ISSN: 0973-7510
E-ISSN: 2581-690X
The present study investigates the antimicrobial potential of the crude extracts of two fungal strains isolated from the Vellar estuary water against clinical human pathogens. The two potent fungal isolates were identified as Fusarium oxysporum RAS 2 and Aspergillus niger RAS 3 through 18S rRNA sequencing. 5 ml of n-butyl alcohol (nBA) and ethyl acetate (EA) crude extracts confirmed significant antimicrobial properties exhibited a wide range of zone of inhibitions from 10 to 38 mm against bacterial and fungal pathogens which were collected from Government Medical College Hospital, Chidambaram, when compare to control measures. The EA and NA extract of F. oxysporum RAS 2 exhibited the highest activity (30 ± 0.7 mm) and (25 ± 0.5 mm) against Shigella sp., and E. coli, respectively. Both extracts of A. niger RAS 3 showed the highest activity (38 ± 0.8 mm) and 27 ± 0.5 mm against B. subtilis. In antifungal activity, the ethyl acetate extract of F. oxysporum RAS 2 and A. niger RAS 3 exhibited the highest activity against A. niger. The n-butyl alcohol extract of F. oxysporum RAS 2 showed the highest activity (35 ± 0.7 mm) against C. albicans, while A. niger RAS 3 demonstrated significant activity (36 ± 0.8 mm) against A. niger. FTIR investigation of the EA extracts revealed the existence of an additional ester functional group at the range 1735, which correlated with enhanced antimicrobial activity compared to other extracts lacking this group. Further characterization of these metabolites is necessary to confirm their antimicrobial properties. This study concludes that fungi from the Vellar estuary produce compounds with antimicrobial activity, making them a promising source to develop novel natural antimicrobial metabolites.
F. oxysporum, A. niger, Vellar Estuary, Antimicrobial Activity, FTIR
Fungi can survive at diverse environments, including polar regions, hot springs, deserts, saline waters, oceanic trenches, and highly acidic conditions.1 Among these, mycoextremophiles can thrive in hypersaline waters, such as salt lakes worldwide.2-4 The products of halophilic microbes are considered as valued sources of new bioactive metabolites, as the extreme conditions of hypersaline environments can activate otherwise silent genes and induce unique biosynthetic pathways.5-7
Fungal metabolites display a broad spectrum of bioactivities, like antimicrobial, anticancer, antioxidant, insecticidal, anti-diabetic, and immunosuppressive effects.8-10 Various fungi produce highly complex chemical structures, with numerous metabolites exhibiting significant biological activities. For instance, Alternaria species have been found to produce R-Dibenzopyrones like alternariol and its 5-O-methyl ether, also known as djalonensone.11 Additionally, three new metabolites were purified from Alternaria raphani alongside fifteen known compounds.12
Aspergillus terreus has yielded emodin, an anthraquinone with moderate antibacterial activity.13 Metabolites from Cladosporium spp. have demonstrated antibacterial,14,15 antifungal, and cytotoxic properties.15Wallemia sebi has shown the ability to kill brine shrimp, protozoa, and cell lines16 and significantly inhibit bacterial growth.17 Furthermore, various Fusarium species were also identified to exhibit antifungal18-21 and antibacterial activities.21-23 Several bioactive compounds, including Terremids A and B, Terlactone A, and new compounds like Terreineol, Terreulactone A, Terrain, Terreic Acid, and Saspulvinones, have been identified in A. terreus isolated from high-salt environments.24
While many fungi have been isolated from the Vellar estuary and tested for their pharmacological effects, fungi from water samples in this region have not been extensively studied for their activity against human bacterial and fungal pathogens. This study focuses on the dominant fungal strains isolated from Vellar estuary water and extracted fungal compounds using ethyl acetate and n-butyl alcohol and evaluated their pharmacological effects, which are discussed in detail here.
Sample collection
Water sample (150 ml) was taken in three sterilized DO bottles at a depth of 30 cm from the Vellar estuary (latitude 11.491134°N, longitude 79.765664°E) in Parangipettai, Tamil Nadu, Southeast coast of India. The water sample pH-7.2, salinity 23 ppt and temperature was 26 °C. The sample was kept in the Mycology Lab at Centre of Advanced Study (CAS) in Marine Biology for further microbiological investigation.
Isolation of test organisms
Potato Dextrose Agar (PDA) medium (120 ml)of pH 5.6 ± 0.2 containing 75 mg of chloramphenicol (to avoid bacterial contamination) was prepared. Six plates were inoculated with serially diluted (101-106) water samples using the pour plate method and incubated at 37 °C for 48 hours for complete growth. Two dominant fungal strains were selected, isolated using the streak plate method, and maintained in pure culture. Biomass cultures were grown in a shaking incubator at 100 rpm at 37 °C in Potato Dextrose Broth (PDB) for five days.
