ISSN: 0973-7510
E-ISSN: 2581-690X
This research aimed to study the compatibility of antagonistic bacteria that effective to control rice bacterial leaf blight disease, including Bacillus pumilus FDKF5 and Bacillus sp. IKM1 with agricultural chemicals and bio-products. Compatibility test of antagonistic bacteria with pesticides by using poison plate method showed that B. pumilus FDKF5 and Bacillus sp. IKM1 were able grow together with eight pesticides including 5 types of insecticide such as Conbalis (pyridaben 20% WP), Centaur (imidachloprid 70% WG), Posch (carbosulfan 20% W/V EC), Microthiol sulfur (sulfur 80% WG) and Abamectin (abamectin 1.8% W/V EC) and 3 types of fungicide including Terraclor Super-X (etridiazole 6% + quintozene 4% W/V EC), Fundasol 50 (benomyl 50% WP) and Octave (prochloraz 50% WP). However, streptomycin sulfate bactericide was inhibited their growth. Co-culture test of antagonistic bacteria with bio-products by using dual culture technique showed that Trichoderma and Beauveria bio-products could overgrowth on colonies of antagonistic bacteria but did not inhibit their growth. While, Metarhizium bio-products could inhibit the growth of both antagonistic bacteria. Dual culture of antagonistic bacteria with Bacillus bio-product showed that Bacillus sp. IKM1 could grow very well while B. pumilus FDKF5 could grow moderately.
Bacillus, bio-products, fungicides, insecticides.
The crucial main food crop is rice (Oryza sativa L.). The crop is widespread all over the world due to its wider adaptability under different environmental conditions. The primary limitations rice production is plant disease and insect pests.1,2 Xanthomonas oryzae pv. oryzae (Xoo) is bacterial leaf blight causal agent, its widely prevalent destroyed disease of rice.3 Hence, endophytic bacteria isolates were test for their efficiency against X. oryza pv. oryzae (Xoo). In vitro test, higher inhibition of Xoo was Bacillus subtilis var. amyloliquefaciens (FZB 24), EPB 9, EPB10, EPCO 29 and EPCO 78.4 While, testing activities of eleven chemicals including blasticidin, celdion, tricyclazole, streptomycin, sumithione, saturn, mipcine, stem F-34, hinosan, kasumin, phytomycin against Xanthomonas campestris pv. oryzae, a cause of paddy bacterial blight. In vitro studies, six chemicals such as phytomycin, streptomycin, blasticidin, kasumin, tricyclazole and sumithione inhibited bacterial growth. There are three chemicals viz blasticidin, kasumin and streptomycin which confident control disease in vivo.5 Moreover, investigation of the anti – activity of different broad spectrums antibiotics against X. oryza pv. oryzae (Xoo) isolates in vitro assays. The results show that five virulent Xoo isolates was checked again six antibiotics including benzyl-penicillin, ampicillin, kanamycin, streptomycin, chloramphenicol and sinobionic.6 The challenge of pest management is to use natural substances to be more effective in controlling pests. Most farmers use expensive insecticides, that making the problem of worse pests and it can cause damage to the environment and may be related to a crisis that cannot be solved for farmers that need to use insecticides.7 Combinations between different crop protection and pests monitor and natural enemies was defined to Integrated Pest Management (IPM).8 The objective of IPM is reduced chemical pesticides damage to health and ecosystem, its incorporate various strategy including biological, chemical, and cultural practice and resistance varieties, for pest population management to be at economic ratio.9 In plant protection, Bacillus thuringiensis, Trichoderma viridae, Metarhizium anisopliae, Beauveria bassiana, nuclear polyhedrosis virus (NPV) and neem are favorite biopesticides.10 Strategies for controlling plant disease may be achieved by using different biocontrol agents. Therefore, the study of the compatibility of chemicals and bio-products to be used in the IPM program such as studies compatibility of Metarhizium anisopliae with neem derivatives. The most compatible was 0.5% (w/v) neem soap with least growth inhibition of M. anisopliae.11 Streptomyces sp. SAI-25, M. anisopliae and neem seed powder are the biopesticides that effective to control Helicoverpa armigera. In pod borer complex management, compatibility between Streptomyces sp. SAI-25, M. anisopliae, and neem seed powder were tested. The results indicated that all of biopesticides were compatible with each other, therefore can mixed to manage H. armigera. Evaluation of compatibility between commercial product of neem oil emulsion (Azadirachta indica) and neem seeds and leaves extract with B. bassiana in vitro. Seed and leaf extracts were less injurious to B. bassiana than oil emulsion. The oil is incompatible with B. bassiana. At high concentrations oil can inhibiting conidia germination, growth and decrease conidia production and viability. In all concentrations of neem seed and leaf extracts were compatible with the entomopathogenic fungi. Reduction of conidia germination and production was affected of seed extract but did not affect to spore viability.12
Moreover, institute to evaluation of compatibility between B. bassiana, fungicides and botanicals. The compatibility of Beauveria isolate BP1.1 was evaluated with different fungicides that are carbendazim (50 WP) and copper oxychloride (50 WP) and botanicals for example aqueous neem leaf extract (1% w/v), aqueous garlic extract (1% w/v) and neem seed kernel extract (5% w/v) at three different concentrations. The isolate of Beauveria BP1.1 showed maximum growth in copper oxychloride (80.50 mm) followed by neem leaf extract (74.75 mm) and neem seed kernel extract (70.75 mm), whereas, the least growth was observed in garlic extract (60.50 mm) and total inhibition was observed in carbendazim.13 While, poisoned plate method was use for compatibility studies of eight fungicides including propineb, carbendazim, tebuconazole, nativo, fosetyl aluminium, tricyclazole, metalaxyl, kresoxim methyl and Bacillus amyloliquefaciens B15. The results show that, all concentration tests of kresoxim methyl and carbendazim compatible with B. amyloliquefaciens B15.14
Thus, evaluation compatibility of Bacillus spp. and Trichoderma spp. and antagonistic activity to S. cepivorum. Dual cultures method show 42-50% and 100% reduced mycelial growth of S. cepivorum by eight isolates of Bacillus spp. and five isolates of Trichoderma spp. Collaboration of Bacillus spp. and Trichoderma spp. can reduce S. cepivorum mycelium growth. Therefore, it can be used together to inhibit S. cepivorum.15
Hence, the present study aimed to assess the interaction of chemical pesticides, bio-products and antagonistic bacteria. Compatibility tests can be used as a guideline to enhance future integrated pest management.
