ISSN: 0973-7510
E-ISSN: 2581-690X
The main goal of this research is to assess the antibacterial effectiveness of commonly used UAE natural herbs, including Turmeric, Henna, Sidr, and Myrrh, against a range of bacteria such as Staphylococcus aureus, Streptococcus agalactiae (group B streptococci), Streptococcus pyogenes (group A streptococci), Escherichia coli, Enterococcus faecalis, Proteus mirabilis, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Agar diffusion technique was utilized in this study. The herbs were extracted and prepared in serial dilution, a standardized amount of the test microorganisms were inoculated on the agar plates. Subsequently, extracted herbs were placed in the wells that formed on the surface of the media. The agar plates are then incubated at 37°C under appropriate condition. Typically, the herbs extract diffuses in the media, inhibiting the germination of the bacteria, the zone of inhibition is subsequently measured. Significant inhibitory effects were observed with henna herbal extract against 75% of Gram-positive bacteria, while 25% of the bacteria showed inhibition when using sidr extract. Myrrh exhibited an antibacterial effect against most tested bacteria. Comparatively less of an impact was seen by turmeric extract on both Gram-negative and Gram-positive. The antibacterial efficacy of the four plant extracts suggests that Henna displayed the highest effectiveness, followed by Sidr and Myrrh, with Turmeric showing the least potency. Additionally, strains such as E. coli, E. faecalis, P. aeruginosa, K. pneumoniae, and P. mirabilis demonstrated resistance to the plant extracts, while S. aureus, S. pyogenes, and S. agalactiae appeared to be the most susceptible strains. These findings underscore the potential of plant extracts in treating bacterial infections, offering insights for the development of novel compounds with enhanced activity against both resistant and susceptible bacteria, thereby addressing the limitations of current antibiotic agents.
UAE Natural Herbals, Gram-negative and Gram-positive Organisms, Antimicrobial Effect, Agar Diffusion Assay
To address antibiotic resistance, there has been an increasing demand to develop a novel antibiotic drug derived from diverse sources in recent years. Antibiotic resistance is a major problem nowadays, causing failures of treatment of diseases caused by microorganisms resistant to many drugs and raising concerns for world health. Therefore, the development of new antimicrobial remains a crucial objective, with natural products continuing to be a major source.1
Herbs have existed from time immemorial and have been used for many uses, including for the treatment of many diseases. So, in our research, we dealt with some herbs to test if those herbs do effects different types of Gram-negative and positive bacteria.
Henna is a flowering plant that has derived from the henna tree or Lawsonia inermis also called the Egyptian privet. It’s a woody plant with short to long size. The main color of the flower ranges from yellow to pink color which gives small fruit. But the powder reddish-brown dye color of henna that comes from its leaves. The origins of the henna tree are in Asia, Australia, and North Africa.2
The plant’s enormous amount of nutrients and chemicals may have anti-inflammatory, hypotensive, antibacterial, astringent, and antiviral effects, that can be used as a medicine to treat many diseases. In conventional medicine, they used roots, stem bark, seeds, and flowers of henna to treat burns, wounds, reduce fever, and others.3
Turmeric also called Indian saffron, diferuloylmethane, or Curcumin in other languages. Derived from the Curcuma longa plant that belongs to the Zingiberaceae family. It’s native to South Asia.4
Turmeric is used for cooking spices and in the medical field turmeric have an anti-inflammatory and antioxidant effect, as for traditional medicine, they used the rhizome part for clinical purposes like arthritis, poisoned food or digestive disorder, cancers, cleaning wounds, skin problems, In Ayurvedic medicine (Indian medicine) they use turmeric to treat several respiratory conditions. But for now, they use it in the manufacture of sunscreen and dermal creams. Via boiling the herb-dried then grinding it to become a yellow powder that color comes from the polyphenolic compound.5
Sidr is a dense, deciduous, and spreading tree with a branching trunk of light brown zigzag branches, reaching a height of approximately 2 to 4 meters. Its leaves are small, elliptical, and have a thick scale.6
In desert, semi-desert, and coastal regions, the Sidr plant thrives. Its leaves, abundant in magnesium, iron and calcium, possess medicinal properties effective against various illnesses. Its powerful antimicrobial and anti-inflammatory attributes are utilized in crafting wound antiseptics, and its resin-derived oil is used in deodorants.7
Myrrh herb is a mucilage-like substance secreted from a certain type of plant called (Commiphora myrrha). Traditionally, it has been used to address a range of ailments including injuries, buccal ulceration, gastric illness, bacterial diseases, and inflammation conditions. Its versatile properties encompass acting as mucolytic, emmenagogs, carminative, antiparasitic, astringent, and antibacterial. The phytochemical analyses revealed a spectrum of compounds within it, including terpenoids, for example, steroids, diterpenoids, triterpenoids, essential oil, and sesquiterpenoids. Additionally, aromatherapy, cosmetics, and perfumes all use its original oil.
