ISSN: 0973-7510
E-ISSN: 2581-690X
Fructooligosaccharides (FOS) or oligofructose are unique prebiotics due to their fructosyl units with β (2-1) glycosidic linkage. This natural prebiotic travels intact through the GI tract and provides energy to stimulate the growth of beneficial probiotic microbiota especially Lactobacillus spp. These strains selectively perform anaerobic fermentation of FOS to release diversified favourable SCFAs that has manifold health benefits to human. The aim of this study was to determine the effect of prebiotic FOS on probiotic strains to produce SCFAs. The fermentability of prebiotic microbial FOS (MFOS) in vitro was assessed by comparing with commercial FOS (CFOS) on two probiotic strains Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103 growth, CFU and type of metabolite synthesized determined by plate count method and gas chromatography, respectively. The results showed that the prebiotic FOS has influenced the growth of Lactobacillus sp. as their counts increased significantly with MFOS than in controls. The effect of 2% MFOS and CFOS on the growth of Lactobacillus plantarum OUBN2 was 5.08 x 1010 and 3.48 x 1010 CFU/mL whereas, Lactobacillus rhamnosus GG ATCC 53103 was 4.97 x 1010 and 4.7 x1010 CFU/mL, respectively. GC profiles of short-chain fatty acid indicated maximum yield of butyric and propionic acid with concentration in L. plantarum OUBN2 as 44.52 µg/mL and 51.88 µg/mL and L. rhamnosus GG ATCC 53103 was 37.98 µg/mL and 47.08 µg/mL of butyric acid on 2% MFOS and CFOS, respectively. Supplementation of FOS could boost the number of colonic Lactobacillus spp., simultaneously fermenting the FOS to release the health promoting SCFAs namely butyric acid.
Butyric Acid, Fructooligosaccharides (FOS), Gas Chromatography, Lactobacillus spp., Short Chain Fatty Acids (SCFAs)
Prebiotics are distinguished compounds that stimulate the growth and activity of beneficial bacteria in colon thereby enhancing overall human health.1,2 Nondigestible oligosaccharides, like GOS and FOS approved by FDA are recognized as prebiotics, improves probiotics by selectively stimulating their functions.3,4 Fructooligosaccharides (FOS) represent a specific category of oligosaccharides that has garnered considerable commercial attention as prebiotics. FOS has repeated units of fructose with β (2-1) glycosidic linkage that can be acted upon by the probiotics.5,6
FOS are highly favoured as functional and nutritional food ingredients due to their ability to promote the positive health benefit. Endo Inulinolytic organisms when supplemented with the second most abundant plant storage polysaccharide namely inulin can result in the formation of microbial fructooligosaccharides as its end product. Cultivated plants notable for their richness in inulin, are asparagus, soybeans, dahlia and Jerusalem artichokes.7,8 They are versatile components extracted from fruits and vegetables like banana, jicama, onion, chicory roots, garlic, and leek as well as in cereal grains like wheat, corn, and barley. Currently, they are marketed under brand names like Raftilose and Nutraflora, these compounds are derived from various natural sources.9-12
Trillions of bacteria, fungi, and viruses coexist symbiotically with the host in the gastrointestinal (GI) tract, reaching extraordinary densities. This mutual relationship holds potential for mutual benefit.13-16 Probiotic bacteria need to withstand low pH levels and survive the acidic environment of the stomach, tolerate bile salts found in the intestinal tract, adhere to intestinal mucosal cells, and display clinically authenticated well-being of host.9,17,18 There is an expanding research and recognition about the ability of probiotic bacteria to ferment oligosaccharides that may hold significant importance.19-22 Remarkably, certain strains of Lactobacillus and Bifidobacterium spp., which are typically regarded as beneficial constituents of the colon’s microbiota, possess the capability to metabolize prebiotic oligosaccharides.23 Extensive research, both in vitro and in vivo, has established that the presence of FOS or similar oligosaccharides tends to promote the growth or abundance of these bacteria.24,25
Small organic monocarboxylic acids, known as short-chain fatty acids (SCFAs) or volatile fatty acids, are generated through the anaerobic fermentation of indigestible polysaccharides like FOS by the microbiota residing in the large intestine.26 These SCFAs typically contain chains of up to six carbon atoms and serve as the primary end products of the anaerobic fermentation.23,27 Acetate (C2), propionate (C3) and butyrate (C4) the common abundant short-chain fatty acids (SCFAs), are primary byproducts generated in the colon through bacterial fermentation of dietary fibers and resistant starch. Beyond, their traditional roles in providing energy, patient with the inflammatory bowel diseases (IDB), irritable bowel syndrome (IBS), type 2 diabetes (T2D), obesity, autoimmune disorders, or cancer commonly experience disruptions in the intestinal microbiome balance and a reduction in the abundance of bacteria that produce metabolites like SCFAs.28-32 SCFAs, particularly butyrate, have a well-documented impact on the immune system by promoting Treg differentiation and regulating inflammation.33,34 It also contribute to the colonocytes growth and differentiation indicating their advantage.