Antagonistic bacteria
Two isolates of antagonistic bacteria including Bacillus pumilus FDKF5 and Bacillus sp. IKM1 obtained from Microbiology Laboratory, Department of Biology Faculty of Science, Mahasarakham University.
Compatibility test of antagonistic bacteria and pesticides
Study on compatibility between antagonistic bacteria and pesticides such as insecticides, fungicides and bactericide were tested by poison plate technique. Antagonistic bacteria including B. pumilus FDKF5 and Bacillus sp. IKM1 were culture on nutrient broth (NB) shaking at 150 rpm for 24 – 48 hr. After that bacterial suspension were prepared adjust concentration to 1×106 cfu/ml. Bacterial suspension was swab on NA petri plates. Whatman no.1 paper disc with 0.5 cm diameter was sterilized and then drop 30 ul of each pesticides with the concentrations according to company recommend (Table 1). Paper disc with pesticides were place on NA petri plates with antagonistic bacteria 4 pieces per plate in the cross direction. Incubated at 28°C and observe the clear zone for 5 days. Three replicates were maintained along with the control plate with dH2O instead of pesticides.
Table (1):
Pesticides used to study compatibility with B.pumilus FDKF5 and Bacillus sp. IKM1
Chemicals |
Trade name/ Company |
Active ingredient |
Concentration |
|---|---|---|---|
Insecticides |
|||
abamectin |
Abamectin Asia Agrotech Co., Ltd.,Thailand |
1.8% W/V EC |
20 ml/20l |
carbosulfan |
Posch Pitsulin Co., Ltd.Thailand |
20% W/V EC |
50 ml/20l |
imidachloprid |
Centaur |
70% WG |
10 ml/20 l |
pyridaben |
Conbalis Contact Group Co., Ltd., Thailand |
20% WP |
15 g/20 L |
sulfur |
Microthiol sulfur Sotus International Co., Ltd.,Thailand |
80% WG |
60 g/20 l |
Fungicides |
|||
etridiazole + quintozene |
Terraclor Super-X Sotus International Co., Ltd.,Thailand |
6% + 4% W/V EC |
40 ml/20l |
benomyl |
Fundasol 50 Angro Thai Chemical Supplies Ltd., Thailand |
50% WP |
30 g/20 l |
prochloraz |
Octave FMC Chemical (Thailand) Ltd |
50% WP |
20 g/20 l |
Bactericide |
|||
streptomycin sulfate |
Streptomycin sulfate Face Agro Co., Ltd., Thailand |
10 g/20 l |
Compatibility test of antagonistic bacteria and fungal bio-products
Trichoderma sp. was isolates from Trichoderma bio-product retrieve from Biological Control Laboratory, Department of Plant Pathology, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University. Compatibility between Trichoderma bio-product and B. pumilus FDKF5 or Bacillus sp. IKM1 using dual culture technique. First, place 0.5 cm diameter mycelium disc of Trichoderma sp. on PDA surface, 2 cm distance from the edge plate. After that B. pumilus FDKF5 or Bacillus sp. IKM1 was streak with an inoculation loop paired with 4.5 cm distance from Trichoderma sp. Observation the growth of both microorganisms for 5 days and then bacteria colony were re-streak on NA petri dish for test viability.
Beauveria bassiana and Metarhizium anisopliae were isolated from Beauveria bio-product and Metarhizium bio-product obtained from TAB Innovation Co., Ltd. Thailand. Dual culture technique was used by streak B. bassiana or M. anisopliae in a half of petri dish and incubated at 28°C for 3 days. After that B. pumilus FDKF5 or Bacillus sp. IKM1 were streak on the remaining half of petri dish. Incubation at 28°C for 5 days, observe microorganisms growth.
