ISSN: 0973-7510
E-ISSN: 2581-690X
The aim of the present study was to isolate, cultivate and characterize Magnetotactic Bacteria detected in Microcosms collected from north India, and to compare the Genomic DNA of isolated DNA with the ‘Magnetospirillium gryphiswaldense MSR-1’ for the analysis of the size of genomic DNA. The microcosms collected from North India were subjected to study the physiochemical properties such as dissolved oxygen and pH. The isolation of Magnetotactic Bacteria was done by applying Capillary Racktrack method and to grow in double gradient medium. The genomic DNA was isolated and analyzed using pulse field gel electrophoresis. The outcomes of the study show the co-relation between physiochemical properties and the growth rate of the Bacterial strain was 34 times more than the dry weight of iron concentration. The genomic DNA analysis using pulse field gel electrophoresis shows that estimated the size of genomic DNA, consistent with ‘Magnetospirillium gryphiswaldense MSR-1’ The isolated strain significantly shows similarity to the a-subgroup of Proteobacteria. The bacteria were highly applicable in various fields of Science and Technology due to there antitoxic property.
Biodiversity, Iron homeostasis, Microbial Genomics, Magnetotactic Bacteria, PFGE.
The Magnetotactic Bacteria (MTB) are unique among bacterial strains due to the presence of magnetosomes, which is an intracellular structure comprising a magnetic mineral crystal surrounded by a lipid bilayer membrane of 3-4mm thickness1. MTB are motile aquatic prokaryotes which swim along geomagnetic field lines2. MTB does not have proper taxonomic significance and reported as fastidious prokaryotes belongs to heterogeneous group with numerous cellular morphologies together with coccoid, rod-shaped, vibrio, helical that have magnetotactic behaviour3. All MTB’s are Gram-negative; motile, flagellated, exhibit negative tactic behaviour and their growth depend on atmospheric oxygen concentration1. They are present in huge amount just below the oxic-anoxic interface of water body and have the potential to participate in biogeochemical cycling of important elements including iron, sulfur, nitrate3-4.
MTB is unique among bacteria due to the presence of nanosized membrane bounded crystals of magnetic iron minerals present inside the cytoplasmic membrane called magnetosomes, an interesting example of a highly controlled biomineralization process5. Usually magnetotactic bacteria mineralize either iron sulphide magnetosomes which contain greigite (Fe3S4) or iron oxide magnetosomes containing crystals of magnetite (Fe3O4)2,6-8. Also, few more minerals with iron sulphide have been identified in magnetosomes such as mackinawite (tetragonal FeS) and cubic FeS2,9-10. The magnetosome mineral composition seems to be under strict chemical control because even in the presence of hydrogen sulphide in growth medium, the cell of several MTB synthesizes Fe3O4 continuously11.
Consequently, magnetosomes have been suggested in numerous biotechnological and biomedical applications such as carriers in magnetic drug targeting, to increase the efficiency of tumor treatment by hyperthermia, in MRI applications12-14. Moreover, numerous applications for molecular biology of magnetosome have been reported such as procedures for the extraction of DNA and mRNA from different biological samples such as bacterial cells, blood and tissues15-17. A number of magnetosome-based immunoassays were developed to detect antigens, environmental pollutants, hormones and toxic substances18-20.
In view of the numerous application of MTB, the present study was conducted to give a comparative data such as high iron uptake capability, temperature range, and oxyanion reduction as well as compared the Genomic DNA with the ‘Magnetospirillium gryphiswaldense MSR-1 for identifying the new MTB strain from the Northern Indian.
Procedure for Sample Collection and Microcosms Setup
Twenty-eight samples of water and sediment were collected from various aquatic habitats in Uttar Pradesh (Table 1)21. The magnetic property was determined by placing the magnetic poles of a laboratory stirring bar magnet perpendicular to the outside glass wall of the collection bottles (several centimeters above the sediment surface)5. The water specimen was collected from the container wall which was near to the bar magnet after 5 to 6.5 hours and observed under microscope by applying the hanging drop method. Coordination of geographic condition and name of sample sites are represented in Table 1. Dissolved oxygen (DO) concentration was measured using a Hach HQ10 portable dissolved oxygen meter and Hach sensION1+PH1 portable pH meter and the total iron content of the cell mass was determined by atomic absorption spectroscopy using CH 201 AAS5,21.
