ISSN: 0973-7510
E-ISSN: 2581-690X
Pluronic F-127 (PF-127) hydrogel is a versatile biomaterial with promising applications in drug delivery, tissue engineering, and regenerative medicine. PF-127 has antiadhesive activity that prevents bacterial adhesion by creating a hydrated layer on the bacterial surface. This property makes PF-127 suitable for preventing implant-associated infections. In this study, we aimed to evaluate the antibacterial properties of PF-127 using field isolates of Staphylococcus aureus (Gram-positive bacteria) and Escherichia coli (Gram-negative bacteria) and compare them with different antibiotic standards. The antimicrobial potential was assessed using disk diffusion assays with four standard concentrations (20%, 25%, 30%, and 40%). The test microorganisms were inoculated on agar plates, and sterile filter paper disks infused with PF-127 hydrogels were placed alongside standard antibiotic disks. After incubation, the inhibition zones were measured to determine antimicrobial activity. Our results showed that PF-127 lacked intrinsic antimicrobial activity against S. aureus and E. coli at the tested concentrations. In conclusion, PF-127 hydrogel is a promising neutral carrier hydrogel system for loading antibiotics and antimicrobial compounds. Its unique properties, such as biocompatibility and thermo-responsive behaviour, combined with its antiadhesive activity, make it an ideal candidate for various biomedical applications.
Hydrogel, Triblock Copolymer, Drug Delivery System, Poloxamer 407, Synthetic Copolymer, Antibacterial Potential
Pluronic F-127 (also known as Poloxamer 407 or PF-127) is a hydrophilic thermoreversible hydrogel system that has been used for drug delivery across different routes of administration.1,2 It is a thermosensitive, biocompatible, and bioabsorbable polymer with great prospects in tissue engineering applications.3 PF-127 is a non-ionic surfactant made of ethylene oxide and propylene oxide blocks. It is a white, waxy, free-flowing granule that is both tasteless and odourless.1 The interaction of polyethylene oxide block with water molecules through hydrogen bonds is responsible for its water solubility. PF-127 is currently gaining importance as a system for dermal and transdermal drug delivery.2 It exists as monomolecular micelles when dispersed in the liquid at low concentrations. However, as the concentration increases, multimolecular aggregates are formed.2
Another important characteristic of PF-127 that makes it unique is the thermoreversible properties: fluid state at low temperatures enabling easy administration, and gel state at high temperatures, facilitating the prolonged release of loaded agents.4 Therefore, PF-127 at 18–50% concentrations will form hydrogel above 10°C and re-liquefy when cooled below this temperature.5 Furthermore, concentrations above 15% can undergo a reversible thermal transition from micellar liquids to gels facilitating different drug delivery applications.6
PF-127 has low toxicity, reverse thermal gelation and good solubilizing properties, so it is used as an efficient drug delivery system.1 However, thermosensitive PF-127 by itself is not utilized as an antimicrobial agent.7 Usually, peptides and polysaccharides are combined with PF-127 to enhance their antimicrobial and biological properties.8,9 It has been used as a drug delivery agent for various anti-neoplastic drugs,10 lignocaine,11 and as a dressing material for thermal burn wounds.12 PF-127 is also used as a carrier for drug delivery in ophthalmology.13
PF-127 hydrogel has demonstrated immense potential in the field of biomaterials and drug delivery.14 Its unique properties, including thermo-responsive behaviour, biocompatibility, and biodegradability, make it ideal for various applications, especially in regenerative medicine. Pluronic F-127 hydrogel is expected to play a crucial role in the development of innovative biomedical solutions.14 Although several studies have been conducted to incorporate antimicrobial agents into PF-127, it is still not clear whether PF-127 possesses inherent antimicrobial activity at various concentrations that are currently in use.15,16
Therefore, the present study was conducted to evaluate the antibacterial properties of PF-127 using the field isolates of Staphylococcus aureus (Gram-positive bacteria) and Escherichia coli (Gram-negative bacteria) and to compare it with different antibiotic standards.