Compatibility test of antagonistic bacteria and bacterial bio-products
Bacillus sp. was isolates from Bacillus bio-product retrieve from Biological Control Laboratory, Department of Plant Pathology, Faculty of Agriculture at Kamphaeng Saen, Kasetsart University. Dual culture test by streak Bacillus sp. bio-product and B. pumilus FDKF5 or Bacillus sp. IKM1 in parallel with 0.5 cm distance. Incubation at 37°C and observe growth and rating with – not growth, + slightly growth, ++ moderate growth and +++ considerably growth.
Compatibility test of antagonistic bacteria and pesticides
Compatibility test of antagonistic bacteria B. pumilus FDKF5 and Bacillus sp. IKM1 and pesticides the results show that two antagonistic bacteria can growth associated with all pesticides test including insecticides (abamectin 1.8% W/V EC, carbosulfan 20% W/V EC, imidachloprid 70% WG, pyridaben 20% WP and sulfur 80% WG) and fungicides (etridiazole 6% + quintozene 4% W/V EC, benomyl 50% WP and prochloraz 50% WP), except bactericide streptomycin sulfate shown zone of inhibition (clear zone) (Fig. 1, 2).
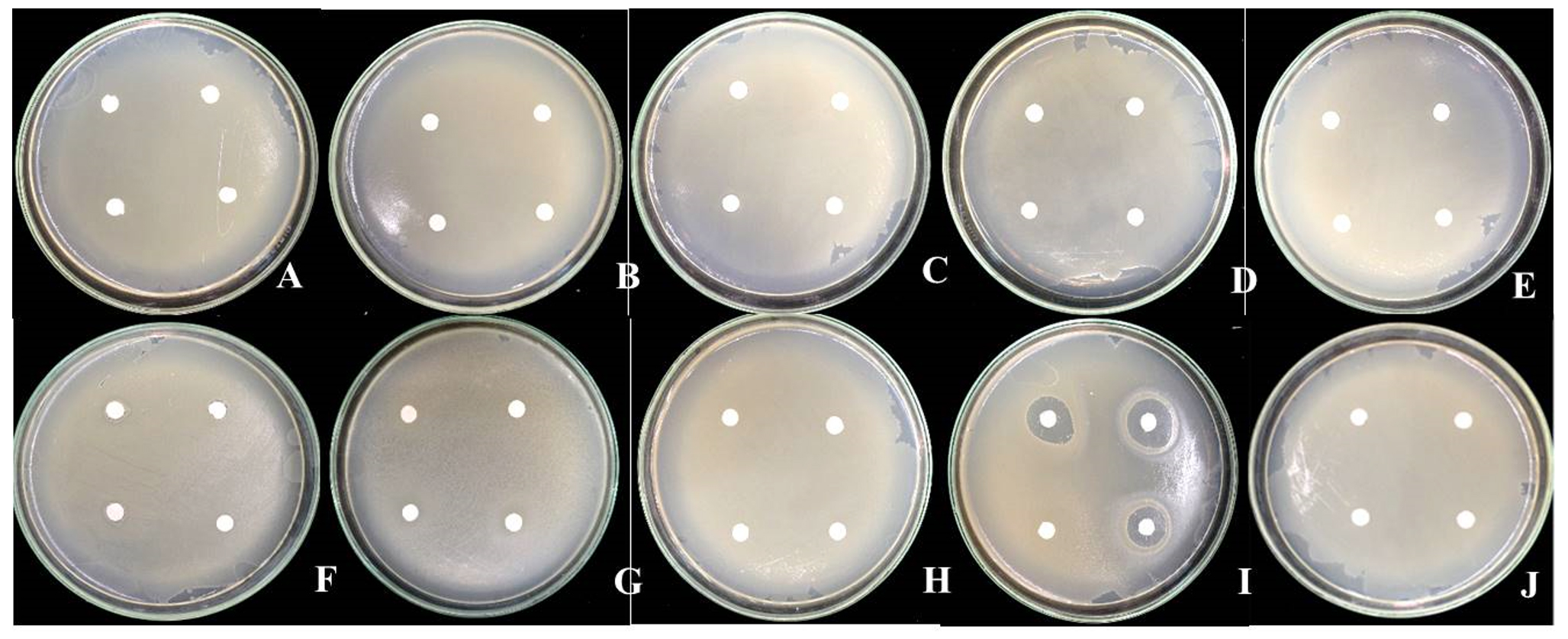 Fig. 1.Compatibility test of antagonistic bacteria Bacillus pumilus FDKF5 and pesticides
