ISSN: 0973-7510
E-ISSN: 2581-690X
The main objective of the study was to observe the antioxidant, cytotoxicity and anticancer activity of 12 marine endophytic fungi extracts. Marine endophytic fungi were isolated from 11 species of seaweeds, collected from Kovalam (covelong), Chennai and Mandapam, Ramanathapuram district in Tamil Nadu, India. The isolated fungi were extracted with ethyl acetate solvent for bioactivity assay. DPPH assay was performed for antioxidant study. The extracts from Chaetomium sp., Phomopsis sp., Acremonium sp., Aspergillus niger, Xylaria sp., Cladosporium sp., and Fusarium sp., showed greater antioxidant potential compared to other fungal extracts. The cytotoxicity assay was carried out in the Vero cell line and the anticancer activity was found in HEp2 cell line (Human Epidermoid carcinoma cells) and in A549 cell line (Human Lung Carcinoma – Epithelial cells). We observed that Phomopsis sp. fungal extract was more toxic to both the cancer cells, but, comparatively, the same extract exhibited less toxicity against the normal Vero cells. The IC50 values for other 11 different fungal extracts against Vero cells were lesser than the IC50 value of the respective extract against A549 cancer cells, indicating the toxicity of these 11 different fungal extracts.
Endophytic fungi, marine-derived secondary metabolites, cytotoxicity, anticancer activity.
Endophyte (Gr. endon, within; phyton, plant) – the word was first coined by de Bary1. Endophytes are an endosymbiotic group of microorganisms that can be readily isolated from any microbial and plant growth medium and they may colonize in plants and microbes. Endophytic organism plays a key role in discovery of novel bioactive secondary metabolites, these metabolites are alkaloids, quinones, steroids, saponins, tannins, terpenoids and phenolic acid. Bioactive secondary metabolites of endophytic organisms serve as a vital sources for antimicrobial, anti-insect, anticancer and many more properties2.
The endophytic microorganisms can be bacteria, fungi, actinomycetes, or viruses. While they express a variety of symbiotic lifestyles ranging from parasitism to mutualism. Terrestrial endophytic organisms – chiefly fungi and bacteria which reside the living interior parts of plant tissue without causing any harmful effect to the host. There are roughly 3,00,000 various plant species inhabiting our planet and it can be expected that each one has a complex community endophytic microorganism3.
Marine endophytes – Bacteria and fungi have a firm relations with soft-bodied marine organisms, these organism does not have a clear structural protection mechanisms, and thus rely on chemical protection by the production of secondary metabolites. Fungi found in almost all the corners of marine habitat, namely marine plants such as algae, driftwood, mangrove plants and in marine invertebrates – sponges, corals, ascidians, holothurians and vertebrates (mainly fungi)4. Apart from all the various marine organisms, algae is utmost ubiquitous sources of marine endophytic fungi for biochemical research. Marine endophytic fungi, which colonize internal tissue of their hosts without causing any diminishing effect, have substantiated to be crucial sources of bioactive natural products with a subtle structure and dynamic pharmaceutical activity5.
Marine-derived secondary metabolites (SM) are low molecular mass, organic compounds. That are dissimilar to primary metabolites, secondary metabolites are not precisely involved in the growth, development or reproduction of the producing organism6. The marine environment is an affluent source of secondary metabolites with implicit application in specialized product development. The amelioration of secondary metabolites from marine resources has been a key factor for intensive research due to their bioactive nature7.
Marine-derived secondary metabolites and anticancer activity
Cancer is characterized as the uncontrolled growth of abnormal cells that affect various organs of the body and invasion into normal tissues. Cancer cells can disseminate to other sections of the body and produce new tumors. Dissemination of cells becomes uncontrolled and ends in death. The annual death toll from cancer is expected to rise to 17.5 million by 2050, simply due to projected population growth and ageing, as well as lifestyle and environmental influences, including smoking and exposure to carcinogenic agents8. Numerous current therapeutic methods are ineffective in treating certain cancers, and multidrug-resistant tumors intensify treatment challenges. The discovery of potential and active novel chemotherapeutic agents isolated from natural sources is a key factor in curing debilitating diseases such as cancer.
There are more than 1000 Marine fungal-derived structurally unique secondary metabolites have already been published. Despite the absence of marine-derived fungal secondary metabolites in current clinical research, plenty of secondary metabolites are designated as a chemotherapy agent due to the anticancer activity9. There is a particular concern and focus in discovering and studying the potential of marine derived fungal secondary metabolites and screening for anticancer activity, leads to the identification of more effective compounds.
Collection of seaweeds
Seaweeds are collected from Kovalam (covelong), Chennai – Coordinates: 12.7925°N 80.2530°E and Mandapam, Ramanathapuram district – Coordinates: 9.28°N 79.12°E. Seaweeds were first observed, then collected according to differentiation in morphology and colour. Collected seaweed samples are then transferred gently into Polythene zip-lock sample cover. Collected seaweed samples were quickly taken to a laboratory and then seaweed was gently washed in sterile water. Seaweed samples are then gently pressed with a sterile tissue paper to remove wetness from seaweed samples. Seaweed samples were further sterilized to remove external microorganisms for the isolation of endophytic fungi.
Isolation of endophytic fungi from seaweeds.