Pluronic F-127 hydrogel preparation
PF-127 hydrogel was prepared by dissolving the powder (Pluronic® F-127, Sigma-Aldrich, USA) in an ice-cold 0.9% sterile normal saline solution (20% – 20g/100ml, 25% – 25g/100ml, 30% – 30g/100ml, and 40% – 40g/100ml). Once the powder was added to the solution, it was shaken vigorously to dissolve it and kept at 4°C overnight (12 hours) to ensure complete dissolution. The final preparation was filter-sterilized using a 0.22-mm pore-size syringe filter and stored at 4°C under sterile conditions till further use.
Bacterial isolates
One isolate each of S. aureus (Gram-positive bacteria) and E. coli (Gram-negative bacteria), was isolated from the clinical samples obtained from the Referral Veterinary Polyclinic-Teaching Veterinary Clinical Complex, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India.
In vitro antimicrobial potential (Disk diffusion assay – Kirby-Bauer method)
The antimicrobial potential of PF-127 was measured using four standard concentrations (20%, 25%, 30%, and 40%). A standardized inoculum of S. aureus (Gram-positive bacteria) and E. coli (Gram-negative bacteria) was prepared by adjusting the turbidity of the bacterial culture with 0.5 Macfarland standard. Muller-Hinton agar was used to perform the assay. The test inoculum was evenly applied over the agar surface using sterile cotton swabs. This procedure was repeated at least three times. Sterile filter paper disks were infused with different concentrations of PF-127 hydrogels by overnight incubation at 4°C. In addition, standard antibiotic discs such as meropenem 10 mcg, co-trimoxazole 25 mcg, erythromycin 15 mcg, tetracycline 30 mcg, vancomycin 30 mcg, and cefotaxime/clavulanic acid 30/10 mcg were used as reference values.
The hydrogel discs along with the standard antibiotic discs were placed on the agar plate and incubated for 24 h at 37°C. The antimicrobial activities were evaluated according to the Kirby–Bauer method by measuring the inhibition zone diameter (mm).17 The zone of inhibition was measured, which represents the antimicrobial activity against the test microorganisms. The diameter of the clear zone around the hydrogel disk indicates the level of inhibition. The values were expressed as mean±SD after repeating the experiments three times. The results were interpreted according to the standard criteria described by the Clinical and Laboratory Standards Institute (CLSI). The isolates were classified as sensitive (S), intermediate (I), or resistant (R) according to the reference tables.
In vitro antimicrobial potential of PF-127
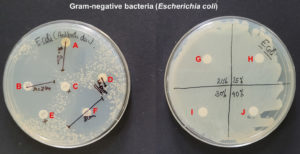
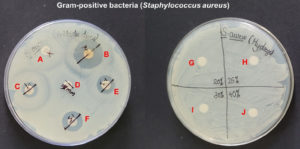
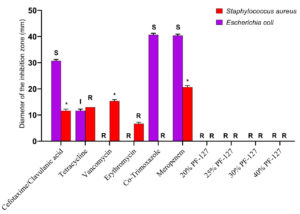
The disk diffusion assay using the Kirby-Bauer method was used to assess the in vitro antimicrobial potential of PF-127 based on the diameter of the zone of inhibition (Figure 1 and 2). None of the concentrations of PF-127 produced a zone of inhibition when inoculated with S. aureus (Figure 2) and E. coli (Figure 1) indicating a complete lack of antibacterial activity. The findings indicate that PF-127 does not possess intrinsic antibacterial activity at concentrations ranging between 20% and 40% (Figure 3). Therefore, PF-127 can be used as an ideal neutral carrier hydrogel system for loading antibiotics and other antimicrobial compounds.