Purification method of Magnetotactic Bacteria
The Modified “Capillary Racktrack” (CRT) method was used to purify and enrich the MTB obtained by magnetic collection. To conform the magnetic property of bacterial isolates, the isolates when spread on the surface of semi-solid medium of agar (0.8%) and incubate under magnetic field created by placing bar magnet near the petridish5, 22- 23.
Media Preparation for Isolation
For the preparation of isolation media, the protocol given by Wolin et al. was used24. Cultivation was carried out under aerobic conditions- (O2):(S2-) inverse double gradient media, and anaerobic conditions. The oxygen-sulfide gradient media were composed of a plug-agar which was overlaid with slush-agar. Slush-agar was consisted of the various semi-solid isolation media; finally the screw-capped culture tubes of glass were left loose for 24-48 hours before inoculation to establish sulfide and oxygen gradients.
The cells growth was measured spectroscopically at 565 nm wavelength using iCAP 7200 ICP-OES (Thermo Fisher Scentific). The cell numbers were counted by Neubauer cell-counting chamber. For the exponential phase, the generation time “g” (h) and the division rate, “v” (h-1) were calculated, using the equation given by Tajer et al.2014. For the analysis of iron, 1 ml of each samples of culture was harvested by centrifugation (10000 rpm for 15 minutes at 4°C). The supernatant was decanted, acidified with 10 µl of nitric acid to pH = 2.0-3.0 and analyzed spectroscopically. The triplicate experiments were performed and as a control, cell free culture medium was used5,21,25-26 .
Genomic DNA Isolation and PFGE
DNA was extracted from 2×109 bacterial cell and subjected to restriction digestion according to method proposed by Dean and Bazylinski,1999. The prepared genome DNA isolated from strains were loaded into the well of 1.0% Pulsed-Field Certified Agarose (Bio-Rad) gel and sealed with warm agarose. Gels were run at 14°C, 6 V/cm, with a 120° reorientation angle with TBE buffer and both the buffer and the gel were cooled to 14°C prior to the start of each run. Switch times and gel run times varied according to the size of fragments being resolved. The gel were stained with 0.1µl-1 ethidium bromide for 10 min and de-stained in distilled water for 30 min. Gels were photographed with a Gel Doc 1000 System (Bio-Rad). The calculation for the size of fragments generated from restriction digestions were done with standard curved created by plotting the logarithm of the fragment molecular weight in kilobases versus distance traveled in mm from the wells. Lambda DNA concatemers (Sigma-Aldrich) were used as size markers. Molecular weights of all fragments produced by digestions of genomic DNA of the isolated strain and “Magnetospirillum gryphiswaldense MSR-1” with PacI, PmeI, and SpeI, were calculated by pulsed-field gels and the total molecular weight of the genomes of each organism was compared after adding the sizes of the fragments produced by each restriction endonuclease27-28.
Identification, purification and characterisation of Magnetotactic Bacteria
To date, the difficulties are faced while isolation and cultivation of MTB in pure culture because the lifestyle of MTB are exceptional as they are fastidious anaerobes. In this study, twenty eight Microcosms were sampled and scanned. The Microcosms were getting saturated into aerobic and anaerobic zones after incubation in a laboratory under the mentioned circumstance for quite a few months. The population of MTB remarkably increases in some microcosms without any chemical enrichment whereas in the rest of the microcosms the MTB was not observed even after enriching with chemicals. The data obtained from the experiment show the presence of MTB in a few samples of the microcosms. MTB was not obtained from the sample collected from several locations of northern India, namely Barua Sagar Tal (BST1-BST5), Moti Jheel (MTJ1-MTJ4), Yamuna River (YAM1 and YAM2), Sandhi Bird Sanctuary (SBS1-SBS4), Ganga River (GAN1 and GAN2)., and the amount of MTB was negligible in Shekhal Jheel (SKL5-SKL7). The most appreciable amount of MTB was obtained from the sample collected from the Keetham Lake (KEEM1-KEEM4). The MTB strains isolated from KEEM4 was a gram-negative bacterium and express the motility due to the presence of a single polar flagellum. The properties of MTB are summarized in Table 1 depicting that only few MTB can tolerate the extreme environment such as high alkaline and saline environment. The data obtained in the study suggest that the environment with low to moderate iron content are most appropriate for MTB, which has a correlation between the blooms of MTB. Through analyzing the data obtained on providing the same environment and incubated under similar condition, put forward that the blooms of these bacteria prejudiced by parameter precisely.