Identification of test organisms
Lactophenol Cotton Blue stain was dropped on a clean glass slide and a loopful of fungal culture was placed over the stain and gently spread apart. A cover slip was placed over the sample, which was then examined under a microscope. Based on the colony mycelium and the standard manual the fungal strains were identified.25
DNA isolation and PCR
Three days old fungal mycelium was taken for DNA isolation. The fungal surface hyphae (0.1 g) were scraped from each culture and ground in 300 µL lysis buffer (200 mM Tris-HCl pH 5.8, 250 mM NaCl, 25 mM EDTA, 0.5% SDS) using a glass-glass homogenizer. Cell debris was pelleted by centrifugation at 13,000 rpm. The supernatant was transferred into a new tube and equal volume of absolute ethanol was added and mixed gently. This mixture was kept at 4 °C for overnight. The next day, the DNA was precipitated by centrifugation at 15,000 rpm and the DNA pellet was re-suspended in 100 µL of TE buffer (pH 8.0).26
PCR reactions were performed using 10 ng of genomic DNA in a thermal cycler (Applied Bioscience). The reaction mixture included EmeraldAmp® GT PCR Mastermix (1X), primer mix (0.4 µM), (18S rRNA F: 5′-CAGCAGCCGCGGTAATTCC-3′; 18S rRNA R: 5′-CCCGTGTTGAGTCAAATTAAGC-3′), template DNA (200 ng), sterile double-distilled water and the final volume was 25 µl. The PCR condition was as follows: initial denaturation at 94 °C for 5 min, followed by 30 cycles of 94 °C for 50 sec, 58 °C for 50 sec, and 72 °C for 50 sec, with a final extension of 72 °C for 10 min. The amplicons were analysed in a 1% agarose gel contains ethidium bromide stain.
DNA sequencing
The amplicons were sequenced bidirectionally at Rajiv Gandhi Centre for Aquaculture (RGCA) by Sanger sequencing method (Applied Bioscience). The noisy peaks were edited by BioEdit (V.7.2.5), resulting in clear sequences of 598 bp and 635 bp. The edited 18S rRNA gene sequences were compared using BLASTn for similarity testing, and the sequences were submitted to NCBI GenBank.
Extraction of bioactive compounds
150 ml of mass-cultured broth of the two fungal strains was taken and equal volume of EA and nBA was poured separately without contamination. The mixtures were kept in a shaking incubator at 100 rpm at 37 °C for one hour. After one hour, the mixtures were filtered in a filter paper (11 µm). Subsequently, the compounds were extracted by a rotary evaporator (IKV®RV10). The semi-solid crude extract then dissolved in 5 ml of the respective solvents for further analysis.
Antibacterial and antifungal activity
Authenticated clinical bacterial strains, including B. subtilis, E. coli, Proteus sp., Pseudomonas sp., S. paratyphi, S. typhi, Shigella sp., and V. harveyi, and fungal strains, such as A. flavus, A. fumigatus, A. niger, C. albicans, and Rhizopus sp., were obtained from Government Medical College and Hospital, Chidambaram and were sub-cultured in Mycology lab in CAS in Marine Biology.
Muller-Hinton Agar (MHA) was prepared in petri plates, and the pathogens were swabbed under sterile conditions. Wells were punctured in the plates, and 100 µl of each fungal extract was loaded to the wells. Plates were kept at 37 °C for one day. After the incubation period was over, the inhibition zones were noted. Triplicate plates were maintained for all the experiments.
Fourier Transform Infrared (FTIR) analysis of extracts
Crude extracts were analysed by FTIR. The spectrum was recorded in triplicate in the infrared range from 650-4000 cm-1 using an FTIR spectrometer (Agilent Cary 630) at the Department of Chemistry, Annamalai University.
Identification of test organisms
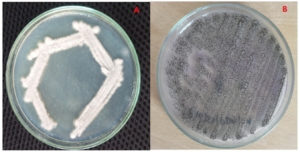
The isolated fungal strains were identified using a standard mycological manual by comparing the phenotypic features after LCB staining. Based on 100% similarity and 99% query coverage in the BLAST search, the fungal strains were confirmed as F. oxysporum (RAS 2) (PQ097244) and A. niger (RAS 3) (PQ097245). The pure culture plates are shown in Figure 1.
Antibacterial activity
The antibacterial effects of the two crude fungal extracts are presented in Table 1. The EA extract of F. oxysporum RAS 2 exhibited the highest activity (30 ± 0.7 mm) against Shigella sp., while A. niger RAS 3 showed the highest activity (38 ± 0.8 mm) against B. subtilis. The n-butyl alcohol extract of F. oxysporum RAS 2 showed the highest activity (25 ± 0.5 mm) against Shigella sp. and E. coli, whereas A. niger RAS 3 exhibited significant activity (27 ± 0.5 mm) against B. subtilis.