Figure 1. Antibacterial activity of meropenem 10 mcg (A), co-trimoxazole 25 mcg (B), erythromycin 15 mcg (C), tetracycline 30 mcg (D), vancomycin 30 mcg (E), cefotaxime/clavulanic acid 30/10 mcg (F), 20% PF-127 (G), 25% PF-127 (H), 30% PF-127 (I), and 40% PF-127 (J) against Escherichia coli (Gram-negative bacteria)
Figure 2. Antibacterial activity of co-trimoxazole 25 mcg (A), meropenem 10 mcg (B), cefotaxime/clavulanic acid 30/10 mcg (C), erythromycin 15 mcg (D), tetracycline 30 mcg (E), vancomycin 30 mcg (F), 20% PF-127 (G), 25% PF-127 (H), 30% PF-127 (I), and 40% PF-127 (J) against Staphylococcus aureus (Gram-positive bacteria)
Figure 3. Comparative antibacterial activity of different antibiotic standards (meropenem 10 mcg, co-trimoxazole 25 mcg, erythromycin 15 mcg, tetracycline 30 mcg, vancomycin 30 mcg, and cefotaxime/clavulanic acid 30/10 mcg) with different concentrations of PF-127 hydrogels (20%, 25%, 30%, and 40%) against Staphylococcus aureus (Gram-positive bacteria) and Escherichia coli (Gram-negative bacteria). S – Sensitive, I – Intermediate, R – Resistant, and * – Reference value not available in CLSI guidelines
In vitro antimicrobial activity of standard antibiotics
The diameter of the zone of inhibition for each antibiotic standard (meropenem 10 mcg, co-trimoxazole 25 mcg, erythromycin 15 mcg, tetracycline 30 mcg, vancomycin 30 mcg, and cefotaxime/clavulanic acid 30/10 mcg) were measured in mm (Figure 3). The values were interpreted based on the standard values set by the CLSI. According to the CLSI reference tables, E. coli was found to be resistant to vancomycin and erythromycin and sensitive to co-trimoxazole, meropenem, and cefotaxime/clavulanic acid. On the contrary, the S. aureus isolate was found to be resistant to erythromycin, tetracycline, and co-trimoxazole as per the CLSI guideline.
Pluronic block copolymers are a category of hydrogel that can induce functional alterations at the cellular level.18 The biological activity of Pluronics is dependent on their ability to incorporate into the cell membranes, thereby undergoing subsequent translocation into the target cells.18,19 This affects cellular functions, including gene expression, ATP synthesis, apoptotic signal transduction, and drug efflux transporter activity.19
The antibacterial activity and biocompatibility of nitric oxide (NO) donor S-nitrosoglutathione (GSNO) releasing PF-127 were evaluated in an in vitro study.20 They demonstrated that the GSNO-hydrogel combination exhibited concentration-dependent cytotoxicity to Vero cell lines and significant antimicrobial activity against Pseudomonas aeruginosa with a minimum bactericidal concentration of 0.5 µg/mL. Furthermore, GSNO-hydrogel was found to be non-toxic to Vero mammalian cells and can be used as a delivery system for NO-based antimicrobials. The incorporation of gallic acid into a dual-responsive temperature/pH-based PF-127 hydrogel enhanced the antimicrobial properties and was used for the treatment of atopic dermatitis.21 The combined application of PF-127 and Ceragenin CSA-13 was reported to reduce the toxic effect of the latter and is indicated for topical applications.16 The polymeric micelle combination of PF-127 and Cremophor EL was investigated as a drug delivery system for norfloxacin as an antibiotic drug model.22 Their findings indicated that the micelle combination exhibited good antibacterial activity against clinically isolated bacterial strains and could act as a controlled drug delivery system for hydrophobic antimicrobial drugs.
PF-127 was found to possess antiadhesive activity (abhesive activity) that prevented the adhesion of S. aureus and S. epidermidis to polymethyl methacrylate.23 This abhesive activity against staphylococcal adherence was higher when the concentration of PF-127 was increased. A similar finding was also observed when Gram-negative bacteria like Pseudomonas aeruginosa were used. PF-127 inhibited the adherence of P. aeruginosa to contact lenses in a concentration-dependent manner.24 The findings indicate that PF-127 prevents bacterial adhesion (both Gram-positive and Gram-negative bacteria) via a nonspecific mechanism that creates a hydrated layer on the bacterial surface.23,24 Therefore, PF-127 is ideal for the prevention of implant-associated infections.