Table (1):
Name, location, physiological characteristics and total iron concentration of the microcosms
S.No |
Sampling Site |
Name of Microcosms |
Geographical coordination |
Dissolve Oxygen Concentr-ation (DO)mg/l |
pH of Microcosms before incubation (ms) |
Total iron concentration of water collected from the microcosms [Fe(mg/l)] |
|---|---|---|---|---|---|---|
1 |
BARUA SAGAR TAL (Barua Sagar, Near Jhasi,UP) |
BST2 BST3 BST4 BST5 |
[25.368°N 78.748°E] |
ND ND 0.58 0.69 1.49 |
6.01 6.05 6 6 6 |
0.001 0.004 0.002 0.003 0.009 |
2 |
SHEKHAL JHEEL (Aligarh ,UP) |
SKL1 SKL2 SKL3 SKL4 SKL5 SKL6 SKL7 |
[27.8575°N 78.2185°E] |
ND 7.03 6.38 6.39 6.4 ND ND |
6.33 6.01 6.44 6.44 8.44 8.83 8.44 |
0.0263 0.0512 0.0243 0.0398 0.1343 0.293 0.310 |
3 |
MOTI JHEEL (Kanpur, UP) |
MTJ1 MTJ2 MTJ3 MTJ4 |
[26.28°N 80.18°E] |
1.51 8.01 8.04 8.01 |
7.14 6.69 6.51 6.32 |
0.0316 0.0331 0.001 0.00 |
4 |
YAMUNA RIVER (Tajgang, Agra,UP) |
YAM1 YAM2 |
[27.9°N 78.3°E] |
7.98 7.94 |
7.4 7.3 |
0.0263 0.0328 |
5 |
KEETHAM LAKE (Agra, UP) |
KEEM1 KEEM2 KEEM3 KEEM4 |
[27.253295°N77.843875°E] |
2.92 2.81 0.59 1.39 |
8.01 8.28 8.18 8.18 |
0.4321 0.2906 0.3440 0.1429 |
6 |
SANDI BIRD SANCTUARY (Hardoi , UP) |
SBS1 SBS2 SBS3 SBS4 |
[27.1805°N79.5813°E] |
3.47 ND ND 7.4 |
6.3 6.34 6.0 6.25 |
0.0361 0.0313 0.0260 0.0211 |
7 |
GANGA RIVER (Narora ghat, UP) |
GAN1 GAN2 |
[28.1148° N 78.2253°E] |
8.97 8.97 |
7.03 6.96 |
0.0823 0.0631 |
Cultivation of Isolated Strain
The bacterial strain was then isolated and culture using the previously described isolation medium. The strain formed sharp band in glass screw capped tube of culture medium and was microaerophilic zone of the medium. The Oxygen consumption by bacterial cell and diffusion of O2 through the lid of tube facilitate the movement of the bacterial band of the strain along the culture tube until optimal niche was achieved. The data of this study confirm that the semi-solid O2 gradient media was best in isolation. Since the Polystyrene plastic tubes are not airtight and the oxygen could diffuse through, hence the use of polystyrene plastic tube are not suitable for establishing the oxygen gradient in the semi-solid medium. Addition of water in a little amount from the respective aquatic habitat to the isolated media were important because these bacteria live and adopt by a complex pattern of chemical gradient in their aquatic habitats, which are difficult to mimic under laboratory condition5,29. A bacterium was identified from the sample KEEM4 responds under magnetic field towards the South Pole of the Bar Magnet (Fig. 1).

Fig. 1. The motility of bacterial colony towards South Pole
The bar magnet as indicated by arrow (→) showed the movement of bacterium towards south pole.