The production of short-chain fatty acids using fructooligosaccharides largely remained intact as they reach the colon. Later utilized by diverse populations of gut bacteria. Data on Lactobacillus spp. growth indicated that fructooligosaccharides promoted their growth efficiently. Many studies conducted in pure cultures confirmed that Lactobacillus spp. exhibited a greater capacity to utilize the fructooligosaccharides substrate when compared to glucose. Potentially elevating the consumption of prebiotics in the diet could enrich the composition of good gut bacteria and positively influence the nutritional quality of the human diet.35
Despite significant commercial and research attention given to fructooligosaccharides and probiotic bacteria, there remains a notable gap in understanding which strains effectively metabolize the fructooligosaccharides to produce sort out short-chain fatty acids under in vitro conditions. This study focuses in elucidating the efficacy of probiotic strain Lactobacillus spp. to ferment the prebiotic FOS to produce health promoting SCFAs.
Prebiotic substances (MFOS and CFOS)
For this study, two different substances were studied for their prebiotic potential. Endo-Inulinolytic bacteria Bacillus tequilensis VAK19 (Accession number ON586735.1) isolated from rhizosphere soil of Colocasia was inoculated on modified Czapek Dox media containing dahlia inulin (1%) as carbon substrate. The obtained product fructooligosaccharides was recovered from 24 h cultured broth and was lyophilized. This was labelled as microbial fructooligosaccharides (MFOS) and was used as a prebiotic agent for the study. NutraFlora® FOS a Commercial fructooligosaccharides (CFOS) was purchased and used along with dextrose as control.
Probiotic organism and inoculum preparation
Lactobacillus plantarum OUBN2 was collected from the Centre for Microbial and Fermentation Technology (CMFT), Department of Microbiology, Osmania University. Lactobacillus rhamnosus GG ATCC 53103 was isolated from the probiotic supplements purchased from the local market. Both the strains were activated and passaged thrice in MRS or deMan Rogosa, and Sharpe broth cultured at 37 °C for 24 h.36 To prepare the inoculum, 100 mL of MRS broth was prepared with the ingredients including peptone (1 gm), beef extract (1 gm), yeast extract (0.5 gm), glucose/FOS (1 gm), tween 80 (0.01 gm), ammonium citrate (0.02 gm), sodium acetate (0.05 gm), magnesium sulphate (0.01 gm), manganese sulphate (0.005 gm), dipotassium hydrogen phosphate (0.2 gm), pH 6.5. Media was autoclaved for inoculum preparation and culturing of probiotic organisms.
Effect of prebiotics on growth of probiotic organisms
Prebiotic influence on Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103 growth was studied using MFOS, CFOS and the results were compared by growing these strains in presence of glucose. The prepared inoculum of Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103 were cultured in a modified MRS broth where glucose was substituted with 1%, 1.5% and 2% of the oligosaccharide (MFOS) produced from the fermented broth of Bacillus tequilensis VAK19 and CFOS.37 A control group was cultured with the culturing broth not supplemented with prebiotics but with glucose. The growth patterns or cell density of the probiotics were monitored by measuring the turbidity at 600 nm for 0, 12 and 24 h of incubation time. Growth rate was calculated using the equation.38
Growth rate = (OD of latest Fermentation time – OD of its previous Fermentation time) / OD of its previous Fermentation time
0.1 mL of the culture was inoculated on MRS agar media to count the CFU (Colony Forming Units) reported as CFU/mL. pH of the fermented broth was determined using a calibrated pH meter.
Identification of SCFA metabolites in the fermented broth produced by Lactobacillus spp. using Gas Chromatography
The postbiotic SCFAs namely acetate, propionate and butyrate were determined using gas chromatography (GC).