Seaweeds samples were cut into little fragments of exactly 1 x 1 cm and washed three times with sterile seawater to eliminate surface debris. Little fragment of seaweed sample was immersed in EtOH 70% for 60 – 80 seconds for surface sterilization. Small fragments of seaweed tissue were soaked dried with a sterile cotton cloth (or rinse with sterile artificial sea water) to cease the sterilization with EtoH 70%. Fragment of seaweed sample was kept carefully on the surface of a Petri plate containing potato dextrose agar medium with sterilized forceps so, that the freshly cut edges are in direct contact with the agar surface. Seal the Petri plates with parafilm, label and store it at 20 – 25°C. Fungal growth from the cut seaweed fragments usually initiate’s after 2-3 days until 14 days after the onset of the experiment [10]. Different fungal growth were observed from different seaweed sample. The individual fungal growth was isolated by removing hyphal tips growing out of the cut seaweed fragments with a sterile loop onto a fresh Petri plate containing fresh potato dextrose agar medium. The isolated marine endophytic fungi from the host marine algae were identified through microscopic observation and fungal genus were compared with standard monographs. They were distinguished from each other by their cultural characteristics such as colony morphology, growth rates, hyphal mat characteristics, and pigmentation of the fungal colony and medium11-16. All the marine endophytic fungal isolates were documented and maintained in potato dextrose agar slants.
Large-scale cultivation of endophytic fungi from seaweeds.
Small fragments of fungal mycelium were transferred aseptically with a sterile bent inoculation loop in a 1000 ml Erlenmeyer flask containing the sterilized Potato Dextrose Broth medium. Fungal growth is carried out at room temperature under stationary condition. Based upon the fungal growth, Potato Dextrose Broth medium was incubated for 3–4 weeks.
Collection of crude extracts from endophytic fungi
Fungal cultured Potato Dextrose Broth was first filtered with a Büchner funnel and mycelium remains were discarded. Fungal cultured Potato dextrose broth was once again filtered through a glass funnel using Whatman qualitative filter paper, fungal culture filtrate collected in 1000 ml beaker. The Fungal cultured filtrate was then transferred into a separation funnel, equal volumes of ethyl acetate was added into a separation funnel and mixed rigorously. An aqueous phase and ethyl acetate phase was formed in a separating funnel, the aqueous phase was affably separated from the separation funnel and discarded. Ethyl acetate phase was then separated and collected in 1000 ml Erlenmeyer flasks, collected ethyl acetate extract was then transferred to 1000 ml Round-bottom flask. Ethyl acetate fungal extract was then dried under vacuum using a rotary evaporator at 40°C, this process takes about 40 minutes. At the end of the process Ethyl acetate solvent was collected in receiving round-bottom flask and the fungal extract was retained in evaporating round-bottom flask. Fungal extracted was observed as a dry solid or oily residue in evaporating round-bottom flask17.
Fungal extracted was removed from the round-bottom flask by eluting the fungal extract with ethyl acetate solvent. Eluted fungal extracted was dispensed in sterile vials and then fungal extracts were air dried at room temperature. After air drying, dried fungal extracts were stored in the freezer for furthered bio-activity screening.
1, 1-Diphenyl-2-picryl-hydrazyl (DPPH) Assay
Buffered methanol with acetic acid were used for the preparation of the DPPH stock solution, 40 ml of 0.1M acetate buffer (pH 5.5) added to 60 ml of methanol to prepare buffered methanol. All the chemical and solvents used in the study were analytical grade. The reaction tubes were wrapped in aluminium foil and kept at 30°C for 30 minutes in darkness, due to the photosensitivity of the chemical used in the study. The optical density of the sample was measured by spectrophotometric at 517 nm and the data obtained were presented as percentage scavenging activity18.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Gas chromatography –Mass spectrometry were performed by using a JEOL GCMATE II GC-MS spectrometer. The initial temperature of this instrument was at 50°C for 2 minutes. After this period the temperature of the oven was increased to 250°C. At the rate of 10°C/min maintained for 15 minutes. The temperature of the injection port was assured at 220°C and flow rate of helium at 1ml/min. Analyte ionization voltage was 70 electron volt (eV). The samples were injected in split less method. The Mass spectral scans range was at the rate of 50-650 (m/z). Application of computer in analysing GC-MS electron –ionization mass spectra, utilizing the National Institute of Standards and Technology (NIST) software Ver.2.1 MS data library, acquired spectrum were compared and to identify the compounds19.
Bioactivity Assay
Cell cytotoxicity assay in Vero cell line.
The weighed fungal crude extracts were first dissolved in 25µL of DMSO and then mixed on the vortex mixer. 5 ml of MEM was added to the DMSO dissolved fungal crude extracts. The extracts were filtered through Whatman syringe filter (pore size-0.2µm). The filtered crude extract was made up into 1 ml stock. 100µL of diluted fungal extracts was added in the 96 well plates. The 96 well plates were kept in 5% CO2 incubator at 36°C. Vero cells observed subsequently 24 hours, 48 hours and 72 hours.
Anticancer activity in HEp2 and A549 cell lines.
The weighed fungal crude extracts were first dissolved in 25µL of DMSO and then mixed on a vortex mixer. 5 ml of MEM was added to the DMSO dissolved fungal crude extracts. The dissolved fungal crude extracts were filtered through Whatman syringe filter (pore size-0.2µm). The filtered crude extract was made up into 1ml stock. 100µL of diluted fungal extracts was added in the 96 well plates. The 96 well plates were kept in 5% CO2 incubator at 36°C and HEp2 and A549 cells were observed after 24 hours, 48 hours and 72 hours.