Scientific investigation has underscored its multifaceted biological activities, which include anti-inflammatory, antioxidant, antimicrobial, anthelmintic in addition to its activity against diabetes and malignancy. Recently, research has even demonstrated its efficacy against pulmonary diseases such as coronavirus infections.
Considering the ongoing progress of medication production, there is hope that its diverse phytochemical constituents could be further explored. Potential avenues include its application against parasites. Additionally, deeper elucidation of its interactions with pharmaceutical drugs holds promise for optimizing therapeutic outcomes.8
Methanolic leaf extracts of plants, including T. procumbens, T. cordifolia, and C. igneus, had strong antibacterial action against a variety of Gram-positive and Gram-negative pathogens, according to research by Pallavi et al. Comparing these studied plant extracts to the common antibiotic ciprofloxacin utilized in this investigation, they showed increased efficacy.9
Using natural antibacterial substances from renewable resources improves the resilience and performance of textiles and considerably mitigates the ecological consequences conventionally linked to treatments with chemicals.10
Different Gram-negative and Gram-positive organisms such as (Streptococcus agalactiae (group B streptococci), Streptococcus pyogenes (group A streptococci), S. aureus, E. coli, E. faecalis, P. aeruginosa, K. pneumoniae, and P. mirabilis), were enrolled in the in vitro analysis to assess the antimicrobial effect of the extracted herbal compounds.
Extraction of herbal
The plant materials used for determining antimicrobial activity were collected in different forms: Henna (Lawsonia inermis Linn) in powder form, Turmeric (Curcuma longa) in powder form, Sidr (Ziziphus Jujuba) in leaves form, and Myrrh in gum-resin form.
The collected plant parts underwent a series of steps for preparation. They were initially washed, disinfected, and cleaned with tap water, followed by air drying until ready for use.
The herbal extraction method followed the protocol outlined by Hemeg et al. Distilled water served as the solvent for the dried plant material, whether in powder, leaf, or gum resin form.
To initiate the extraction process, 10 grams of the specified plant material were added to 100 mL D. W. in a sterile container. The solution allowed to soak for 24 hours at room temperature or in a cool, dry environment. After the designated extraction period, the residual plant material was separated from the solvent. Initially, rough clarification was achieved through decanting, followed by filtration using Whatman filter paper to obtain a clear filtrate. The resulting extract yields were then transferred into small sterile containers and stored in the refrigerator at temperatures between 2-4°C until required for further use.11
Preparation of organisms
Eight organisms were used to assess each herbal extract’s antimicrobial efficacy. Four organisms were Gram-positive (group A streptococci, group B streptococci, Enterococcus faecalis and S. aureus) and four organisms were Gram-negative (Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae). All organisms were obtained and well-identified by subculture.
In the sub-cultivation of bacteria, a loop was used to capture a bacterial colony, then used to inoculate into fresh nutrient agar and blood agar (line for isolation), then placed in the incubator at 37°C overnight (18-24 hours) aerobically and then refrigerator (2-4°C).
Antibacterial activity
Serial dilution is employed to prepare three concentrations of each extract: 100%, 75%, and 50%. The total volume for each extract concentration was 1000 mL.
The inoculum suspension was prepared using pure colonies of each microorganism from agar plates. The turbidity was adjusted to 0.5 McFarland standard (1.5 × 108 CFU per mL) for Gram-negative bacteria or McFarland tube number 1.0 (3.0 × 108 CFU/mL) for Gram-positive bacteria.
In the agar diffusion assay, the bacteria suspension was spread evenly across the entire Mueller Hinton agar plate surface. Three wells were aseptically punched into the agar, and each well received (100 µL) of the herbal extract at different concentration corresponding to the 50%, 75%, and 100% diluted extracts. The inoculated plates were then incubated aerobically at 37°C overnight. The herbal extract diffused into the agar, inhibiting the growth of the test organisms used.