Although the abhesive activity of PF-127 has multiple utilities in the biomedical field, they lack intrinsic antimicrobial activity against staphylococci (S. aureus and S. epidermidis) when used at 4% and 15% concentration.23 Our study confirmed that PF-127 lacked intrinsic antimicrobial activity against S. aureus and E. coli at four different concentrations (20%, 25%, 30%, and 40%). The synergism exhibited by the combination of PF-127 and antibiotics is due to a two-step phenomenon. First, the antiadhesive activity of PF-127 decreases the number of adherent bacteria. In addition, the qualitative modification of the bacterial binding site enhances the susceptibility of residual adherent bacteria to antibiotic action.23
Other poloxamers such as poloxamer 331 and poloxamer CRL8131 have previously exhibited antimicrobial activity against mycobacteria.25,26 In addition to exhibiting a synergistic effect with rifampin, poloxamer 331 inhibited the growth of Mycobacterium avium complex (MAC) isolates indicating anti-mycobacterial activity.25 Similarly, another poloxamer CRL8131 exhibited intrinsic anti-mycobacterial activity against Mycobacterium tuberculosis and produced synergistic effects when used in combination with antibiotics such as rifampin, isoniazid, and streptomycin.26
The unique drug delivery characteristics and thermoreversible nature make PF-127 a promising delivery system that can be used along with various pharmaceutical agents as well as with different routes of administration.1 The findings of the present study revealed that PF-127 by itself does not have any antimicrobial properties. However, Pluronic-lysozyme conjugates possess antiadhesive and antibacterial properties. PF-127-lysozyme conjugate has exhibited antibacterial activity against Bacillus subtilis.27 For biomedical and therapeutic applications, the balance between the antimicrobial and viscoelastic properties of PF-127 needs to be considered.7 A hybrid hydrogel platform was prepared by combining PF-127/chlorhexidine nanoparticles (NP) and chitosan methacrylate-gallic acid (CSMA-GA) polymers possess antimicrobial and antioxidant activities to enhance the antibacterial properties of PF-127.28 These hybrid hydrogel combinations were found to possess strong reactive oxygen species (ROS) scavenging ability and high antibacterial efficiency during in vitro studies, and it promoted angiogenesis and significantly reduced inflammation during the in vivo studies. In another study that combined several polymers and ZnO with PF-127, it was observed that PF-127 did not exhibit any antimicrobial or antifungal activities against the various bacterial and fungal strains used.7
PF-127 hydrogel holds great promise as a versatile biomaterial. Its tunable properties, biocompatibility, and thermo-responsive behaviour make it an attractive candidate for drug delivery, tissue engineering, wound healing, and other biomedical applications. Previous studies have identified the antiadhesive activity of PF-127 against Gram-positive (S. aureus and S. epidermidis) and Gram-negative (P. aeruginosa) bacteria. In addition, poloxamers other than PF-127 have exhibited intrinsic antimicrobial (anti-mycobacterial) activity against MAC and M. tuberculosis. However, findings from this study confirm that PF-127 lacks intrinsic antimicrobial activity against S. aureus (Gram-positive bacteria) and E. coli (Gram-negative bacteria) at concentrations ranging between 20% and 40%. Therefore, PF-127 can be used as an ideal neutral carrier hydrogel system for loading antibiotics and other antimicrobial compounds due to their antiadhesive property that enhances the susceptibility of microorganisms against the loaded compound.
ACKNOWLEDGMENTS
The authors would like to thank Director, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, India, and the All-India Network Program on Diagnostic Imaging and Management of Surgical Conditions in Animals (AINP-DIMSCA) for providing the necessary research facilities to carry out this work. The authors are also grateful to Dr. Mufeeda Beegum and Dr. Ambily R for their help in this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9(3):339-358.
- Almeida H, Amaral MH, Lobao P, Lobo JM. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): main features and their applications in topical and transdermal administration of drugs. J Pharm Pharm Sci. 2012;15(4):592-605.
Crossref - Brunet-Maheu JM, Fernandes JC, de Lacerda CA, Shi Q, Benderdour M, Lavigne P. Pluronic F-127 as a cell carrier for bone tissue engineering. J Biomater Appl. 2009;24(3):275-87.
Crossref - Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709-2728.
Crossref - Yang HC, Chang HY. Novel air leak test using surfactant for lung surgery. J Thorac Dis. 2018;10(12):6472-6474.
Crossref - Shriky B, Kelly A, Isreb M, et al. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J Colloid Interface Sci. 2020;565:119-130.