MTT assay
MTT assay is one the prominent in-vitro assay utilised for the assessment of cell viability, proliferation of cells and cell cytotoxicity. The main principle of the MTT is that yellow coloured compound 3-(4, 5-Dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium Bromide) is converted into blue Formazan derivative (2E, AZ) – (4, 5-Dimethylthiazol-2-yl)-3, 5-diphenylformazan by mitochondrial reductase. Formazan crystals are then solubilised with Dimethyl Sulfoxide (CH3)2 SO and released solubilised formazan reagent is measured spectrophotometrically. Since the reduction of MTT can occur only in metabolically active cells, the level of activity is a measure of the viability of the cells20,21.
Collected seaweeds
Totally 11 seaweeds were collected from two different coastal areas in Tamil Nadu, south India. 4 seaweeds were collected from Kovalam (Covelong), Chennai, Coordinates – 12.7925°N 80.2530°E and 7 seaweeds were collected from Mandapam, Ramanathapuram district, Coordinates -9.28°N 79.12°E were shown in (Table 1 & 2), (Fig. 1).
Fig. 1. (A) Chaetomorpha antenina. (B) Grateloupia lithophila. (C) Ulva lactuca. (D) Garcilaria corticata. (E) Garcilaria solicornia. (F) Turbinaria conides. (G) Gercilaria edulis. (H) Halimedia macroloba. (I) Stoechospermum marignatum. (J) Gelidiella acerosa. (K) Sargassum swartzii.
Table (1):
Seaweeds collected from Kovalam (covolong), Chennai
|
Kovalam (covolong), Chennai
|
Chaetomorpha antenina. |
| Grateloupia lithophila. | |
| Ulva lactuca. | |
| Garcilaria corticata |
Table (2):
Seaweeds collected from Mandapam, Ramanathapuram district
|
Mandapam, Ramanathapuram district |
Garcilaria solicornia |
