimum.
Detection of antibiotic
Extraction of antibiotic was carried out by following the method described earlier (Rosales et al., 1995) with slight modifications. Briefly, pre-inoculum of bacterium was prepared in 5 ml of KB broth and allowed to grow at 30°C for 19h. A 100ml of Syncase medium ( Na2HPO4 5gm, K2HPO4 5gm, Glucose 5gm, NH4Cl 1.18gm, Na2SO4 0.089, MgCl2.6H2O 0.042gm, MnCl2.H2O 0.004 gm, Casein hydrolysate 10gm dissolved in 1000ml of distilled water) was inoculated with 1% of pre-inoculum incubated at 30°C for 4 days on rotator shaker. The fermentation culture was centrifuged at 3500 rpm for 20 minutes. The collected supernatant of Pseudomonas sp. was acidified to pH 2.0 with 1N HCL. Extraction was done with equal volume of ethyl acetate. Ethyl acetate fraction was reduced to dryness in vacuum. Residue was redissolved in 2 ml methanol separately. 10µl samples were chromatographed on Silica-G TLC plates and Chloroform: methanol (50:50) solvent system was used. TLC was visualized under UV and sprayed with Para-anisaldehyde and compared bands with reference compound. Antifungal activity of each crude extract was checked by well plate assay method.
HPLC apparatus
HPLC system consists of a Waters pump 515, rheodyne injection value with a 20 µl loop and an analytical column (250mm × 4.6mm, 5µm). The spectrophotometric dual › absorbance detector 2487. The reagents for mobile phase preparation i.e. acetonitrile : water acidified with 0.5% glacial acetic acid ( 50: 50) were of HPLC grade and all mobile phases used were filtered and degassed on a Millipore HPLC filteration system with 0.22 micron filter paper. All samples were run at a flow rate of 1ml/ min and detected at a wavelength of 270 nm.
Results and discussion
In this study, we demonstrated that 2, 4-diacetylphloroglucinol producing bacteria can be isolated from the apple rhizosphere and can be effective in the biocontrol of apple phytopathogenic fungus. The fluorescent bacterial antagonist was gram negative, rod shaped and produces greenish pigment on King’s B medium. It was found to inhibit the mycelial growth of all fungal pathogens tested. Pseudomonas aeruginosa An-F showed maximum inhibition against Fusarium oxysporum 45.23%I followed by Phytophthora cactorum 40.38%I. It also inhibit Dematophora necatrix 20%I and Pythium ultimum 13.15%I (Table1).
Table 1 Potential of An-F for antifungal activity.
| % inhibition | ||||
| Strain | Dematophora | Fusarium | Phytophthora | Pythium |
| An-F | 20 | 45.23 | 40.38 | 13.15 |
Investigation on microbial metabolites is gaining greater momentum in the agrochemical industry as a source for the development on new pesticide products. Production of siderophores, phosphatase, HCN, ammonia and plant growth regulators i.e. IAA, gibberellins and cytokinins evidently suggests a potential plant growth promoting ability of the strain An-F (Table 2).
Table 2. Potential of An-F strains for plant growth promoting activities.