Crossref - Lupu A, Rosca I, Gradinaru VR, Bercea M. Temperature Induced Gelation and Antimicrobial Properties of Pluronic F127 Based Systems. Polymers. 2023;15(2):355.
Crossref - Bercea M. Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities. Polymers. 2022;14(12):2365.
Crossref - Bertila XJ, Rupachandra S. Insights into current directions of protein and peptide-based hydrogel drug delivery systems for inflammation. Polymer Bulletin. 2022.
Crossref - Miyazaki S, Takeuchi S, Yokouchi C, Takada M. Pluronic F-127 gels as a vehicle for topical administration of anticancer agents. Chem Pharm Bull (Tokyo). 1984;32(10):4205-4208.
Crossref - Chen-Chow PC, Frank SG. In vitro release of lidocaine from Pluronic F-127 gels. Int J Pharm. 1981;8(2):89-99.
Crossref - Nalbandian RM, Henry RL, Wilks HS. Artificial skin. II. Pluronic F-127 Silver nitrate or silver lactate gel in the treatment of thermal burns. J Biomed Mater Res. 1972;6(6):583-590.
Crossref - Miller SC, Donovan MD. Effect of poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm. 1982;12(2-3):147-52.
Crossref - Akash MS, Rehman K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J Control Release. 2015;209:120-138.
Crossref - Lee SH, Lee JE, Baek WY, Lim JO. Regional delivery of vancomycin using pluronic F-127 to inhibit methicillin resistant Staphylococcus aureus (MRSA) growth in chronic otitis media in vitro and in vivo. J Control Release. 2004;96(1):1-7.
Crossref - Leszczynska K, Namiot A, Cruz K, et al. Potential of ceragenin CSA-13 and its mixture with pluronic F-127 as treatment of topical bacterial infections. J Appl Microbiol. 2011;110(1):229-238.
Crossref - Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. American Society for Microbiology. 2009;15:55-63.
- Giuliano E, Paolino D, Fresta M, Cosco D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines. 2018;6(1):7.
Crossref - Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98-106.
Crossref - Pelegrino MT, De Araujo Lima B, Do Nascimento MHM, Lombello CB, Brocchi M, Seabra AB. Biocompatible and Antibacterial Nitric Oxide-Releasing Pluronic F-127/Chitosan Hydrogel for Topical Applications. Polymers. 2018;10(4):452.
Crossref - Chatterjee S, Hui PC, Kan CW, Wang W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci Rep. 2019;9(1):11658.
Crossref - Tanase MA, Raducan A, Oancea P, et al. Mixed Pluronic-Cremophor Polymeric Micelles as Nanocarriers for Poorly Soluble Antibiotics-The Influence on the Antibacterial Activity. Pharmaceutics. 2021;13(4):435.
Crossref - Veyries ML, Faurisson F, Joly-Guillou ML, Rouveix B. Control of staphylococcal adhesion to polymethylmethacrylate and enhancement of susceptibility to antibiotics by poloxamer 407. Antimicrob Agents Chemother. 2000;44(4):1093-1096.
Crossref - Portoles M, Refojo MF, Leong FL. Poloxamer 407 as a bacterial adhesive for hydrogel contact lenses. J Biomed Mater Res. 1994;28(3):303-309.
Crossref - Hunter RL, Jagannath C, Tinkley A, Behling CA, Nolte F. Enhancement of antibiotic susceptibility and suppression of Mycobacterium avium complex growth by poloxamer 331. Antimicrob Agents Chemother. 1995;39(2):435-439.
Crossref - Jagannath C, Allaudeen HS, Hunter RL. Activities of poloxamer CRL8131 against Mycobacterium tuberculosis in vitro and in vivo. Antimicrob Agents Chemother. 1995;39(6):1349-54.
Crossref - Muszanska AK, Busscher HJ, Herrmann A, van der Mei HC, Norde W. Pluronic-lysozyme conjugates as anti-adhesive and antibacterial bifunctional polymers for surface coating. Biomaterials. 2011;32(26):6333–6341.
Crossref - Xu Z, Liu G, Zheng L, Wu J. A polyphenol-modified chitosan hybrid hydrogel with enhanced antimicrobial and antioxidant activities for rapid healing of diabetic wounds. Nano Research. 2023;16(1):905-916.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.