Growth rate and generation time
The growth and iron uptake of MTB isolated from KEEM4 are shown in Fig. 2 and Fig. 3. According to Fig. 2 and Fig. 3, the strain expresses the co-relation between the amount of iron uptake and the rate of growth by KEEM4. The iron uptake enhances the biomass at exponential growth phase, the concentration of dissolved iron got diminish callously while it has a minor diminish at stationary phase and the iron uptake persistently dawdling thereafter (Generation Time, Division rate and iron uptake of MTB isolated from Keetham lake are shown in Table 2). After using different sources of Iron, it was observed that the ferric citrate is better since it could be easily prepared and do not have a precipitation problem, these observation are also supported by previous studies5. The data from this study revealed the co-relation between iron uptake and growth, since high amounts of iron is absorbed at the initial iron concentration. The absorption of dissolved Oxygen by bacterial cell was about 34 times much higher than the dry weight, which is only 2.94% in total. The entire the quality and performance of isolated KEEM 4 shows the resemblance with Magnetospirillum gryphiswaldense. Moreover, the findings from the present study exposed that under microaerobic condition the isolated MTB is able to tolerate and reduce selenate oxyanion and the reduction of selenate is a valuable capability for this strain, which has not been reported in other MTB. Studies from ‘Tajer et al.’ showed the valuable interpretation of selenate in MTB isolated from Iran5. He concluded that the selenate reduction through MTB is an important biochemical process in an aquatic environment and oxyanion forms of selenium are toxic to living organism and bacteria; however, some microorganisms can apply their metabolic capacity in different ways to transform the oxyanions to other non-toxic chemical5. Nowadays, various methods have been used to remove toxic selenium from water waste of industries 30-31.
Table (2):
Generation Time, Division rate and iron uptake of MTB isolated from Keetham Lake
| Generation Time, Division rate and iron uptake of MTB isolated from Keetham lake | ||
|---|---|---|
| Generation Time (h) | 67hrs | |
| Division Rate (h-1) | 0.014 | |
| Iron concentration in Medium | Before growth | 7.12±0.07 |
| After growth | 5.01±0.01 | |
| Dry weight of iron | 3.56 | |
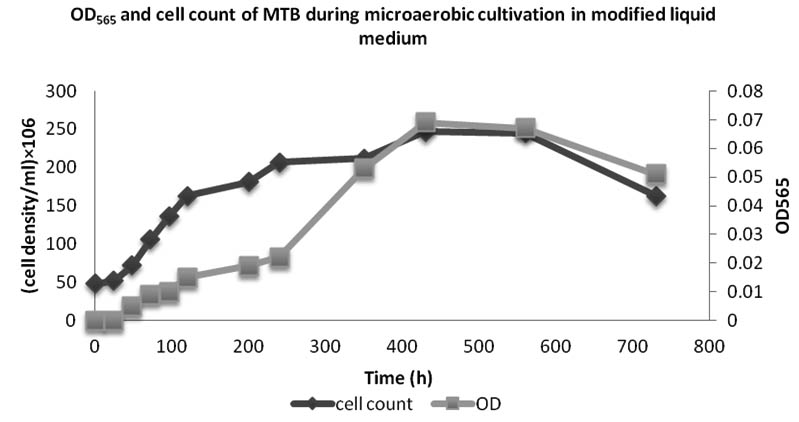
Fig. 2. OD565 and cell count of bacteria isolated from Keetham Lake during microaerobic cultivation in modified liquid medium
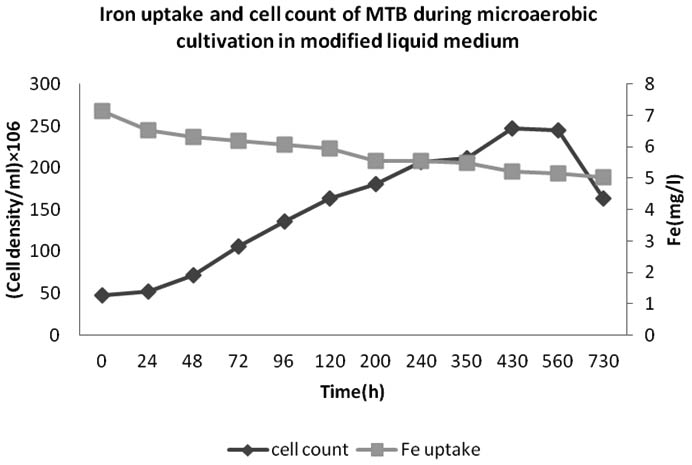
Fig. 3. Iron uptakeand cell count of bacteria isolated from Keetham Lake during microaerobic cultivation in modified liquid medium
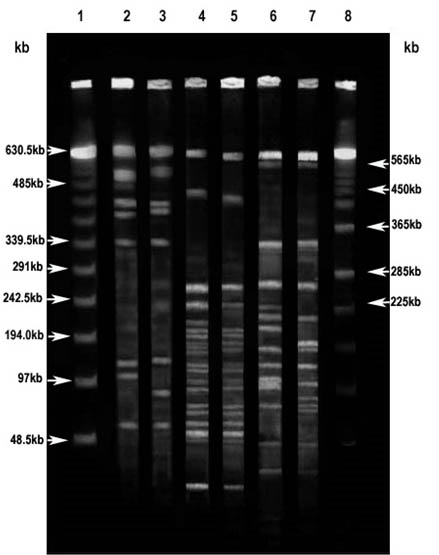
Fig. 4. PFGE of genomic DNA from magnetotactic bacterial strains Magnetospirillum gryphiswaldense MSR-1 ( MSR-1) and MTB isolated from Keetham lake (KEEM 4)