After culturing the L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 in presence of microbial fructooligosaccharides, commercial fructooligosaccharides and glucose of different concentration (1%, 1.5% and 2.0%), 3.0 mL of the 2.0% supernatant were individually taken in separate tubes. 3.0 mL of 1M HCl was added, vortexed and the contents were mixed thoroughly. The tubes were immersed in an ice water bath for chilling. The contents were centrifuged at 10,000 rpm for 15 min at 4 °C. Aspirate 2.0 mL of the clear supernatant into another fresh tube and add 2.0 mL of ethyl acetate. Invert the tubes completely and mix the contents for 3 min. Centrifuge at 10,000 rpm for 15 mins at 4 °C. Aspirate the clear supernatant using a sterile syringe and filter it through 0.2 µm filter membrane. Collect the filtrate in a screw capped vial and was immediately subjected to Gas Chromatography analysis.39 1.0 µL of each sample was injected into an Zebron Capillary column with a dimension of 30 m x 0.32 mm x 0.25 µm with 250 °C as inlet temperature. The analysis was performed at 250 °C using flame ionization detector (FID) for 13.5 min per sample. Ethyl acetate was used as the sample probe cleaner with nitrogen as carrier gas supplied at 2 mL/min velocity.
Stock solution of acetic acid (2 gm or 190.48 µL/L), propionic acid (0.5 gm or 50.51 µL/L), butyric acid (0.5 gm or 52.08 µL/L) and valeric acid (0.1 gm or 10.65 µL/L) were prepared using ethyl acetate. Working standard solution were diluted (1 in 10) from the above solutions using ethyl acetate and were subjected to gas chromatography.39
The identification of the metabolites (SCFAs) was determined using the GC chromatogram by comparing the retention times to the standards used for the study. The metabolite concentrations were calculated based on the area covered underneath the specific peak.
The prebiotic potential or efficacy of microbial fructooligosaccharides (MFOS) to stimulate the growth of probiotic organisms was studied along with a commercial FOS (CFOS).40 The probiotic organisms that were used for the study were Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103. This study included determination of growth of L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 in presence of different concentration of MFOS, CFOS and dextrose by monitoring the absorbance of the turbidity at 600 nm, calculating the colony forming units/mL (CFU/mL), change in the pH of the culture media and determining the concentration of short-chain fatty acids (SCFA) by Gas chromatography (GC).
Growth of L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 on MFOS and CFOS
FOS has been reported as a strong prebiotic stimulant especially stimulating the growth of probiotic organisms that colonizes the gut. In our study, the MFOS produced by Bacillus tequilensis VAK19 an endo-inulinolytic bacteria was used as carbon source for the growth of L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 strains.
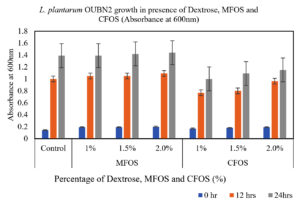
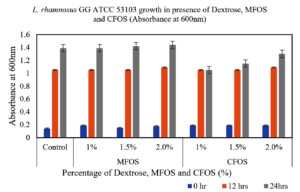
From the Figures 1 and 2, it can be observed that, the MFOS at different concentrations (1%, 1.5% and 2%) were able to stimulate the growth of both L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103, also the same effect was observed in control supplemented with dextrose in the MRS media at different fermentation time (0, 12 and 24 h). Whereas there was a less stimulatory effect of the CFOS when supplemented at different concentrations (1%, 1.5% and 2%).
Figure 1. Growth of L. plantarum OUBN2 at different concentrations of MFOS, CFOS and dextrose at 0, 12 and 24 h
Figure 2. Growth of L. rhamnosus GG ATCC 53103 at different concentrations of MFOS, CFOS and dextrose at 0, 12 and 24 h
Both the probiotics exhibited notable growth rate of 4.5 to 5.1 times when compared to its initial cell density between 12 to 24 h of fermentation using MFOS as their carbon source whereas, CFOS the growth rate has slightly decreased with cell density ranging between 3.5 to 4.73 times (Table).
Table:
Growth rate of Lactobacillus spp. strains over fermentation time supplemented with MFOS and CFOS at different concentration
| Bacterial strain | Concen (%) | 0-12 h | 12-24 h |
|---|---|---|---|
| Lactobacillus plantarum OUBN2 | MFOS 1% | 4.53 | 0.32 |
| MFOS 1.5% | 4.53 | 0.35 | |
| MFOS 2% | 4.55 | 0.30 | |
| CFOS 1% | 3.5 | 0.29 | |
| CFOS 1.5% | 3.44 | 0.36 | |
| CFOS 2% | 4.05 | 0.20 | |
| Control | 6.14 | 0.39 | |
| Lactobacillus rhamnosus GG ATCC 53103 | MFOS 1% | 4.5 | 0.30 |
| MFOS 1.5% | 4.83 | 0.35 | |
| MFOS 2% | 5.1 | 0.32 | |
| CFOS 1% | 4.5 | 0.01 | |
| CFOS 1.5% | 4.52 | 0.09 | |
| CFOS 2% | 4.73 | 0.19 | |
| Control | 6.5 | 0.32 |
Survival of probiotics in FOS supplemented media showed a positive response by both the Lactobacillus strains throughout 24 h of fermentation time is in agreement with a study reported by Scott et al.,41 Narbad et al.42 reported that sufficient time is required for the probiotics to cleave the complex structure of polysaccharides to release and supply the monosaccharides as sole carbon substrate for their growth. Also, our findings align with the key findings of Nobre et al.12 which stated that microbial FOS from Aspergillus ibericus significantly enhanced the growth of probiotics when compared to commercial FOS.