Fig. 1.Compatibility test of antagonistic bacteria Bacillus pumilus FDKF5 and pesticidesA: pyridaben 20% WP, B: imidachloprid 70% WG, C: carbosulfan 20% W/V EC, D: sulfur 80% WG, E: abamectin 1.8% W/V EC, F: etridiazole 6% + quintozene 4% W/V EC, G: benomyl 50% WP, H: prochloraz 50% WP, I: streptomycin, J: dH2O
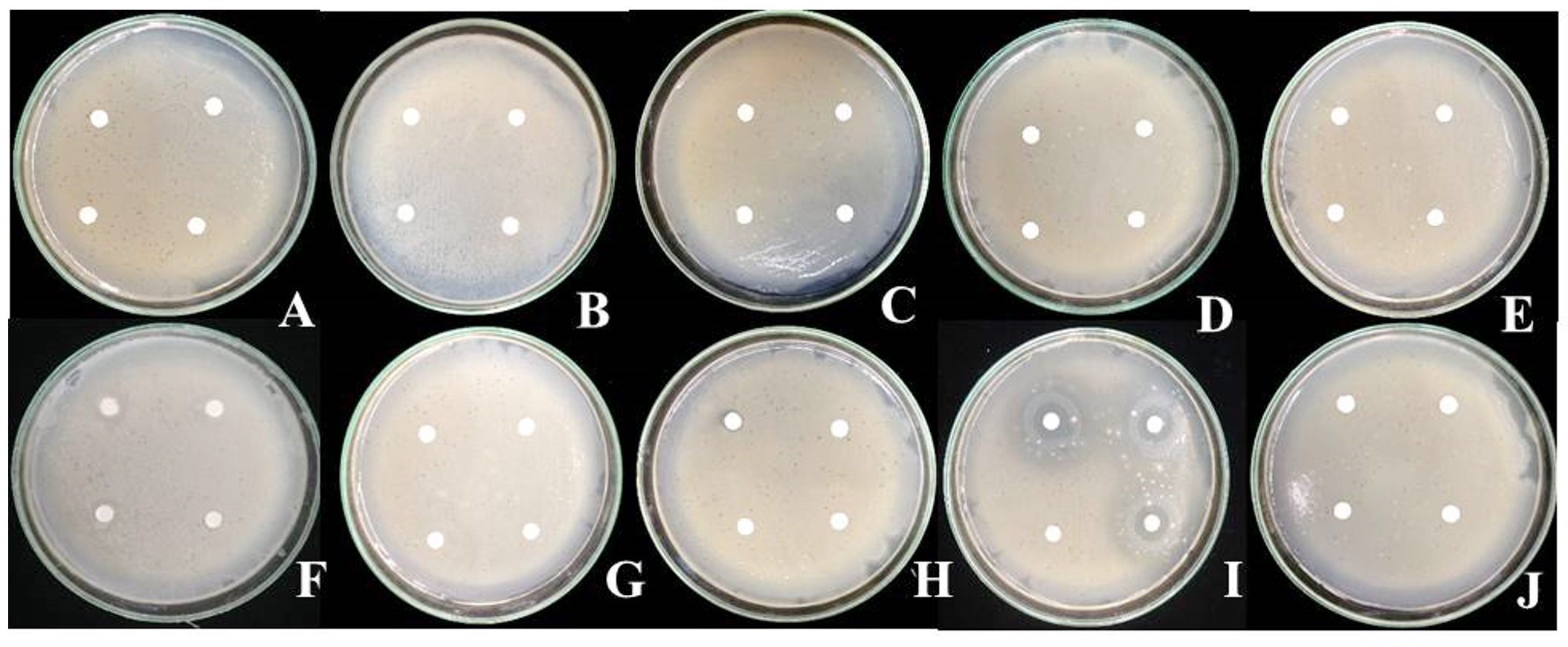 Fig. 2. Compatibility test of antagonistic bacteria Bacillus sp. IKM1 and pesticides
Fig. 2. Compatibility test of antagonistic bacteria Bacillus sp. IKM1 and pesticidesA: pyridaben 20% WP, B: imidachloprid 70% WG, C: carbosulfan 20% W/V EC, D: sulfur 80% WG, E: abamectin 1.8% W/V EC, F: etridiazole 6% + quintozene 4% W/V EC, G: benomyl 50% WP, H: prochloraz 50% WP, I: streptomycin, J: dH2O
Compatibility test of antagonistic bacteria and fungal bio-products
Compatibility test of antagonistic bacteria B. pumilus FDKF5 and Bacillus sp. IKM1 and Trichoderma bio-product the results show that Trichoderma sp. over growth on B. pumilus FDKF5 or Bacillus sp. IKM1 but not inhibit their growth. When re-streak B. pumilus FDKF5 or Bacillus sp. IKM1 on NA found normal growth and normal colony (Fig. 3).