Previous reports accounted that, the MTB has the ability to uptake vast amount of iron from the environment (up to 4.32% of the dry weight), which was coupled with their formation of magnetosome; while, the total iron content in Escherichia coli has been 0.0049- 0.0213% of the dry weight5. These observations support the theory that MTB is causative to the biogeochemical cycling of Iron. It has been reported that they have precious role in contributing the iron flux to sediments of water bodies, however the involvement in iron cycling revolving in environment, still has to be addressed in huge amount5, 31-32.
Comparative genomic DNA analysis using PFGE
The pattern of restriction fragment obtained through PFGE run of MSR-1 and KEEM-4 genomic DNA are shown in Fig. 4. On digesting the genomic DNA of both the strains with PacI, among the eight fragments, five were just about identical sized fragments obtained from the genomic DNA of strain KEEM-4. Approximately 382, 130 and 102 kb of distinctive fragments were observed for strain MSR-1, and just about 399, 131, and 83 kb fragments were observed for strain KEEM-4. Out of twenty-two fragments, sixteen corresponding fragments were obtained from KEEM-4 on digesting the genomic DNA of both the strains with PmeI. Inimitable fragments were noticed at around 618, 450, 195, 177, 154, and 129 kb for MSR-1 and 588, 426, 193, 150, and 136 kb for MSR-1.
On digesting the genomic DNA of both the strains with SpeI among the sixteen fragment significantly eleven were just about identical sized fragments were obtained from the genomic DNA of strain KEEM-4. Distinctive fragments for MSR-1 were found at 223, 198, 135, 94, and 92 kb, and for KEEM-4 at the region of 147, 138, 96, and 90 kb. The fragment ensuring from genomic DNA of MSR-1 and KEEM-4 on with PacI, PmeI, and SpeI put forward to estimate the genomic size of the strains on comparative bases. Respectively, the data estimates the size of genomic DNA was an average of 3.5 ± 0.2 Mb for MSR-1 and 3.4± 0.2 Mb for KEEM-4 (Fig. 4).
The use of Pulse Field Gel Electrophoresis has momentous and precious outcomes for organization and the size of the genomic DNA. The observation shows that the linear DNA is missing in both genomic DNA of isolates and Magnetospirillium gryphiswaldense MSR-1. It is shows that very large circular DNA molecules can become indiscriminately linearized at some stage for preparation of the genomic DNA for the electrophoresis. The information throws the presence of multiple chromosomes or large replicons in certain organisms27, 33-34. To linearized the chromosome under alkaline condition, lysis environment did not progress the appearance of the bands, since these bands migrated at approximately 3.4 Mb for strains MSR-1 and KEEM-4, similar to the genome sizes estimated from the addition of restriction fragments for these strains and consisted with the previous observation of Dean and Bazylinski that the strains contain a single circular chromosome27. This outcome is remarkable because current revision illustrate that a enormous deal of diversity concerning genome size and organization belong to the class Proteobacteria, in the a-subgroup that includes practically all the magnetite-producing magnetotactic bacteria, as well as strains MSR-1 and KEEM-427, 34-35. Members of the ±-subgroup of the Proteobacteria not only show great differences in genome size, but many possess mega-base sized replicons, linear chromosomes, and multiple chromosomes34-35.