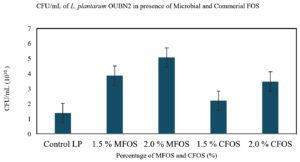
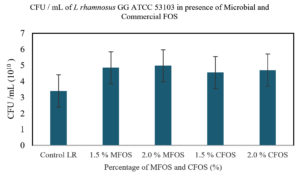
CFU/mL of L. plantarum OUBN2 and Lactobacillus GG ATCC 53103 on MFOS and CFOS
L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 exhibited growth in presence of different concentrations of MFOS and CFOS. It can be observed from the Figure 3 that the CFU/mL of L. plantarum OUBN2 was 5.08 x 1010 at 2% of MFOS and 3.48 x 1010 at 2% CFOS which indicates that at the concentration of 2% it is a good carbon source and has stimulated the probiotic organism’s growth. Similarly, in another study reported by Katarzyna et al.,37 that Lactobacillus plantarum LOCK 0860 has shown significant growth when 2% inulin was supplemented in the culture media.
From Figure 4, the effect of MFOS and CFOS on the growth of L. rhamnosus GG ATCC 53103 was determined by calculating the CFU/mL. It can be inferred that, at a concentration of 1.5% and 2% of MFOS the CFU/mL was 4.85 x 1010 and 4.97 x 1010 respectively. In presence of 1.5% and 2% of CFOS the CFU/mL was 4.55 x 1010 and 4.7 x1010 respectively.
A key finding in a study by Rahman et al.43 observed that while formulating yogurt with 1.5% inulin as supplement showed maximum counts of Lactobacillus spp. with 8.17 ± 0.01 log CFU/mL along with enhanced physical properties. Hence, it can be observed from this study that the concentration of 2% either MFOS or CFOS, the growth of the Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103 was stimulated indicating that they are efficient prebiotic stimulants when compared to control (2% of dextrose).
pH change of MFOS and CFOS supplemented culture media of Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103
Prebiotics are well known to alter gut environment, where during their fermentation acidic products are produced that can reduce the pH of the gut. Walker et al. and Duncan et al.,44,45 reported that a pH unit of one is sufficient to change the gut microbiota inhabitants. This can change the composition of acid labile microbiota species of gut and stimulate the SCFA producing microbes that are entitled to have butyrogenic effect as reported by Walker et al.44
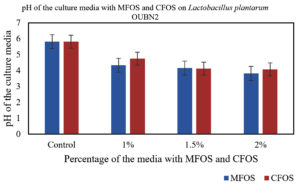
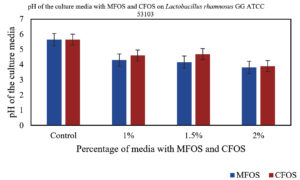
In our study, when 24 h culture medium inoculated with Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103 in presence of MFOS and CFOS at 1%, 1.5% and 2% was observed that, there was change in the pH of the medium. The initial pH of the modified MRS media was 6.5. The decrease in pH from the initial time to 24 h was due to the release and accumulation of short-chain fatty acids or volatile fatty acids namely acetic acid, propionic acid or butyric acid in the culture broth, is in accordance with Azmi et al.,39 whereas in the control with 2% dextrose the pH change was minimum (Figures 5 and 6).
Figure 5. Change in the pH of the culture media with different concentrations of MFOS and CFOS inoculated with Lactobacillus plantarum OUBN2
Figure 6. Change in the pH of the Culture media with different concentrations of MFOS and CFOS inoculated with Lactobacillus rhamnosus GG ATCC 53103
In a study reported by Pagar et al.,46 illustrated that, a 2% concentration of okara substantially boosted the growth of L. plantarum. This accelerated growth led to a drop in pH to 4.24 over 24 h, strongly suggesting the active fermentation of prebiotics by probiotics. This resulted in production of various volatile organic acids, primarily short-chain fatty acids (SCFAs) agree with our study.