Antimicrobial activity was assessed by measuring the diameter of the inhibition zone in millimeters.
The antibacterial efficacy of herbal extracts, determined through the agar well diffusion method, varied between 10 mm and 36 mm in diameter.
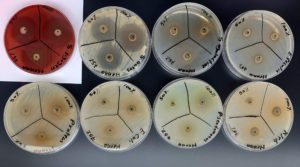
Among the four herbal extracts, Henna showed the largest zone of inhibitions (36 mm), exhibiting sensitivity to Staphylococcus aureus, Group A Streptococci, Group B Streptococci, and Klebsiella pneumoniae. In Figure 1, the zones of inhibition at concentrations of 100%, 75%, and 50% were 35 mm, 30 mm, and 30 mm, respectively, against Staphylococcus aureus. For Group A Streptococcus (Streptococcus Pyogenes), the diameters were 36 mm, 35 mm, 32 mm, while for Group B Streptococcus (Streptococcus agalactiae), they were 17 mm, 15 mm, 14 mm. Against Klebsiella pneumoniae, the diameters were 12 mm, 10 mm at concentrations of 100% and 75%.
Figure 1. Illustrates the zone of inhibition, which indicates the sensitivity of Group A Streptococci, S. aureus, Group B Streptococci, and Klebsiella pneumoniae to Henna at 100% and 75% concentrations. In contrast, Enterococcus faecalis, Proteus mirabilis, Escherichia coli, and Pseudomonas aeruginosa exhibit resistance
Myrrh showed antibacterial effect against all bacteria, the range of the size of the zone of inhibition varied between 11 and 16 mm, except Escherichia coli at a concentration of 50%, show no effect (Table).
Table:
Illustrates the correlation between the inhibition zones of test organisms and different concentrations of the herbal extracts. The results indicate that the effectiveness of the herbal extracts varies significantly depending on the type of bacteria and concentration used. Henna and Myrrh exhibit broader antibacterial properties compared to Turmeric and Sidr
| Organism | Correlation of inhibition zone and type of Herbals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumeric | Sidr | Henna | Myrrh | |||||||||
| 100% | 75% | 50% | 100% | 75% | 50% | 100% | 75% | 50% | 100% | 75% | 50% | |
| S. aureus | 0 | 0 | 0 | 20 | 20 | 20 | 35 | 30 | 30 | 12 | 12 | 11 |
| Group A Streptococci | 12 | 12 | 10 | 0 | 0 | 0 | 36 | 35 | 32 | 15 | 14 | 13 |
| Group B Streptococci | 0 | 0 | 0 | 22 | 20 | 19 | 17 | 15 | 14 | 13 | 11 | 11 |
| Enterococcus faecalis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 14 | 14 |
| E. coli | 12 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 15 | 14 | 0 |
| K. pneumoniae | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 10 | 0 | 15 | 14 | 13 |
| P. aeruginosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 13 | 12 |
| P. mirabilis | 14 | 14 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 14 | 13 |
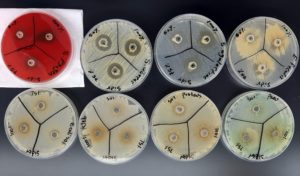
Sidr displayed inhibitory effects against Staphylococcus aureus (20 mm at all concentrations) and Group B Streptococcus (Streptococcus agalactiae) with diameters of 22 mm, 20 mm, 19 mm at concentrations of 100%, 75%, and 50%. Escherichia coli showed sensitivity only at a concentration of 100%, with a 12 mm diameter (Figure 2).
Figure 2. Illustrates the zone of inhibition, which indicates the sensitivity of Staphylococcus aureus, Streptococcus agalactiae, and Escherichia coli to Sidr at 100% concentrations. In contrast, Streptococcus pyogenes, Enterococcus faecalis, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa exhibit resistance
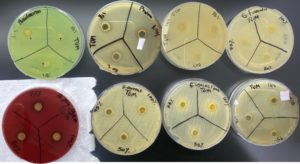
Turmeric exhibited a comparatively lower antibacterial effect. Against Group A Streptococcus (Streptococcus Pyogenes), the diameters were 12 mm, 12 mm, 10 mm. For Proteus mirabilis, they were 14 mm, 14 mm, 13 mm, and against Escherichia coli, only at a concentration of 100%, a 12 mm diameter was observed (Figure 3).