Table (1):
Antibacterial effect of two fungal extracts
| No. | Pathogen | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| EA extract of F. oxysporum RAS 2 | nBA extract of F. oxysporum RAS 2 | EA extract of A. niger RAS 3 | nBA extract of A. niger RAS 3 | ||
| 1. | B. subtilis | 28 ± 0.6 | 22 ± 0.3 | 38 ± 0.8 | 27 ± 0.5 |
| 2. | E. coli | 24 ± 0.5 | 25 ± 0.6 | 25 ± 0.5 | 25 ± 0.5 |
| 3. | Proteus sp. | 27 ± 0.6 | 24 ± 0.4 | 28 ± 0.6 | 25 ± 0.5 |
| 4. | Pseudomonas sp. | 24 ± 0.5 | 25 ± 0.5 | 29 ± 0.6 | 26 ± 0.5 |
| 5. | S. paratyphi | 25 ± 0.5 | 24 ± 0.3 | 28 ± 0.6 | 26 ± 0.5 |
| 6. | S. typhi | 10 ± 0.2 | 17 ± 0.3 | 19 ± 0.4 | 21± 0.5 |
| 7. | Shigella sp. | 30 ± 0.7 | 25 ± 0.5 | 35 ± 0.8 | 24 ± 0.5 |
| 8. | V. harveyi | 24 ± 0.5 | 19 ± 0.4 | 28 ± 0.5 | 25 ± 0.4 |
Antifungal activity
The ethyl acetate extract of F. oxysporum RAS 2 and A. niger RAS 3 exhibited the highest activity against A. niger. The n-butyl alcohol extract of F. oxysporum RAS 2 showed the highest activity (35 ± 0.7 mm) against C. albicans, while A. niger RAS 3 demonstrated significant activity (36 ± 0.8 mm) against A. niger. Detailed results are presented in Table 2.
Table (2):
Antifungal effect of two fungal extracts
| No. | Pathogen | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| EA extract of F. oxysporum RAS 2 | nBA extract of F. oxysporum RAS 2 | EA extract of A. niger RAS 3 | nBA extract of A. niger RAS 3 | ||
| 1. | A. flavus | 14 ± 0.3 | 22 ± 0.4 | 24 ± 0.5 | 30 ± 0.7 |
| 2. | A. fumigatus | 25 ± 0.5 | 25 ± 0.5 | 30 ± 0.6 | 28 ± 0.6 |
| 3. | A. niger | 32 ± 0.8 | 31 ± 0.6 | 36 ± 0.9 | 36 ± 0.8 |
| 4. | Rhizopus sp. | 29 ± 0.6 | 28 ± 0.5 | 35 ± 0.8 | 32 ± 0.7 |
| 5. | C. albicans | 25 ± 0.5 | 35 ± 0.8 | 35 ± 0.7 | 30 ± 0.6 |
Fourier-Transform Infrared Spectroscopy (FTIR) analysis
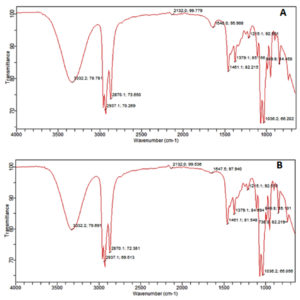
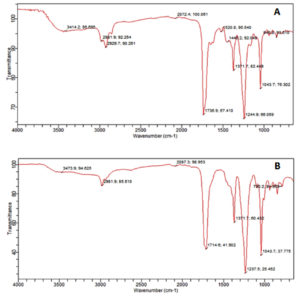
The result of FTIR confirmed the presence of different chemical functional groups in the crude samples. The detailed results are provided in Table 3 and illustrated in Figure 2 and 3.