GC profile of the SCFA produced by Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103 in presence of MFOS and CFOS
Dextrose is the commonly used carbon source in the MRS media for the cultivation of probiotic strain. However, these strains have the ability to metabolize various carbon sources and their ability to degrade the same was well studied and reported by Saeed and Salam.47
From the above results it indicated that, by supplementing the prebiotics at 2% (w/v), it was favourable for the growth and metabolism of probiotics. Hence, this concentration was used for studying and quantifying the SCFA production by GC, is in accordance with Katarzyna et al.37
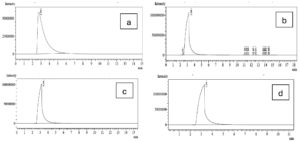
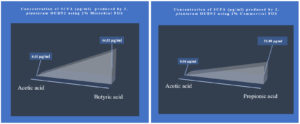
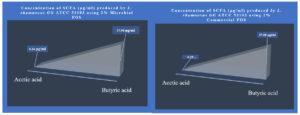
The probiotic strains namely L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 when cultured in presence of 2% of prebiotic stimulants MFOS and CFOS, after 24 h the obtained fermented broth was centrifuged and the short-chain fatty acids were extracted, and the samples were analysed using GC chromatogram. The concentrations of SCFAs were calculated using the peak area covered by the standard acetic acid, propionic acid, butyric acid and valeric acid GC profiles (Figure 7) and its yield was dependent on the type of carbon source used and the probiotic monoculture that was experimented.
Figure 7. GC Profile of SCFAs. (a). Acetic acid, (b). Propionic acid, (c). Butyric acid, (d). Valeric acid
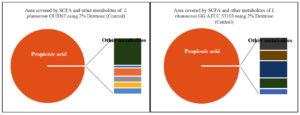
The metabolites namely SCFAs (µg/mL) produced by L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 was 45.608 µg/mL and 46.90 µg/mL (Figures 8 and 9) respectively as propionic acid with less amount of acetic acid, when cultured on 2% dextrose media. Other types of metabolites were found to be in trace amounts (Figure 10).
Figure 8. GC profile of the metabolites produced by (a). L. plantarum OUBN2 and (b). L. rhamnosus GG ATCC 53103 in presence of 2% dextrose (Control)
Figure 9. Metabolite concentration SCFA (µg/mL) produced by Probiotic strains. (a) L. plantarum OUBN2 and (b). L. rhamnosus GG ATCC 53103 on 2% dextrose (Control) – GC analysis
Figure 10. Area covered by SCFA (%) and other metabolites of L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 using 2% Dextrose (Control)
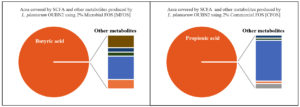
Short-chain fatty acid metabolites concentration (SCFA µg/mL) produced by L. plantarum OUBN2 on 2% MFOS and 2% CFOS was 44.52 µg/mL and 51.88 µg/mL respectively as butyric acid and propionic acid48 (Figures 11 and 12), other metabolites were found to be in less amounts (Figure 13). According to Rahayu et al.,49 supplementation of FOS with L. plantarum DAD-13 has maximized the yield of SCFAs namely acetate, butyrate and propionate which is in accordance with our report. In another study by Sandra et al. and Fakuda et al.,50,51 reported that when L. plantarum ST-III along with FOS when supplemented resulted in increased production of a C2 metabolite namely acetate.
Figure 11. GC profile of the metabolites produced by L. plantarum OUBN2 in presence of MFOS and CFOS (a and b)
Figure 12. Metabolite concentration (SCFA µg/mL) produced by Probiotic strain Lactobacillus plantarum OUBN2 on 2% MFOS and CFOS – GC analysis
Figure 13. Area covered by SCFA (%) and other metabolites of Lactobacillus plantarum OUBN2 using 2% MFOS and CFOS
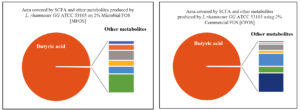
Similarly, the short-chain fatty acid metabolites concentration (SCFA µg/mL) produced by L. rhamnosus GG ATCC 53103 on 2% MFOS and 2% CFOS was 37.98 µg/mL and 47.08 µg/mL respectively as butyric acid52 (Figures 14 and 15), and also the other metabolites were found to be in trace amounts (Figure 16).