Taken together, the study focused towards the cultivation and growth of the bacteria belongs to the ±-subgroup of Proteobacteria and the co-relation between physiochemical properties. The isolated strain plays an important role in the iron biogeochemical cycle. The unseen assistance of the study opens the drastic scope in biomedical science and waste water treatment as the strains identified through this study have the property to reduce the toxic selenate. The comparative analyses of genomic DNA between known and unknown Magnetotactic bacteria through PFGE estimate the co-relation accuracy.
In conclusion the isolated magnetotactic bacterial strain may be useful in number of application as other known magnetotactic bacteria do; however further study is desirable to establish the phylogeny and to investigate more application magnetotactic bacteria.
ACKNOWLEDGMENTS
The study was carried out at IFTM University, Moradabad, UP, INDIA. The research paper is a piece of P.hD work of one of the author Renu Singh.
- Balkwill D, Maratea D, and Blakemore RP. Ultrastructure of a magnetotactic spirillum. Journal of Bacteriology, 1980; 141: 1399-1408.
- Bazylinski DA, Frankel RB. Magnetosome formation in Prokaryotes. Nature Reviews, Microbiology, 2004; 2: 217-229.
- Bazylinski DA, Frankel RB, Heywood BR, Mann S, King JW, Donaghay PL. Controlled biomineralization of magnetite (Fe3O4) and greigite (Fe3S4) in a magnetotactic bacterium. Applied and Environmental Microbiology, 1995; 61: 3232–3239.
- Bazylinski DA, Dean AJ, Schüler D, Phillips EJ, Lovley DR. N2-dependent growth and nitrogenase activity in the metalmetabolizing bacteria, Geobacter and Magnetospirillumspecies. Environmental Microbiology, 2000; 2: 266–273.
- Tajer Mohammad Ghazvini P, Kermamshahi RK, Ahmad NG, and Sadeghizadeh, M. Isolation. characterization of a NovalMagnetotactic Bacterium from Iron Uptake and Producing Magnetic Nanoparticles in Alphaproteobacterium MT-KTN90. Microbiology, 2014; 7: e19343.
- Farina M, Esquivel DMS, Lins de BH (1990) Magnetic iron-sugar crystals from a magnetotactic microorganism. Nature 343: 256-258.
- Heywood BR, Bazylinski DA, Garratt-Reed A, Mann S, Frankel RB. Controlled biosynthesis of greigite (Fe3S4) in magnetotactic bacteria. Naturwissenschaften, 1990; 77: 536–538.
- Frankel RB, Blakemore RP, Wolfe RS. Magnetite in freshwater magnetotactic bacteria. Science , 1979; 203: 1355–1356.
- Meldrum FC, Heywood BR, Mann S, Frankel RB, Bazylinski DA. Electron microscopy study of magnetosomes in a cultured coccoid magnetotactic bacterium. Proceedings of the Royal Society of London. Series B, Biological sciences Royal Society, 1993; 251: 231–236.
- Towe KM, and Moench TT. Electron-optical characteri- zation of bacterial magnetite. Earth and Planetary Science Letters, 1981; 5: 213-220.
- Po‘sfai M, Buseck PR, Bazylinski DA, Frankel RB. Reaction sequence of iron sulphide minerals in bacteria and their use as biomarkers. American Mineralogist, 1998; 83: 1469-1481
- Guo L, Huang J, Zhang X, Lic Y, Zheng L. Bacterial magnetic nanoparticlesas drug carriers. Journal of Materials Chemistry, 2008; 18: 5993–5997.
- Sun C, Lee JSH, Zhang MQ (2008) Magnetic nanoparticles in MR imaging anddrug delivery. Advanced Drug Delivery Reviews 60: 1252-1265
- Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles forhyperthermia. International Journal of Hyperthermia, 2008; 24: 467-474.
- Sode K, Kudo S, Sakaguchi H, Nakamura N, Matsunaga T. Application of bacterial magnetic particles for highly selective mRNA recovery system. Biotechnology Techniques, 1993; 7: 688-694
- Yoza B, Arakaki A, Matsunaga T. DNA extraction using bacterial magneticparticles modified with hyperbranched polyamidoamine dendrimer. Biotechnology, 2003a; 101: 219-228
- Yoza B, Arakaki A, Maruyama K, Takeyama H, Matsunaga T. Fullyautomated DNA extraction from blood using magnetic particles modified with a hyperbranched polyamidoamine dendrimer. Journal of Bioscience and Bioengineering, 2003b; 95: 21-26.