Figure 3. Shows the sensitivity of the Proteus mirabilis, Streptococcus Pyogenes, and Escherichia coli to Tumeric at 100% concentration. While Pseudomonas aeruginosa, Enterococcus faecalis, Staphylococcus aureus, Streptococcus agalactiae and Klebsiella pneumoniae shows resistance
The antibacterial activity evaluation of these plant extracts is summarized in Table and charts represented in Figures 4-11.
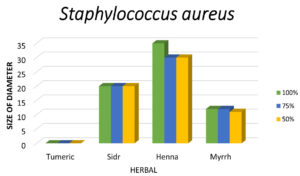
Figure 4. Shows that the largest zone of inhibition of Staphylococcus aureus is observed with Henna, followed by Sidr and Myrrh, with no inhibition seen with Turmeric
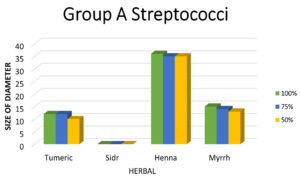
Figure 5. Shows that the largest zone of inhibition of Group A Streptococci is observed with Henna, followed by Myrrh and Turmeric, with no inhibition seen with Sidr
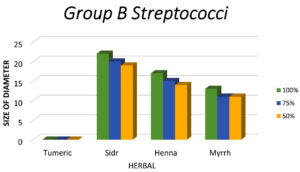
Figure 6. Shows that the largest zone of inhibition of Group B Streptococci is observed with Sidr, followed by Henna and Myrrh, with no inhibition seen with Turmeric
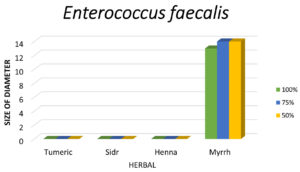
Figure 7. Shows that the zone of inhibition of Enterococcus faecalis is only observed with Myrrh, and no inhibition seen with other herbs
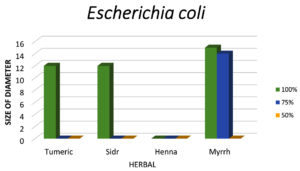
Figure 8. The graph indicates that Myrrh is the most effective herbal extract against E. coli, followed by Turmeric and Sidr, while Henna shows no antibacterial activity
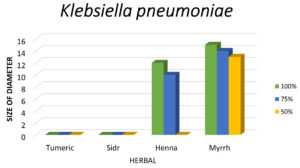
Figure 9. The graph indicates that Myrrh is the most potent herbal extract against K. pneumoniae, followed by Henna, while Turmeric and Sidr show no antibacterial activity
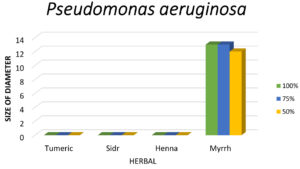
Figure 10. The graph clearly indicates that Myrrh is the only effective herbal extract against P. aeruginosa, while Turmeric, Sidr, and Henna show negligible antibacterial activity
Herbs exhibit promising efficacy against diseases and their causative agents, prompting research into the efficacy of UAE herbs against various bacteria. Our investigation revealed that Henna, Sidr, Myrrh, and Turmeric, extracted via water extraction method, exhibit varying reactions against different Gram-negative and Gram-positive organisms.
A study conducted in Iraq by Kathem K. Al-Rubiay in 2008, demonstrated that the oil and alcohol extraction of Henna displayed antimicrobial activity against Staph aureus, Staphylococcus epidermidis, P. aeruginosa, and -hemolytic streptococci. While our study corroborated its impact against Gram-positive bacteria, it did not yield positive results against Gram-negative bacteria.12
Turmeric exhibited potent antibacterial effects in our study against Gram-positive bacteria such as Group A Streptococcus, and Gram-negative organisms including Proteus mirabilis and E. coli, consistent with findings from a study at the Microbiology Department, Quaid E Azzam University, Pakistan.13
Furthermore, research by Noha Khalil at the college of Pharmaceutical Sciences and Pharmaceutical Industries, Future University, New Cairo, revealed that the oil extract of Myrrh effectively targeted S. aureus, K. pneumoniae, P. aeruginosa, and E. coli. In comparison, our study demonstrated efficacy against additional bacteria including Streptococcus pyogenes, Streptococcus agalactiae, and Enterococcus faecalis.14
Regarding Sidr, a study conducted in Oman observed significant antibacterial activity against Pseudomonas aeruginosa through the extraction of leaves and seeds in ethanol. However, our study showed that Sidr affected Staphylococcus aureus, Streptococcus agalactiae, and Escherichia coli through water extraction.15
Based on the results of our investigation, it is clear that the antibacterial properties of all plants were notably more efficient to Gram-positive in comparison to Gram-negative organisms.