| Turbinaria conides | |
| Gercilaria edulis. | |
| Halimedia macroloba | |
| Stoechospermum marignatum | |
| Gelidiella acerosa | |
| Sargassum swartzii |
Isolated endophytic fungi from seaweeds
A total of 12 marine endophytic funguses was isolated from different genus of seaweeds. A microscopic examination of the isolated marine endophytic fungal spores revealed various genus of endophytic fungi they were shown in (Table 3).
Table (3):
Marine endophytic Fungus isolated from collected seaweed
Collected Seaweeds |
Isolated Marine endophytic fungi |
|---|---|
(A) Chaetomorpha antenina. |
|
(B) Grateloupia lithophila.
|
|
(C) Ulva lactuca
|
|
(D) Garcilaria corticata |
|
(E) Garcilaria solicornia.
|
|
(F) Turbinariaconides |
|
(G) Gercilaria edulis.
|
|
(H) Halimedia macroloba. |
|
(I) Stoechospermum marignatum
|
|
(J) Gelidiella acerosa
|
|
(K) Sargassumswartzii.
|
|
1, 1-Diphenyl-2-picryl-hydrazyl (DPPH) Assay
The antioxidant activity of DPPH of isolated marine endophytic fungal ethyl acetate extracts was shown in (Table 4). DPPH radical react with suitable reducing agents then losing colour stoichiometrically with the number of electrons consumed, which is measured spectrophotometrically at 517 nm. The extracts from Chaetomium sp., Phomopsis sp., Acremonium sp., Aspergillus niger, Xylaria sp., Cladosporium sp., and Fusarium sp., showed greater antioxidant potential compared to other fungal extracts.
Table (4):
Antioxidant activity of crude endophytic fungal Ethyl acetate extract using the DPPH Assay method
| S.no | Name of the Fungi | DPPH activity (%) | |
|---|---|---|---|
| 1. | Chaetomium sp. | 96.41 | |