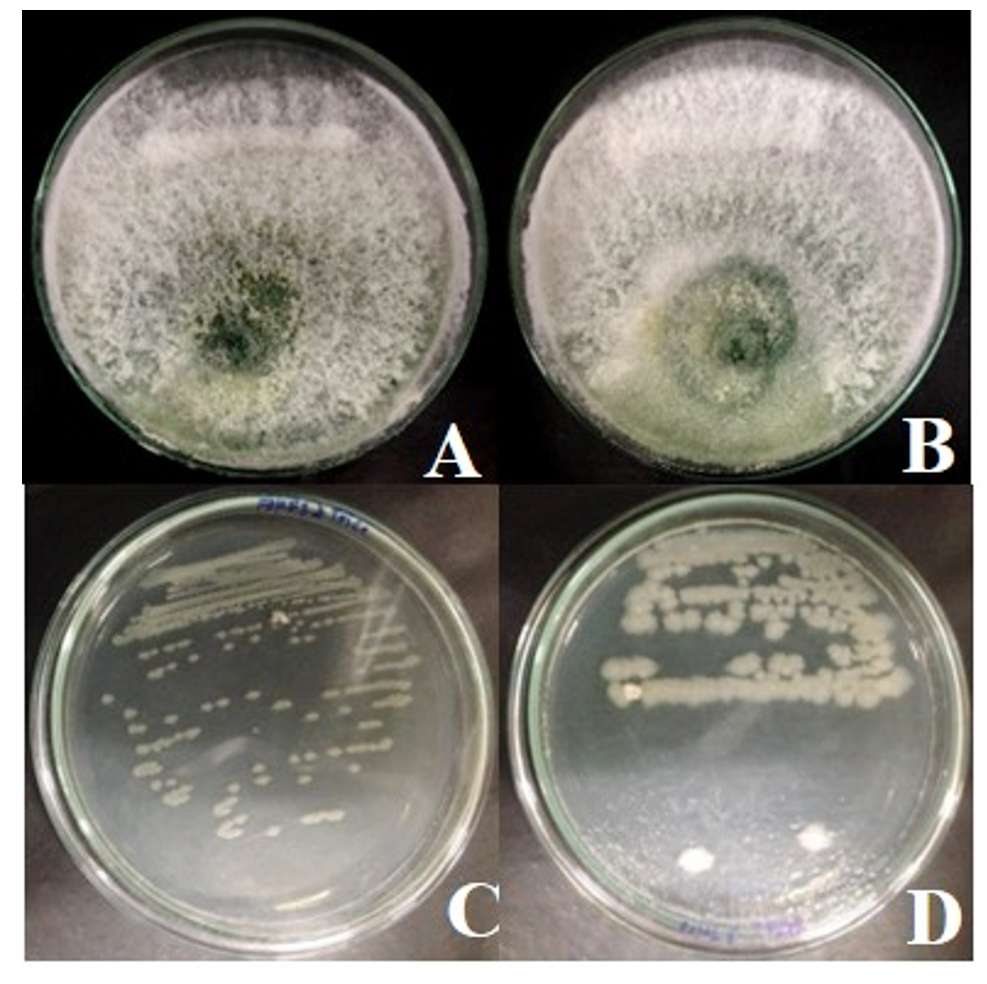 Fig. 3. Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Trichoderma bio-product
Fig. 3. Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Trichoderma bio-productA: Trichoderma bio-products and Bacillus pumilus FDKF5, B: Trichoderma bio-products and Bacillus sp. IKM1, C: Re -streak of Bacillus pumilus FDKF5, D: Re -streak of Bacillus sp. IKM1
Compatibility test of antagonistic bacteria B. pumilus FDKF5 and Bacillus sp. IKM1 and Beauveria bio-product the results show that Beauveria bassiana over growth on B. pumilus FDKF5 or Bacillus sp. IKM1 but not inhibit their growth. When re-streak B. pumilus FDKF5 or Bacillus sp. IKM1 on NA found normal growth and normal colony (Fig. 4).
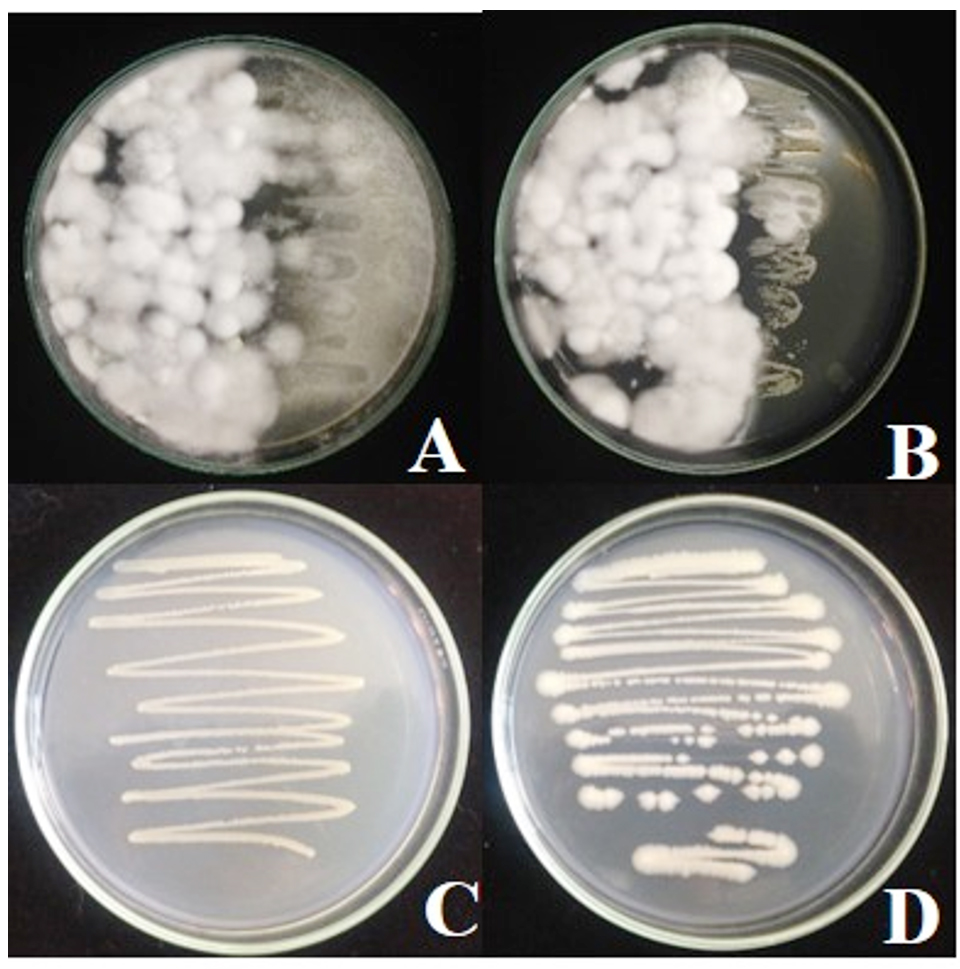 Fig. 4.Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Beauveria bio-product
Fig. 4.Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Beauveria bio-productA: Beauveria bio-products and Bacillus pumilus FDKF5, B: Beauveria bio-products and Bacillus sp. IKM1, C: Re -streak of Bacillus pumilus FDKF5,D: Re -streak of Bacillus sp. IKM1
Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Metarhizium bio-product found that Metarhizium anisopliae growth normally but B. pumilus FDKF5 or Bacillus sp. IKM1 show slightly growth (Fig. 5).
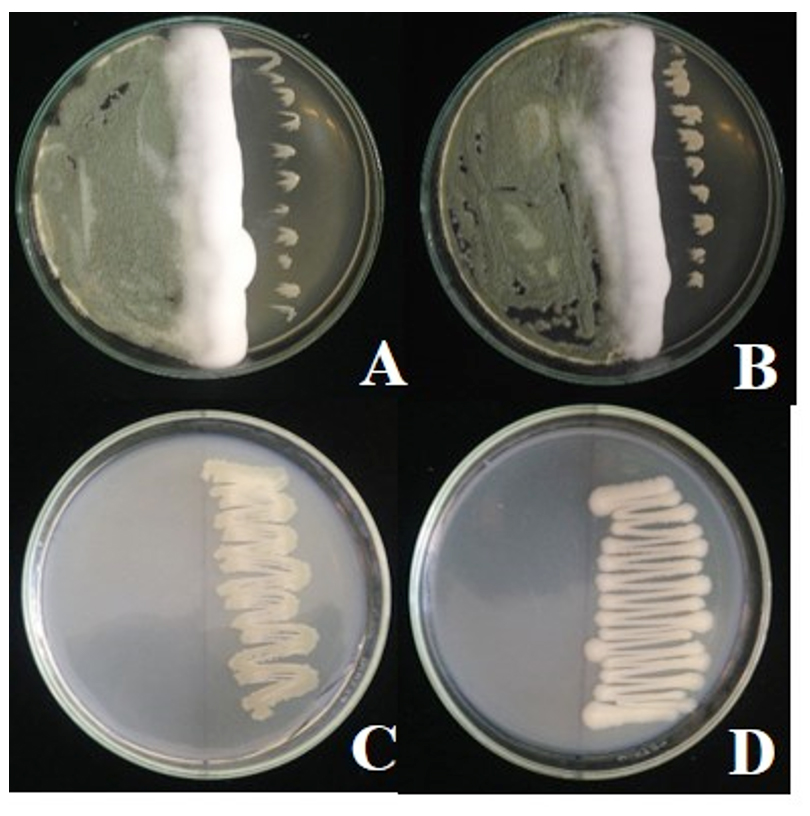 Fig. 5. Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Metarhizium bio-product
Fig. 5. Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Metarhizium bio-productA: Metarhizium bio-product and Bacillus pumilus FDKF5, B: Metarhizium bio-product and Bacillus sp. IKM1, C: Bacillus pumilus FDKF5, D: Bacillus sp. IKM1
Compatibility test of antagonistic bacteria and bacterial bio-products
Compatibility test of antagonistic bacteria B. pumilus FDKF5 and Bacillus sp. IKM1 and Bacillus bio-product the results show that both isolates of antagonistic bacteria can growth associated with Bacillus bio-product. Bacillus sp. IKM1 show considerably growth (+++), while B. pumilus FDKF5 show moderate growth (++) (Fig. 6).