Figure 14. GC profile of the metabolites produced by L. rhamnosus GG ATCC 53103 in the presence of 2% MFOS and CFOS (a and b)
Figure 15. Metabolite concentration (SCFA µg/mL) produced by Probiotic strain L. rhamnosus GG ATCC 53103 on 2% MFOS and CFOS – GC analysis
Figure 16. Area covered by SCFA (%) and other metabolites of L. rhamnosus GG ATCC 53103 using 2% MFOS and CFOS
The findings from our present study revealed that, L. rhamnosus GG ATCC 53103 produced butyrate was found to be in agreement with a previous similar study as reported by Alessandro et al. and Wong et al.53,54 Both the Lactobacillus strains experimented in our study produced butyrate, propionate and acetate using FOS at different levels. However, L. plantarum OUBN2 was a strong butyrate producer when compared to L. rhamnosus GG ATCC 53103 in presence of MFOS. This observation is in agreement with previous studies as reported by Alessandro et al.53
Many probiotic strains are considered as reliable potential natural sources for butyrate production.40 Among them are potential Lactobacillus spp. candidate in the production of SCFA that elevates colonic epithelium function, stimulate appropriate immune and inflammatory responses and could possibly prevent colorectal cancer.55,56 As the SCFAs are organic acids, direct consumption may not be palatable and therefore, recommendation of a prebiotic like FOS with butyrate producing probiotic could improve human health.
The metabolic profiles of L. plantarum OUBN2 and L. rhamnosus GG ATCC 53103 clearly indicated the production of short-chain fatty acid like acetic acid, propionic acid and butyric acid from Gas Chromatography analysis.57 With dextrose supplementation to the probiotic strains, high yield of propionic acid was noted, whereas when the carbon source was replaced with MFOS the postbiotic produced were largely butyrate. Such postbiotic compounds becomes cross feed substrates for other gut microbiome as reported by Belenguer et al., and Falony et al.58,59 The prebiotic MFOS was effectively metabolized that has led to the production of one of the postbiotic agent namely, butyrate as it has been reported about its significance in providing energy to the colonocytes, modulating the immune system, prevents colon cancer,60 was shown to be effectively used to reduce adiposity and act as a communication link between gut and brain observed in a study by Gaman et al.61
Metabolism of prebiotics involve pH change directly enhancing beneficial microbial growth by inhibiting bad gut microbes and production of different types of organic acids like SCFAs. To have maximum benefit of prebiotics and probiotics, understanding the type of human diet, symptoms related to diseases, drugs consumed, age and other factors that can impact population of gut microbes has to be explored more.
Based on our results we conclude that, 2% of FOS supplemented as prebiotic agent has assisted the selected Lactobacillus spp. strains growth enhancement and the formed metabolites have the potential to positively favour in promoting human health. Thus, a synbiotic combination of Lactobacillus spp with FOS could be a sustainable choice in modulating the GI tract microbiota and needs further in vivo experiments.
Metabolites of gut microbiota, especially SCFAs are significant products in understanding and determining the gut microbiota associated diseases. Therefore, quantifying them to understand the type of SCFA produced by a probiotic strain can benefit the host. As can be understood from different studies, when compared to different types of SCFAs, the predominant SCFA with many health benefits is C4 metabolite namely butyrate. From our study, it can be concluded that both the probiotic Lactobacillus strains namely Lactobacillus plantarum OUBN2 and Lactobacillus rhamnosus GG ATCC 53103, were stimulated efficiently by the prebiotic FOS to enhance their growth and activity in fermenting the supplied FOS. The fermented product was found to be butyrate a promising health promoting postbiotic SCFA. In addition, this study can be extended under in vivo to understand the long-term benefits of prebiotics effects on diversified microbial gut probiotics by analysing them as a synbiotic product.
ACKNOWLEDGMENTS
The authors would like to thank the Management and Principal of Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad, for the financial support of the research and publication of this paper as a part of our project. Authors also thank the Centre for Microbial and Fermentation Technology (CMFT), Department of Microbiology, Osmania University, for sharing the probiotic culture.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SV and KA conceptualized and designed the experiments. SV compiled the information from literature sources and drafted the manuscript. KA and BB supervised and reviewed the manuscript. All authors have read and approved the final manuscript for publication.
FUNDING
This project was funded by a Research Seed Money Grant (Grant number 600/BVC/MRP-Seed Money/2022-23/1) from Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259-275.
Crossref - Smolinska S, Popescu FD, Zemelka-Wiacek M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J Clin Med. 2025;14(11):3673.