| 2. | Penicillium sp | 46.94 | |
| 3. | Aspergillus sp. | 49.35 | |
| 4. | Aspergillus terrus | 45.63 | |
| 5. | Phomopsis sp. | 97.86 | |
| 6. | Rhizopus sp. | 48.02 | |
| 7. | Acremonium sp. | 96.14 | |
| 8. | Aspergillus niger | 95.32 | |
| 9. | Xylaria sp. | 97.86 | |
| 10. | Cladosporium sp. | 95.79 | |
| 11. | Fusarium sp. | 95.02 | |
| 12 | Ascomycete’s sp. | 46.41 | |
GC-MS Analysis
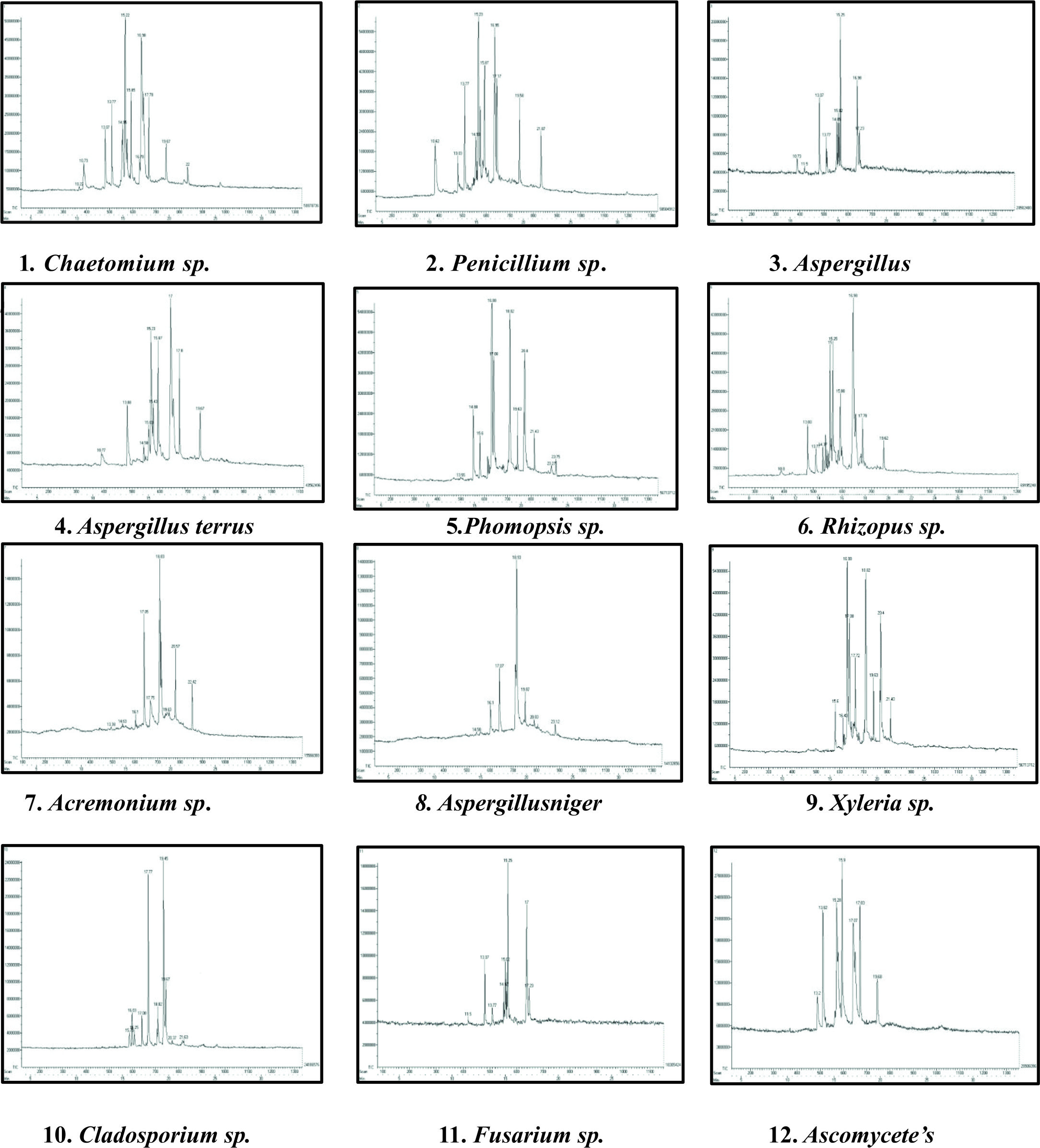
GC-MS chromatogram analysis of the ethyl acetate crude extracts Chaetomium sp., Penicillium sp., Aspergillus sp., Aspergillus terrus, Phomopsis sp., Rhizopus sp., Acremonium sp., Aspergillus niger, Xylaria sp., Cladosporium sp., Fusarium sp., Ascomycetes sp., showed multiple peaks which indicate the presence of many compounds. Comparison of the GC-MS mass spectra constituents with the National Institute of Standard and Technology (NIST) library is shown in (Fig. 2).
Fig. 2. GC-MS chromatogram analysis showed multiple peaks which indicating the presence of many compounds in crude endophytic fungal Ethyl acetate extract.
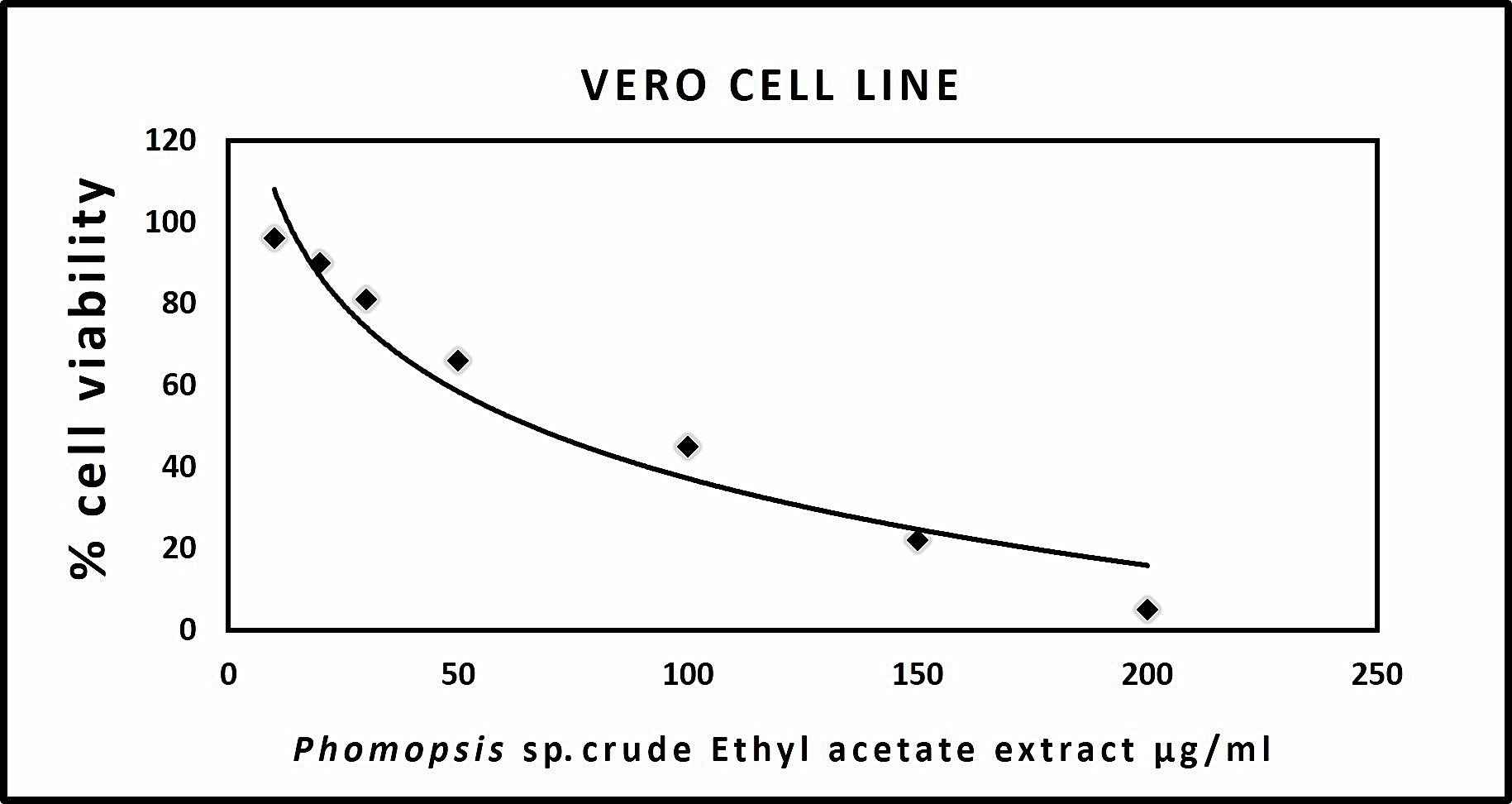
Cell cytotoxicity assay in Vero Cells
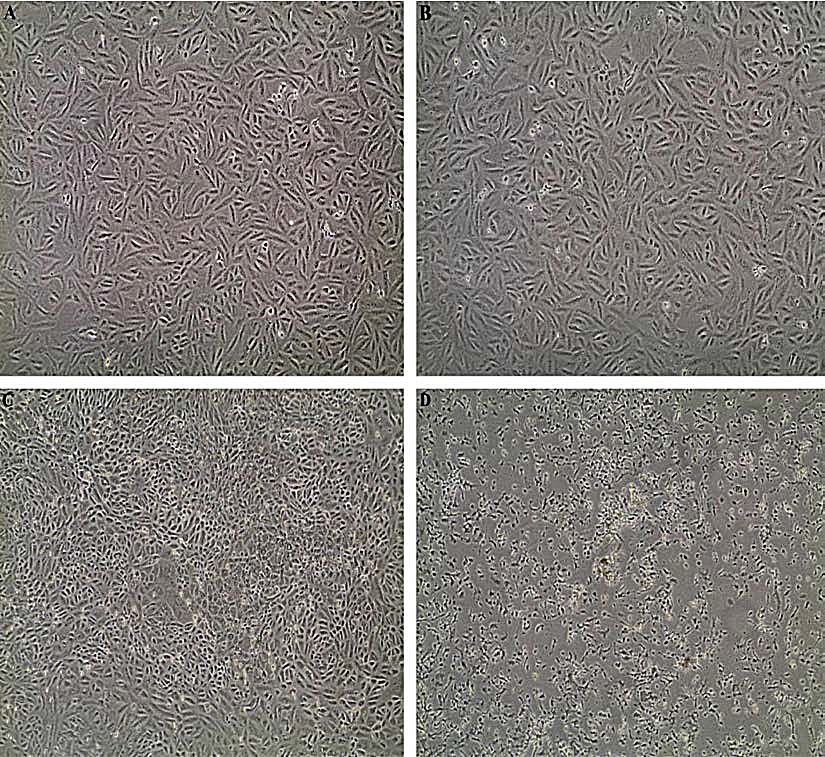
The results acquired through MTT assay for Vero cell line showed a dose-dependent decrease in the percentage of cell viability. The IC50 of each extract was shown in (Table 5). The microscopic observation of cytotoxicity of Phomopsis sp. against Vero cells showed in (Fig. 3). The dose-dependent effect of Phomopsis sp., against Vero cell viability shown in (Fig. 4).
Fig. 3. (A) Vero cell control, (B) Vero cells after 24 hours, (C) Vero cells after 48 hours, (D) Vero cells after 72 hours.