| Siderophore
%SU |
Phosphate solubilizing
µg/ml |
HCN | Ammonia | Plant growth regulators | ||
| Auxins
µg/ml |
Gibberellins
µg/ml |
Cytokinins
µg/ml |
||||
| 57.86 | 49 | + | ++++ | 20 | 630 | 23 |
Biosynthesis of bioactive substances is a specific property of some species or even some strains of microorganisms. This property depends greatly upon the conditions of cultivation of microorganisms. Although good growth may occur in many media, secondary metabolites may only be produced in a specific medium (Bentley and Kiel, 1962). Development of medium that produce high yields of a desired secondary metabolite is still empirical to a considerable degree (Demain, 1973). In our study experiment on media was focused on the identification of a suitable growth medium for Pseudomonas stain An-F that can induce higher level of antifungal activity against apple phytopathogens. Maximum percent inhibition was showed by syncase media against all tested pathogens (Table 3). DAPG is an antibiotic known to be active against a broad spectrum of microorganisms and is involved in the suppression of many plant diseases (Defago 1993; Keel et al., 1990; Keel et al., 1992; Weller and Thomashow 1993). The metabolite DAPG extracted in ethyl acetate fraction of the An-F strain produced bright yellow colour spot at Rf value of 0.8 in the TLC plate. HPLC analysis of extract showed the presence of 2, 4-diacetylphloroglucinol when compared with reference compound (Table 4, Fig.1). Earlier, a number of strains of Pseudomonas have been shown to produce phloroglucinol (Broadbent et al., 1976; Garagulya et al., 1974). The severity of tobacco black root rot was reduced when soil was amended with phloroglucinol (Keel et al., 1992). Phloroglucinol antibiotics are phenolic metabolites produced by bacteria with broad antibacterial and antifungal spectrum and phytotoxic properties (Thomashow and Weller 1996). Keel et al., (1990) and Raaijmakers et al., (1997) reported that phloroglucinol – producing strains of P. fluorescens have been shown to be effective against root pathogens viz., Fusarium oxysporum in tomato, Thielaviopsis basicola in tobacco and Gaeumannomyces graminis var. tritici in wheat. Beside this, phloroglucinol induced defense mechanism against fungal infection (Tomas Lorente et al., 1989). Because of the innate potential of producing siderophore, DAPG, IAA, phosphatase and broadspectrum fungal antibiosis, the strain An-F can be used as biofertilizer as well as a biocontrol agent.
Table 3. Effect of media on production of antifungal activity by An-F strain.
| Media | ||||||
| Fungus Pathogens | Antifungal activity (mm dia. and % inhibition) | |||||
| KMB | PPM | YM | Syncase Media | Succinate Media | NB | |
| %I | %I | %I | %I | %I | %I | |
| Dematophora necatrix | 0 | 23.91 | 27.14 | 31.11 | 28.84 | 20 |
| Fusarium oxysporum | 22.22 | 23.91 | 28.07 | 45.23 | 20.45 | 14 |
| Phytophthora cactorum | 0 | 25.71 | 28.84 | 47.87 | 25 | 40.38 |
| Pyhium ultimum | 25 | 18.36 | 23.07 | 23.91 | 9.75 | 13.15 |
Table 4. Quantity of DAPG produced by Pseudomonas aeruginosa An-F.
S. no. |
Name of sample |
Retention Time (RT) |
Conc. (µg/100ml) |
1 |
DAPG standard |
11.374 |
|
2 |
An-F |
11.196 |
3.02 |
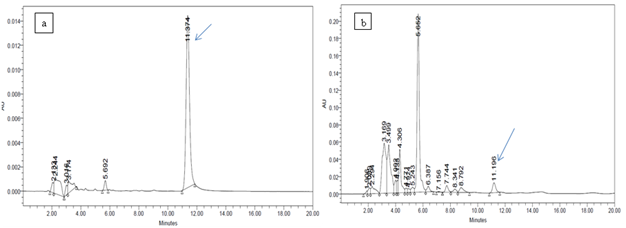
Fig. 1 HPLC chromatograms of authentic 2, 4-diacetylphloroglucinol (a) and extract of Pseudomonas aeruginosa strain An-F (b) after four days of incubation at 300C in Syncase broth.
References
- Bakker and Scippers. Microbial cyanide production in the rhizosphere in relation to total yield reduction and Pseudomonas species mediated plant growth stimulation. Soil Biology and Biochemistry 1987; 19: 451-457.
- Benizri, E., Piutti, S., Verger, S., Pagès, L., Vercambre, G., Poessel, J.L. and Michelot, P. Replant disease: Bacterial community structure and diversity in peach rhizosphere as determined by metabolic and genetic finger printing. Soil Biol. Biochem., 2005; 37: 1738–1746.
- Bentley, R. and Keil, J. G. J. Biol. Chem. 1989; 237-867.
- Bray, R.H. and Kurtz, L.T. Determination of total organic available forms of phosphorus in soil. Soil Science 1945; 23: 343-353.
- Broadbent, D., Mabelis, R.P. and Spencer, H. C-acetylphloroglucinols from Pseudomonas fluorescens. Phytochemistry 1976; 15: 1785.