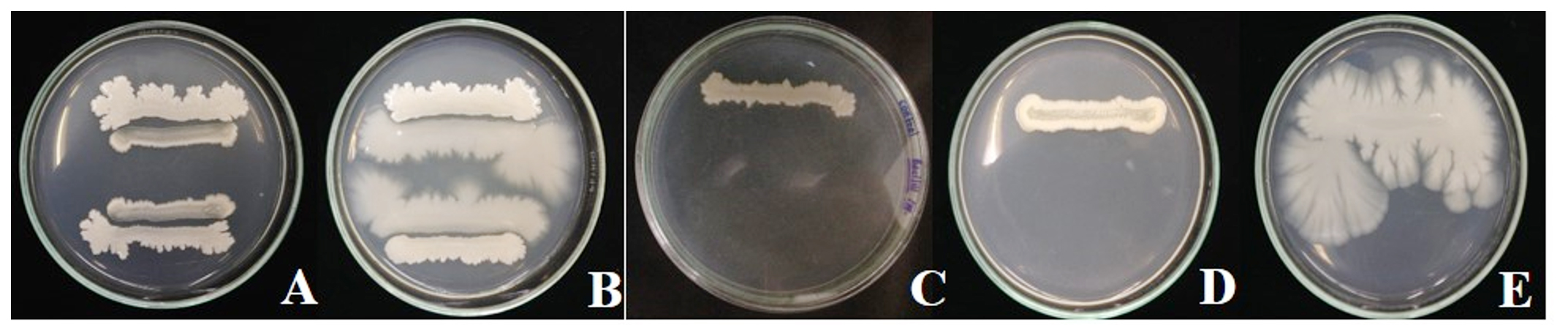 Fig. 6. Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Bacillus sp. bio-product
Fig. 6. Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Bacillus sp. bio-productA: Bacillus sp. bio-product and Bacillus pumilus FDKF5, B: Bacillus sp. bio-product and Bacillus sp. IKM1, C: Bacillus sp. bio-product, D: Bacillus pumilus FDKF5, E: Bacillus sp. IKM1
In South and Southeast Asia, the major essential food crop is rice. It is attacked by insect, disease, weed and vertebrate pests. Integrated pest management (IPM) is combination of many strategies for pest management for economic values, consist of a biological control, chemical control, cultural practices and resistance plant varieties. In this experiment, compatibility between antagonistic bacteria that effective to control rice bacteria leaf blight and insecticides, fungicides, bactericides and bio-pesticides were evaluated.
Two antagonistic bacteria including B. pumilus FDKF5 and Bacillus sp. IKM1 can growth associated with all tested pesticides including insecticides (abamectin, carbosulfan, imidachloprid, pyridaben and sulfur) and fungicides (etridiazole + quintozene, benomyl and prochloraz), except bactericide streptomycin sulfate. Similarity with that studies compatibility of bacterial biocontrol agent Bacillus subtilis with commonly used chemical fungicides such as carbendazim, mancozeb, metalaxyl, wettable sulpher, hexaconazole, difenconazole, tebuconazole and kresoxim methyl. The compatibility tests revealed that among the solid formulation fungicides, the B. subtilis showed more tolerance to carbendazim and among the liquid formulation fungicides hexaconazole and kresoxim methyl showed maximum compatibility up to 3000µl/ l concentration.16
Trichoderma bio-product and Beauveria bio-product show over growth on B. pumilus FDKF5 and Bacillus sp. IKM1 but not inhibit their growth. Moreover, compatibility among the most efficient isolates of Bacillus spp. and Trichoderma spp. was evaluated. Dual culture demonstration of Bacillus spp. and Trichoderma spp., found that eight and five isolates can reduce mycelium growth of S. cepivorum. Combination between Bacillus spp. and Trichoderma spp., there are 12 were incompatible but combination of Bacillus spp. (SF45, SF32 and SF311) and Trichoderma spp. can reduce mycelial growth of S. cepivorum. Thus, shows that both microorganisms can be used together to control white rot disease.15 In vitro, studied compatibility of biocontrol agent including Trichoderma harzianum, Bacillus subtilis and Pseudomonas fluorescens. Absence of inhibition zone indicated that the biocontrol agents were compatible with each other.17 Study of effect of pesticides comprise of imidaclopride, flufenoxuron, teflubenzuron+ phuzalon, endosulfane and amitraz on conidia germination, vegetative growth and sporulation of B. bassiana and test their compatibility too.The research found that B. bassiana is incompatible with flufenoxuron. Flufenoxuron was causal of complete inhibition in B. bassiana growing. B. bassiana DEBI008 was compatible with imidacloprid, thus could use at the same time in IPM program.18
Dual culture of antagonistic bacteria and Metarhizium bio-product, the results show that Metarhizium bio-product growth normally but B. pumilus FDKF5 and Bacillus sp. IKM1 show slightly growth. While, Streptomyces sp. SAI-25, M. anisopliae, and neem seed powder were evaluated their compatible for using to controlling pod borer complex. The result show that, there were compatibility and can be combined for Helicoverpa armigera management.10
Compatibility test of antagonistic bacteria B. pumilus FDKF5 or Bacillus sp. IKM1 and Bacillus bio-product the results show that both isolates of antagonistic bacteria can growth associated with Bacillus bio-product. Bio-control agents could be used as a way not harmful to the environment and effective to control disease and may be advised to the farmers for profitable organic farming. Compatibility study of antagonistic bacteria with agricultural chemicals and bio-products to increase the efficacy of biocontrol agents to controlling rice bacterial leaf blight diseases. Moreover, the pesticides tolerance ability broadened the use as these bio-pesticides in combination with pesticides can be applied under integrated disease management for the management of rice pest.