Table (5):
Half maximal inhibitory concentration (IC50) of crude endophytic fungal Ethyl acetate extract
No. |
Isolated marine endophytic fungi |
IC50 values in Vero cell line |
|---|---|---|
1. |
Chaetomium sp. |
95 μg/ml |
2. |
Penicillium sp |
89 μg/ml |
3. |
Aspergillus sp. |
92 μg/ml |
4. |
Aspergillus terrus |
128 μg/ml |
5. |
Phomopsis sp. |
66 μg/ml |
6. |
Rhizopus sp. |
170 μg/ml |
7. |
Acremonium sp. |
126 μg/ml |
8. |
Aspergillus niger |
119 μg/ml |
9. |
Xylaria sp. |
154 μg/ml |
10. |
Cladosporium sp. |
126 μg/ml |
11. |
Fusarium sp. |
92 μg/ml |
12. |
Ascmycetes sp. |
172 μg/ml |
Anticancer Activity Assay in HEp2 and A549 Cancer Cell Line
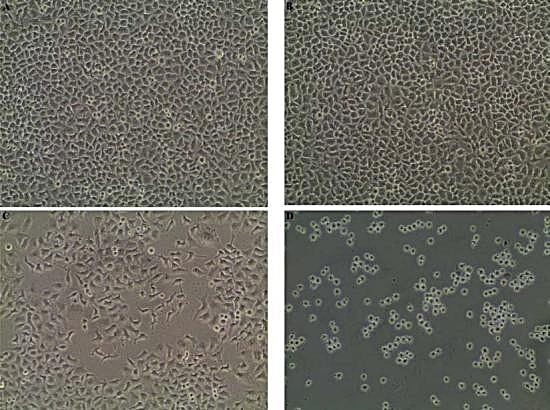
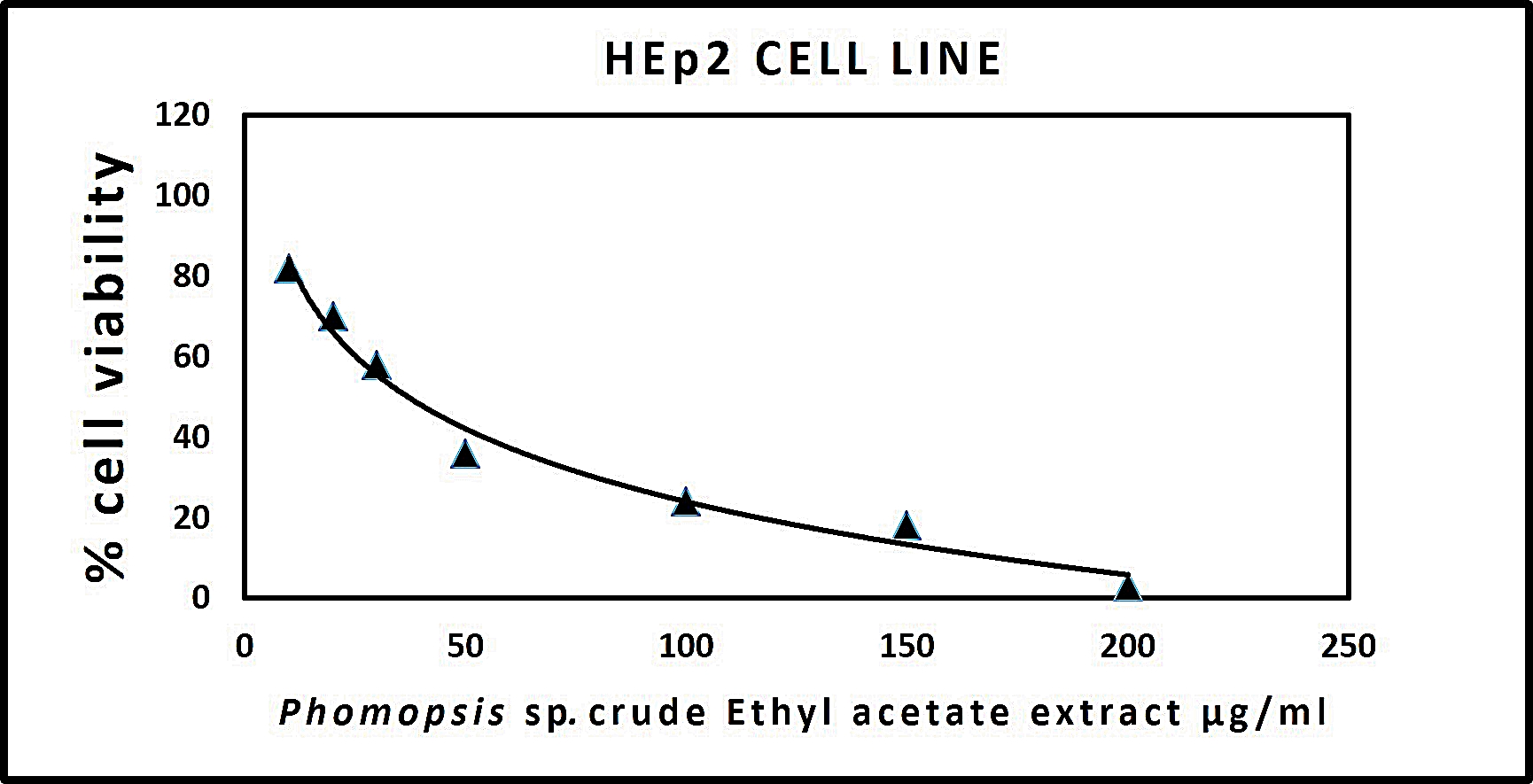
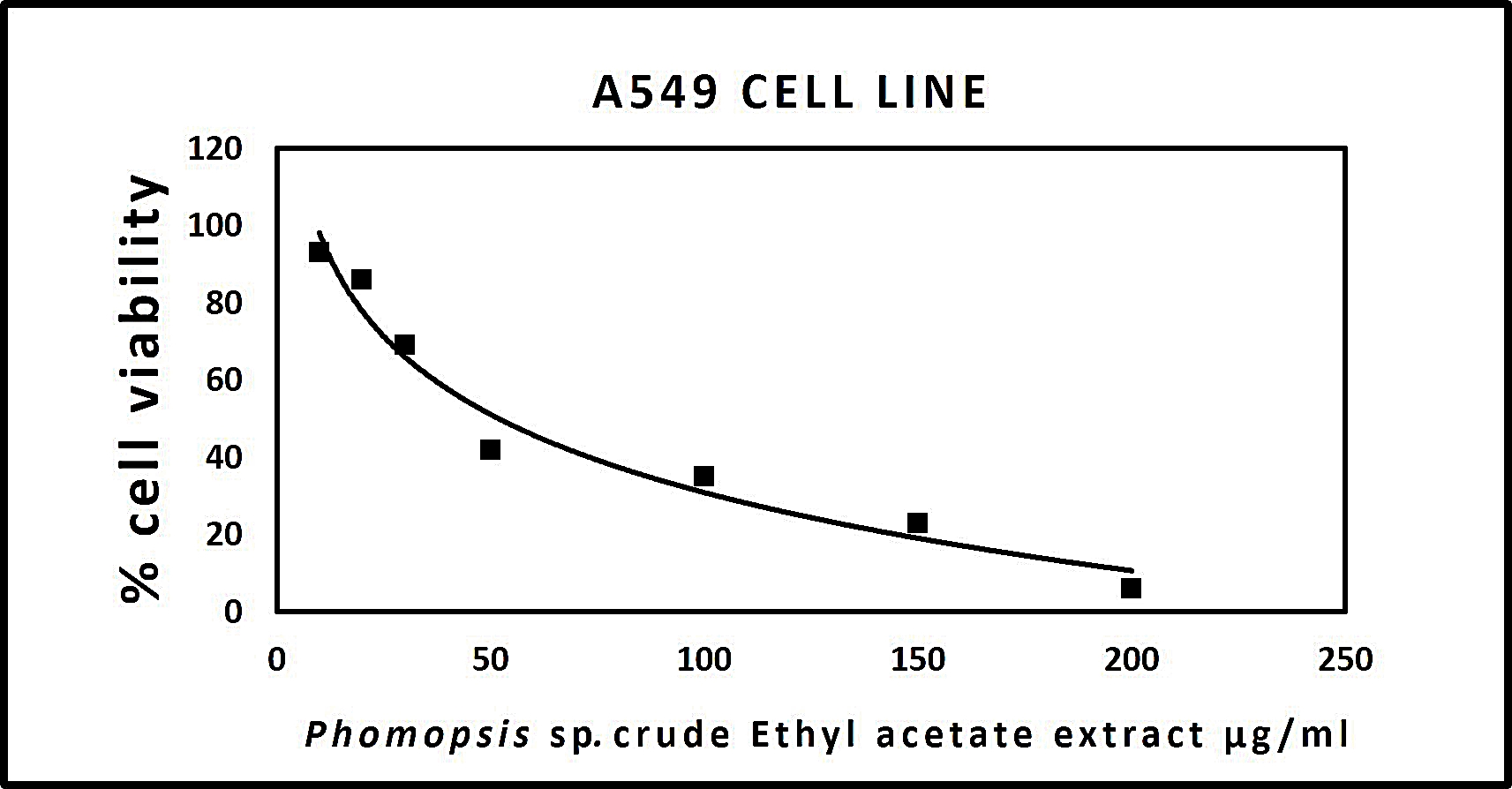
The results procured through MTT assay for HEp2 cell line were compared to the result of A549 cell line shown in (Table 6) and also the microscopic observation of two cancer cells (for Phomopsis sp.,) was shown in (Fig. 5 & 6). The cancer cell lines have shown a dose-dependent decrease in the percentage viability.
The half maximal inhibitory concentration(IC50) value was observed for Phomopsis sp. shown in (Fig. 7 & 8).