Crossref - Kojima Y, Ohshima T, Seneviratne CJ, Maeda N. Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J Oral Biosci. 2016;58(1):27-32.
Crossref - Tester RF, Al Ghazzewi FH. A preliminary study of the synbiotic effects of konjac glucomannan hydrolysates (GMH) and lactobacilli on the growth of the oral bacterium Streptococcus mutans. Nutr Food Sci. 2011;41(4):234-237.
Crossref - Sangeetha PT, Ramesh MN, Prapulla SG. Recent trends in the microbial production, analysis and application of fructooligosaccharides. Trends Food Sci Technol. 2005;16(10):442-457.
Crossref - Bhadra S, Chettri D, Verma AK. Microbes in fructooligosaccharides production. Bioresource Technology Reports. 2022;20:101159.
Crossref - Kaur N, Gupta AK. Applications of inulin and oligofructose in health and nutrition. J Biosci. 2002;27(7):703-714.
Crossref - Ali ME, Nizar NN, editors. Preparation and processing of religious and cultural foods. Woodhead Publishing. 2018;12.
- Crittenden RG, Tannock GW. Prebiotics In: Probiotics: A Critical Review. Horizon Scientific Press, Wymondham. 1999:141-56.
- Roberfroid MB, Van Loo JAE, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr. 1998;128(1):11-99.
Crossref - Yun JW, Lee MG, Song SK. Batch production of high-content fructo-oligosaccharides from sucrose by the mixed-enzyme system of β-fructofuranosidase and glucose oxidase. J Ferment Bioeng. 1994;77(2):159-163.
Crossref - Nobre C, Sousa SC, Silva SP, et al. In vitro digestibility and fermentability of fructo-oligosaccharides produced by Aspergillus ibericus. J Funct Foods. 2018;46:278-287.
Crossref - Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268-1273.
Crossref - Erny D, de Angelis ALH, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2017;150(1):7-15.
Crossref - Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Eng J Med. 2016;375(24):2369-2379.
Crossref - Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337-340.
Crossref - Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69(5):1052S-7S.
Crossref - Hamner S, McInnerney K, Williamson K, Franklin MJ, Ford TE. Bile salts affect expression of Escherichia coli O157:H7 genes for virulence and iron acquisition, and promote growth under iron limiting conditions. PloS one. 2013;8(9):e74647.
Crossref - Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401-1412.
Crossref http://doi.org/10.1093/jn/125.6.1401 - Macfarlane GT, Cummings JH. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ. 1999;318(7189):999-1003.
Crossref - Wang X, Gibson GR. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J Appl Microbiol. 1993;75(4):373-380.
Crossref - Vazquez-Rodriguez B, Santos-Zea L, Heredia-Olea E, et al. Effects of phlorotannin and polysaccharide fractions of brown seaweed Silvetia compressa on human gut microbiota composition using an in vitro colonic model. Funct Foods. 2021;84:104596.
Crossref - Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62(5):1589-1592.
Crossref - Boehm G, Lidestri M, Casetta P, et al. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;86(3):F178-81.
Crossref - Chen J, Sharma S, Quiocho FA, Davidson AL. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc Natl Acad Sci U S A. 2001;98(4):1525-1530.
Crossref - Ashaolu TJ, Ashaolu JO, Adeyeye SAO. Fermentation of prebiotics by human colonic microbiota in vitro and short chain fatty acids production: a critical review. J Appl Microbiol. 2021;130(3):677-687.
Crossref - Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1-8.
Crossref - Bhutia YD, Ganapathy V. Short, but smart: SCFAs train T cells in the gut to fight autoimmunity in the brain. Immunity. 2015;43(4):629-631.
Crossref - Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:1-21.
Crossref - Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1-8.
Crossref - Hu J, Lin S, Zheng B, Cheung PCK. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr. 2018;58(8):1243-1249.
Crossref - Nagpal R, Wang S, Ahmadi S, et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep. 2018;8(1):12649.
Crossref - Rycroft CE, Jones MR, Gibson GR, Rastall RA. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microbiol. 2001;91(5):878-887.
Crossref - Baert F, Matthys C, Mellaerts R, Lemaitre D, Vlaemynck G, Foulon V. Dietary intake of Parkinson’s disease patients. Front Nutr. 2020;7:105.
Crossref - Xu X, Fu H, Quan H, et al. Effects of fructooligosaccharides and Lactobacillus reuteri on the composition and metabolism of gut microbiota in students. Food & Function. 2025;16(4):1562-1575.
Crossref - de Man JD, Rogosa D, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Microbiol. 1960;23(1):130-135.