Acknowledgements
Authors would like to thank the Department of Biology, Faculty of Science, Mahasarakham University.
Conflict of Interest
The authors declares that there is no conflict of interest.
Authors’ Contribution
Author listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
None
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
- Fahad S, Nie L, Hussain S, Khan F, Li L, Liu X, Tabassum A, Wu C, Xiong D, Cui K, Huang J. Rice Pest Management and Biological Control, pp 85-106. E. Lichtfouse, A. Goyal (eds.), Springer International Publishing Switzerland, 2015.
Crossref - Heinrichs EA. Biology and Management of Rice Insects. Wiley Eastern, New Delhi, 1994.
- Kala A, Soosairaj S, Mathiyazhagan S, Raja P. Isolation and Identification of Xanthomonas oryzae pv. oryzae the causal agent of rice bacterial leaf blight and its activities against of six medicinal plants. Asian J. Plant Sci. Res., 2015; 5(6): 80-83.
- Nagendran K, Karthikeyan G, Peeran MF, Raveendran M, Prabakar K, Raguchander T. Management of Bacterial Leaf Blight Disease in Rice with Endophytic Bacteria. World Applied Sci. J., 2013; 28(12): 2229-2241.
- Ashrafuzzaman H. Chemical control of bacterial leaf blight of paddy caused by Xanthomonas campestris pv. oryzae. Plant Patho. Bact., 1987; 955-958.
Crossref - Khan JA, Siddiq R, Arshad HMI, Anwar HS, Saleem K, Jami FF. Chemical control of bacterial leaf blight of rice caused by Xanthomonas oryzae pv. oryzae. Pak. J. Phytopathol., 2012;, 24(2): 97-100.
- Goodell G. Challenges to international pest management research and extension in the third world: do we really want IPM to work? Bull. Entomol. Soc. Am., 1984; 30: 18–26.
Crossref - Chandler D, Alastair S. Bailey, Tatchell G. Mark, Davidson G, Greaves J, Wyn P. Grant. The development, regulation and use of biopesticides for integrated pest management. Phil. Trans R Soc B, 2011; 366: 1987–1998.
Crossref - Sorby K, Fleischer G, Pehu E. Integrated pest management in development: review of trends and implementation strategies. Agriculture and Rural Development Working paper 5. World Bank, Washington, DC, 2003.
- Agale SV, Gopalakrishnan S, Gupta R, Rangarao GV, Srinivas V, Wani SP. Compatibility of Streptomyces sp., Metarhizium anisopliae, and neem seed powder against pigeon pea pod borer complex. Int. J. Curr. Microbiol. App. Sci., 2018; 7(2): 390-397.
Crossref - Kelwatkar NM, Kankale MD, Das SB. Compatibility of Metarhizium anisopliae with neem derivatives in vitro condition. J. Entomol. Zoo Studies, 2017; 5(4): 1689-1692.
- Rogerio AD, Sueli SM, Ayres OM. Compatibility of the fungus Beauveria Bassiana (Bals.) Vuill. (Deuteromycetes) with extracts of neem seeds and leaves and the emulsible oil. Neotrop. Entomol., 2005; 34(4): 601-606.
Crossref - Deb L, Rajesh T, Majumdar D, Tombisana RK. Evaluation of biological compatibility of Beauveria bassiana with fungicides and botanicals. J. Pharmacol. and Phytoche., 2017; SP1: 1120-1124.
- Krishnamoorthy K Kamesh, Sankaralingam A, Nakkeeran S. ompatibility between fungicides and Bacillus amyloliquefaciens isolate B15 used in the management of Sclerotinia sclerotiorum causing head rot of cabbage. Int. J. Chem Studies, 2017; 5(6): 239-243.
- Fuga CAG, Lopes EA, Vieira BS, Cunha WV. Efficiency and compatibility of Trichoderma spp. and Bacillus spp. isolates on the inhibition of Sclerotium cepivorum. Cientםfica, 2016; 44(4): 526-531.
Crossref - Basamma H, Kulkarni S. Studies on compatibility of Bacillus subtilis (Ehrenberg) Cohn. with chemical fungicides. Int. J. Curr. Microbiol. App. Sci., 2017, 6(3): 578-586.
Crossref - Harshita, Sinha A, Khan JB, Trivedi S, Verma A, Rao SG. Compatibility of fungal and bacterial bi0-agents and their antagonistic effect against Fusarium oxysporum f. sp. lycopersici. Int. J. Curr. Microbiol. App. Sci., 2018; 7(7): 2305-2316.
Crossref - Alizadeh A, Mohamad A Samih, Khezri M, Rohallah S Riseh. Compatibility of Beauveria bassiana (Bals.) Vuill. with several pesticides. In. J. Agri. Biol., 2007; 9(1): 31-34.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