- Tanaka T, Takeda H, Ueki F, Obata K, Tajima H, Takeyama H, Goda Y, Fujimoto S, Matsunaga T. Rapid and sensitive detection of 17 beta-estradiolinenvironmental water using automated immunoassay system with bacterial magnetic particles. Biotechnology, 2004; 108: 153-159
- Yoshino T, Matsunaga T. Efficient and stable display of functional proteins on bacterial magnetic particles using mms13 as a novel anchor molecule. Applied and Environmental Microbiology, 2006; 72: 465-471.
- Yoshino T, Kato F, Takeyama H, Nakai K, Yakabe Y, Matsunaga T. Development of a novel method for screening of estrogenic compounds using nano-sized bacterial magnetic particles displaying estrogen receptor. Analytica Chimica Acta, 2005; 532: 105-111.
- Blakemore RP, Maratea D, Wolfe RS. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. Journal of Bacteriology, 1979; 140: 720–729.
- Schüler D, Frankel RB. Bacterial Magnetosome: Micrbiology, biomineralization and biotechnological application. Applied Microbiology and Biotechnology, 1999; 53: 464-473
- Wolfe RS, Thauer RK, Fennig NP. A capillary racetract method for isolation of magnetotacticbacteria. FEMS Microbiology Letters, 1987; 45: 31-36
- Wolin EA, Wolin MJ, Wolfe RS. Formation of methane by bacterial extracts. Journal of Biological Chemistry, 1963; 238: 2882–2886.
- Schüler D, Spring S, Bazylinski DA. Improved technique for the isolation of magnetotacticspirilla from a freshwater sediment and their phylogenetic characterization. Systematic and Applied Microbiology 22: 466-471 Lefèvre CT, Trubitsyn D, Abreu F, Kolinko S, de Almeida LG, de Vasconcelos AT, Lins U, Schüler D, Ginet N, Pignol D, Bazylinski DA (2013) Monophyletic origin of magnetotaxis and the first magnetosomes. Environ Microbiol, 1999; 15(8):2267-74.
- Dean AJ, Bazylinski DA. Genome Analysis of Several Marine, Magnetotactic Bacterial Strains by Pulsed-Field Gel Electrophoresis. Current Microbiology, 1999; 39: 219–225
- Birren B, Lai E. Pulsed field gel electrophoresis: a practical guide. New York: Academic Press Inc 1993.
- Spring S, Bazylinski DA. Magnetotactic Bacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E editors. The Prokaryotes. Springer, 2006; 3: 842–862.
- Soudi MR, Tajer MGP, Khajeh K, Gharavi S. Bioprocessing of seleno-oxyanions and tellurite in a novel Bacillus sp. strain STG-83: A solution to removal of toxic oxyanions in presence of nitrate. Journal ofHazardous Materials, 2009; 165: 71–77.
- Lefèvre CT, Abreu F, Schmidt ML, Lins U, Frankel RB, Hedlund BP, Bazylinski DA. Moderately thermophilic magnetotactic bacteria from hot springs in Nevada. Applied and Environmental Microbiology 2010; 76: 3740–3743.
- Schüler D, Baeuerlein E, Iron-limited growth and kinetics of iron uptake in Magnetospirillumgryphiswaldense. Archives ofMicrobiology, 1996; 166: 301-307.
- Devereux R, Willis SG, Hines ME, Genome sizes of Desulfovibrio desulfuricans, Desulfovibrio vulgaris and Desulfobulbus propionicus estimated by pulsed-field gel electrophoresis of linearized chromosomal DNA. Current Microbiology, 1997; 34: 337–339.
- Jimas-Bilak E, Michaux-Charachon S, Bourg G, Ramuz M, Allardet-Servent A, Unconventional genomic organization in the alpha subgroup of the Proteobacteria. Journal of Bacteriol, 1998; 180: 2749– 2755
- DeLong EF, Frankel RB, and Bazylinski DA, Multiple evolutionary origins of magnetotaxis in bacteria. Science, 1993; 259: 803–806.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