Fig. 5. (A) HEp2 cell control, (B) HEp2 cells after 24 hours, (C) HEp2 cells after 48 hours, (D) HEp2 cells after 72 hours.
Fig. 6. (A) A459 cell control, (B) A459 cells after 24 hours, (C) A459 cells after 48 hours, (D) A459 cells after 72 hours.
Table (6):
50 % Maximal inhibition of inhibitory concentration (IC50) of crude Ethyl acetate extract endophytic fungi
| Isolated Endophytic Marine Fungi | IC50 Values in HEp2 Cell Line and A549 Cell Line | |
|---|---|---|
| HEp2 Cell Line | A549 Cell Line | |
| 1. Chaetomium sp. | 72 μg/ml | 106 μg/ml |
| 2. Penicillium sp. | 89 μg/ml | 114 μg/ml |
| 3. Aspergillus sp. | 112 μg/ml | 134 μg/ml |
| 4. Aspergillus terrus | 136 μg/ml | 165 μg/ml |
| 5. Phomopsis sp. | 37 μg/ml | 52 μg/ml |
| 6. Rizopus sp. | 161 μg/ml | 185 μg/ml |
| 7. Acremonium sp. | 139 μg/ml | 167 μg/ml |
| 8. Aspergillus niger | 151 μg/ml | 166 μg/ml |
| 9. Xylaria sp. | 158 μg/ml | 175 μg/ml |
| 10. Cladosporium sp. | 125 μg/ml | 134 μg/ml |
| 11. Fusarium sp. | 95 μg/ml | 134 μg/ml |
| 12. Ascmycetes sp. | 164 μg/ml | 185 μg/ml |
Fig. 7. Graph representing anticancer activity of crude endophytic fungal Ethyl Acetate Extract in HEp2 Cell Line.