Crossref - Slizewska Katarzyna, Chlebicz-Wojcik A. The in vitro analysis of prebiotics to be used as a component of a synbiotic preparation. Nutrients. 2020;12(5):1272.
Crossref - Wang CY, Chen YW, Tain YL, et al. Fast quantification of short chain fatty acids in rat plasma by gas chromatography. J Food Sci. 2020;85(6):1932-1938.
Crossref - Azmi AFMN, Mustafa S, Hashim DM, Manap YA. Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules. 2012;17(2):1635-1651.
Crossref - Roupar D, Coelho MC, Goncalves DA, et al. Evaluation of microbial-fructo-oligosaccharides metabolism by human gut microbiota fermentation as compared to commercial inulin-derived oligosaccharides. Foods. 2022;11(7):954.
Crossref - Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87(1):30-40.
Crossref - Vardakou M, Palop CN, Christakopoulos P, Faulds CB, Gasson MA, Narbad A. Evaluation of the prebiotic properties of wheat arabinoxylan fractions and induction of hydrolase activity in gut microflora. Int F Food Microbiol. 2008;123(1-2):166-170.
Crossref - Rahman MS, Emon DD, Nupur AH, Mazumder MA, Iqbal A, Alim MA. Isolation and characterization of probiotic lactic acid bacteria from local yogurt and development of inulin-based synbiotic yogurt with the isolated bacteria. Appl Food Res. 2024;4(2):100457.
Crossref - Walker AW, Duncan SH, Leitch ECM, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71(7):3692-36700.
Crossref - Duncan SH, Louis P, Thomson JM, Flint HJ. The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol. 2009;11(8):2112-2122.
Crossref - Pagar R, Deshkar S, Choudhary R, Nagore D, Bhikane N. Synergistic Effects of Okara-Based Prebiotic and Lactobacillus plantarum Probiotic: Development of A Nutraceutical Formulation for Gut Health. Curr Res Nutr Food Sci J. 2025;13(1):317-332.
Crossref - Saeed A H, Salam A I. Current limitations and challenges with lactic acid bacteria: a review. Food Nutr Sci. 2013;4:73-87.
Crossref - Kim H, Kwon J, Choi SY, Ahn YG. Method development for the quantitative determination of short chain fatty acids in microbial samples by solid phase extraction and gas chromatography with flame ionization detection. J Anal Sci Technol. 2019;10(1):1-6.
Crossref - Gunawan D, Juffrie M, Helmyati S, Rahayu ES. Effect of Lactobacillus plantarum DAD-13 and Fructo-oligosaccharides on Short-Chain Fatty Acid Profile and Nutritional Status in Indonesian Stunting Children. Open Access Maced J Med Sci. 2021;9(B):1790-1796.
Crossref - Tejero-Sarinena Sandra, Barlow J, Costabile A, Gibson GR, Rowland I. Antipathogenic activity of probiotics against Salmonella Typhimurium and Clostridium difficile in anaerobic batch culture systems: is it due to synergies in probiotic mixtures or the specificity of single strains? Anaerobe. 2013;24:60-65.
Crossref - Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543-554.
Crossref - Blaak EE, Canfora EE, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Beneficial Microbes. 2020;11(5):411-55.
Crossref - Pessione A Alessandro, Bianco GL, Mangiapane E, Cirrincione S, Pessione E. Characterization of potentially probiotic lactic acid bacteria isolated from olives: Evaluation of short chain fatty acids production and analysis of the extracellular proteome. Food Res Int. 2015;67:247-54.
Crossref - Wong JM, De Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235-243.
Crossref - Feizi H, Plotnikov A, Rezaee MA, et al. Postbiotics versus probiotics in early-onset colorectal cancer. Crit Rev Food Sci Nutr. 2024;64(11):3573-3582.
Crossref - Chen J, Vitetta L. Inflammation-modulating effect of butyrate in the prevention of colon cancer by dietary fiber. Clin Colorectal Cancer. 2018;17(3):e541-e544.
Crossref - Markowiak-Kopec P, Slizewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107.
Crossref - Belenguer A, Duncan SH, Calder AG, et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72(5):3593-3599.
Crossref - Falony G, Vlachou A, Verbrugghe K, Vuyst LD. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol. 2006;72(12):7835-7841.
Crossref - Mahdavi M, Laforest-Lapointe I, Masse E. Preventing colorectal cancer through prebiotics. Microorganisms. 2021;9(6):1325.
Crossref - Gaman A, Kuo B. Neuromodulatory processes of the brain-gut axis. Neuromodulation. 2008;11(4):249-259.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.