- Demain, A. L. Mutation and production of secondary metabolites. Advances in Applied Microbiology 1973; 16: 177-202.
- Garagulya, A.D., Kiprianova, E.A. and Boiko, O.I. Antibiotic effect of bacteria from the genus Pseudomonas on phytopathogenic fungi. Microbiology (Kiev) 1974: 36: 197–202.
- Gorden and Paleg, L.G.,. Quantitative measurement of IAA. Plant Physiology 1957; 10: 37-38.
- Holbrook, A.A., Edge, W.L.W. and Bailey, F. Spectrophotometric method for determination of gibberellic acid in gibberellins, ACS Washington, D.C., 1961; 159-167.
- Keel, C., Schnider, U., Maurhofer, M., Voisard, C., Laville, K., Burger, U., Wirthner, U. P., Haas D. and Defago G. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol. Plant-Microbe Inter. 1992; 5: 4–13.
- Keel, C., Wirthner, P., Obeshansli, T., Voisard, C., Burger, U., Hass, D. and Defago, G. Pseudomonas as antagonists of plant pathogens in the rhizosphere, role of the antibiotic 2,4-diacetylphloroglucinol in the suppression of black root rot of tobacco. Symbiosis 1990; 9: 327–341.
- Lata and Saxena, A.K. Characterization of plant growth promoting rhizobactria. In: Saxena, A.K. (Ed.), Training manual on Biofertilizer Technology. IARI Delhi, 2003; 24-25.
- Letham, M.L. Regulators of cell division in plant tissues. A cytokinnin bioassay using excised raddish cotyledons. Physiologia Plantarum 1971; 25: 391-396.
- Olsen, R., Cole, C.V., Whatanable, F.S. and Dean, L.A. Estimation of available phosphorus by extraction with sodium carbonate. US Department of Agriculture 1954; 939 .
- Raaijmakers, J., Wellers, D. and Thomashow, L. Frequency of antibiotic producing Pseudomonas spp. in natural environments. Appl. Environ. Microbiol. 1997; 63: 881–887.
- Rosales, A.M., Thomashow, L., Cook, R.J. and Mew, T.W. Isolation and identification of antifungal metabolites produced by rice associated antagonistic Pseudomonas sp. Phytopathology 1995; 85: 1028-1032.
- Rumberger, A., Merwin, I.A. and Thies, J.E. Microbial community development in the rhizosphere of apple trees at a replant disease site. Soil Biol. Biochem., 2007; 39: 1645–1654.
- Rutkowski, K., Pacholak, E. and Sawicka, A. Evaluation of the microbiological state of the soil under varied conditions of fertilization and irrigation in a replanted orchard. PartII. Number of fungi and actinomycetes. Prace Kom. Nauk Rol. i Kom. Nauk Leœ . PTPN . 2000b; 89: 185–192.
- Schwyn, B. and Neilands, J.B. Universal chemical assay for the detection and determination of siderphores. Analytical Biochemistry 1987; 28(8): 751-759.
- Thomashow, L.S. and Weller, D.M. Current concepts in the use of introduced bacteria for biological disease control: Mechanisms and antifungal metabolites. 1996; 187–235. In “Plant-Microbe Interactions” (Stacey, G. and Keen, M. eds.). Chapman and Hall, New York, Vol. I
- Tomas-Lorente, F., Iniesta-San Martin, E., Tomas Barberan, F.A., Trowitzsch-Kienast, W. and Wary V. Antifungal phloroglucinol derivatives and lipophilic flavanoids from Helichrysum decumbens. Phytochemistry 1989; 28: 1613–1615.
- Vincent, J.M. Distribution of fungal hyphae in presence of certain inhibitors. Nature 1947; 150-850.
- Weller, D.M. and Thomashow, L.S. Use of rhizobacteria for biocontrol. Curr. Opin. Biotechnol. 1993; 4: 306–311.
- Yao, S., Merwin, I.A., Abawi, G.S. and Thies, J.E. Soil fumigation and compost amendment alter soil microbial community composition but do not improve tree growth or yield in an apple replant site. Soil Biol. Biochem., 2006; 3: 587–599.