The extracts from Chaetomium sp., Phomopsis sp., Acremonium sp., Aspergillus niger, Xylaria sp., Cladosporium sp., and Fusarium sp., showed greater antioxidant potential compared to other. GC-MS chromatogram analysis of the ethyl acetate crude fungal extract showed multiple peaks which indicate the presence of many bioactive compounds. Cell cytotoxicity assay showed different fungal crude extracts exhibited inhibitory concentration 50 (IC 50) at various concentrations in Vero cell line. Anticancer activity assay results exhibited that all the crude fungal extracts except Phomopsis sp., were toxic to both the cancer and normal cells. Further purification of Phomopsis sp., crude fungal extracts will exhibit an efficacious result against cancer cells.
Acknowledgements
We thank the staff of Department of Virology, King Institute of Preventive Medicine and Research for their assistance in cell cytotoxicity and anticancer activity experiments.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding
None.
Data Availability
All datasets generated or analysed during this study are included in the manuscript.
Authors’ Contribution
All authors have made substantial, direct and intellectual contribution to the work and approved it for publication.
Ethics Statement
This research article does not contain any studies involved with human participants or animals.
- Agata Staniek, Herman J. Woerdenbag and Oliver Kayser. Endohytes: exploiting biodiversity for the improvement of natural product-based drug discovery. Journal of Plant Interactions, 2008; 3(2): 75-93.
Crossref - Sushanto Gouda1, Gitishree Das, Sandeep K. Sen, Han-Seung Shin and Jayanta Kumar Patra. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Frontiers in Microbiology, 2016; 7: 1538.
Crossref - Ravindra N. Kharwar, Ashish Mishra, Surendra K. Gond, Andrea Stierle and Donald Stierle, Anticancer compounds derived from fungal endophytes: their importance and future challenges. Natural Product Reports, 2011; 28: 1208–1228.
Crossref - Dian Handayani, Nita Ananda1, Muh. Ade Artasasta1, Rustini Ruslan, Okmes Fadriyanti , Trina Ekawati Tallei.Antimicrobial activity screening of endophytic fungi extracts isolated from brown algae Padina sp. Journal of Applied Pharmaceutical Science, 2019; 9(03): 009-013.
Crossref - Peng Zhang, Xin Li, Bin-Gui Wang. Secondary Metabolites from the Marine Algal-Derived Endophytic Fungi: Chemical Diversity and Biological Activity. Planta. Med., 2016; 82: 832–842.
Crossref - Tina Netzker, Juliane Fischer, Jakob Weber, Derek J. Mattern, Claudia C. Kצnig, Vito Valiante, Volker Schroeckh and Axel A. Brakhage. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol., 2015; 6: Article 299.
Crossref - Shruti Shukla. Secondary metabolites from marine microorganisms and therapeutic efficacy: A mini review. Indian Journal of Geo-Marine Sciences, 2016; 45(10): 1245-1254.
- Ravindra N. Kharwar, Ashish Mishra, Surendra K. Gond, Andrea Stierle and Donald Stierle. Anticancer compounds derived from fungal endophytes: their importance and future challenges. Natural Product Reports 2011; 28: 1208–1228.
- Nelson G.M. Gomes, Florence Lefranc, Anake Kijjoa and Robert Kiss. Can Some Marine-Derived Fungal Metabolites Become Actual Anticancer Agents?. Mar. Drugs, 2015; 13: 3950-3991.
Crossref - Julia Kjer, AbdessamadDebbab, Amal H Aly & Peter Proksch. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nature protocols, 2010; 5(3): 479.
Crossref - E. F. Guba. Monograph of Monochaetia and Pestalotia, pp 342. Harvard University Press. Cambridge, 1961.
- Ellis, M.B. Dematiaceous hyphomycetes., pp 608. Commonwealth Mycological Institute, London, 1971.
- Sutton, B.C. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata, pp 1-696. Commonwealth Mycological Institute, Kew, Surrey, England, 1980.
- A.H.S. Onions, D. Allsopp and H.O.W. Eggins. Smith’s Introduction to Industrial Mycology, pp 398.7th Ed. Edward Arnold, London, 1981.
- T.R. Nag Raj. Coelomycetous anamorphs with appendage-bearing conidia. Pp 1-1101. Mycologue publications, Waterloo, 1993.
- Bills, G.F., & Polishook, J.D. Abundance and Diversity of Microfungi in Leaf Litter of a Lowland Rain Forest in Costa Rica. Mycologia, 1994; 86(2): 187.
Crossref - Kjer, J., Debbab, A., Aly, A.H., & Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nature Protocols, 2010; 5(3): 479–490.
Crossref - Om P. Sharma, Tej K. Bhat. DPPH antioxidant assay revisited. Food Chemistry, 2009; 113: 1202–1205.
Crossref - Radhakrishnan K, James F, Mohan A, Chandra Mohan S. Gas chromatography and mass spectrometry analysis of Canthium parviflorum leaves. Innovare Journal of Science, 2016; 4(6): 22-27.
- Juan C. Stockerta, Richard W. Horobinc, Lucas L. Colomboa, Alfonso Blבzquez-Castro. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labelling perspectives. 2018; 120(3): 159-167.
Crossref - Mosmann, T., Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunology Methods, 1983; 65: 55–63. 55-63.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.