Henna exhibited the highest and most potent activity against Streptococcus pyogenes, followed by Myrrh, which demonstrated antimicrobial efficacy against all test organisms, except E. coli.
Sidr extract displayed antibacterial effects primarily against Gram-positive organisms, particularly S. aureus and S. agalactia, with moderate activity against E. coli.
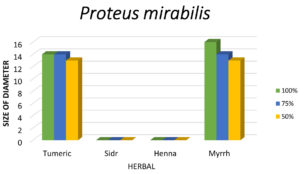
Among all plant extracts tested, only Turmeric and Myrrh showed activity against Proteus mirabilis.
In conclusion, our research indicates that Henna and Myrrh exhibit greater effectiveness than Sidr and Turmeric. This underscores the importance of herbal remedies in combating bacterial infections without introducing harmful chemicals into the body. Our findings reaffirm the wisdom of our ancestors in utilizing these herbs, suggesting their continued use for promoting health and wellness.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Medical Laboratory Sciences, College of Health Sciences, Gulf Medical University, Ajman, United Arab Emirates, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in Vitro Evaluating Antimicrobial activity: A Review. J Pharm Anal. 2016;6(2):71-79.
Crossref - Kothavale SD, Patil AK, Kumbhar RP, Mohite SK. A Review on Henna. Asian Journal of Pharmaceutical Research. 2023;13(1):47-50.
- Singam T, Marsi NB, Abdul-Rashid AH, et al. A review on characteristics and potential applications of henna leaves (Lawsonia inermis). Journal of Computational and Theoretical Nanoscience. 2020;17(2-3):603-12.
- White CM, Pasupuleti V, Roman YM, Li Y, Hernandez AV. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;146:104280.
Crossref - Prasad S, Aggarwal BB. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. Chapter 13. Available from: https://www.ncbi.nlm.nih.gov/books/NBK92752/
- Eid N, Yosri N, El-Seedi HR, Awad HM, Emam HE. Ag@ Sidr honey nanocomposite: chemical profiles, antioxidant and microbicide procurator. Biocatalysis and Agricultural Biotechnology. 2023;51:102788.
- Gibreel HH, Salih RR. The antibacterial effect of some Sudanese plants (Neem, Garad and Sidr). Agriculture and Forestry Journal. 2019;3(2):89-94.
- Batiha GE, Wasef L, Teibo JO, et al. Commiphora myrrh: a phytochemical and pharmacological update. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(3):405-420.
Crossref - Chalivendra P, Ganjikunta RK, Rao KU, Pullakanam RPT. In Vitro Assessment of Natural Herbal Extracts For Antimicrobial Activity. Int J Curr Pharm Res. 2023;15(2):22-25.
Crossref - Hossain MM, Islam T, Jalil MA, et al. Advancements of eco-friendly natural antimicrobial agents and their transformative role in sustainable textiles. SPE Polymers. 2024;5(3):241-276.
Crossref - Hemeg HA, Moussa IM, Ibrahim S, et al. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J Biol Sci. 2020;27(12):3221-3227.
Crossref - Al-Rubiay KK, Jaber NN, Alrubaiy LK. Antimicrobial Efficacy of Henna Extracts. Oman Med J. 2008;23(4):253-256.
- Gul P, Bakht J. Antimicrobial activity of turmeric extract and its potential use in food industry. J Food Sci Technol. 2013;52(4):2272-2279.
Crossref - Khalil N, Fikry S, Salama O. Bactericidal activity of Myrrh extracts and two dosage forms against standard bacterial strains and multidrug-resistant clinical isolates with GC/MS profiling. AMB Express. 2020;10(1).
- Habbal O, Hasson S, El-Hag A, Al-Mahrooqi Z, Al-Hashmi N, Al-Bimani Z, et al. Antibacterial activity of Lawsonia inermis Linn (Henna) against Pseudomonas aeruginosa. Asian Pacific Journal of Tropical Biomedicine. 2011;1(3):173-176.
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.