Table (3):
The functional group details of two fungal extracts
No. |
Frequency range (cm-1) |
EA extract of F. oxysporum RAS2 |
nBA extract of F. oxysporum RAS2 |
EA extract of A. niger RAS3 |
nBA extract of A. niger RA3 |
|---|---|---|---|---|---|
1. |
3332-3473 |
Amide A :N-H stretching of protein |
Amide A:N-H stretching of protein |
Amide A:N-H stretching of protein |
Amide A:N-H stretching of protein |
2. |
2981-2929 |
CH stretch of alkane:mainly lipid |
CH stretch of alkane:mainly lipid |
– |
CH stretch of alkane:mainly lipid |
3. |
2870 |
– |
H-C=O:C-H stretch of aldehyde |
– |
H-C=O:C-H stretch of aldehyde |
4. |
2132 |
– |
C≡C stretch of alkynes |
– |
C≡C stretch of alkynes |
5. |
1736-1746 |
C=O stretch of ester:mainly lipid |
– |
C=O stretch of ester: mainly lipid |
– |
6. |
1640-1647 |
– |
Amide I:C=O stretching of protein |
– |
Amide I:C=O stretching of protein |
7. |
1520 |
C=C stretch of aromatic:mainly carbohydrate |
– |
– |
– |
8. |
1371-1461 |
C-H bend of alkane:mainly lipid |
C-H bend of alkane:mainly lipid |
– |
C-H bend of alkane:mainly lipid |
9. |
1244 |
C-F stretch of alkyl halide:mainly carbohydrate |
– |
– |
– |
10. |
1215-1237 |
– |
C-N stretch of Aliphatic Amine |
C-N stretch of Aliphatic Amine |
C-N stretch of Aliphatic Amine |
11. |
1036-1043 |
C-F stretch of alkyl halide:mainly carbohydrate |
C-F stretch of alkyl halide: mainly carbohydrate |
– |
C-F stretch of alkyl halide: mainly carbohydrate |
The molecular identification of A. niger using 18S rRNA gene sequencing aligns with previous studies27 that used primers (F: 5′-GGAAGGG[G/A]TGTATTTATTAG-3′; R: 5′-TCCTCTAAATGACCAAGTTTG-3′) to amplify a 1452 bp product. In the present study, the same gene was amplified in A. niger and F. oxysporum using different primers (F: 5′-CAGCAGCCGCGGTAATTCC-3′; R: 5′-CCCGTGTTGAGTCAAATTAAGC-3′), resulting in product sizes of 598 bp and 635 bp, respectively. The A. niger RAS 3 (PQ97245) strain showed 100% sequence similarity to A. niger in the NCBI database, corroborating the findings28 where a similar isolate showed 99% sequence similarity in a BLAST search.
The antagonistic activity of crude EA and nBA extracts from A. niger and F. oxysporum was tested against clinical pathogens (bacterial and fungal). Previous studies29 have documented the presence of F. oxysporum in hypersaline soils, and Aspergillus species have been reported as predominant fungi in extreme environments like hypersaline conditions.30,31 In this study, A. niger and F. oxysporum were also isolated from the euryhaline waters of Vellar estuary. The EA extract of A. niger revealed a significant antibacterial activity, with an inhibition zone of 35 ± 0.8 mm for Shigella sp., which is markedly higher than the 15.00 ± 0.8132 for methanol extracts of Paramatrema sp. Similarly, our study found that the EA extract of A. niger produced an inhibition area of 25 ± 0.5 mm in E. coli and 19 ± 0.4 mm in S. typhi. In contrast, previous study33 reported only 12.66 ± 0.58 mm and 15.66 ± 0.58 mm, respectively, for E. coli and S. typhi. When compared to the earlier study32 noted a zone of 24.00 ± 0.81 mm against E. coli with methanol extract of Paramatrema sp., whereas our study recorded a zone of 25 ± 0.5 mm with n-butyl alcohol extract of A. niger and F. oxysporum against E. coli.
The EA solvent extract of A. niger also indicated notable activity in Pseudomonas sp., with a zone of inhibition of 29 ± 0.6 mm, compared to the 24.33 ± 0.47 mm observed32 for methanol extract of Paramatrema sp. Our findings further showed that the EA extract of A. niger produced a zone of 28 ± 0.6 mm against Proteus sp., in contrast to the previous study which showed only 14.66 ± 0.47.32 The antimicrobial potential of A. niger against Bacillus sp. was particularly noteworthy, with a zone of inhibition of 38 ± 0.8 mm, which is significantly higher than the 32 mm reported34 for Penicillium sp. Same authors have reported a lower activity (15 mm) against Pseudomonas sp. for A. niger extracts compared to the 29 ± 0.6 mm observed in our study. Contrarily the earlier report35 exhibited the zones of 13 mm in E. coli and 14 mm in S. typhi against EA extracts of A. niger, the present study found larger inhibition zones of 25 ± 0.5 mm and 19 ± 0.4 mm, respectively. Furthermore, our study showed that the EA extract of F. oxysporum displayed zones of inhibition of 24 ± 0.5 mm against E. coli and 25 ± 0.5 mm against S. paratyphi, compared to the 11.7 ± 0.5 mm and 14.7 ± 0.4 mm respectively.36
The FTIR analysis in the current study revealed that the n-butyl crude extract of A. niger contained functional groups such as amides, alkanes, aldehydes, alkynes, aliphatic amines, and alkyl halides. These findings are consistent with the previous report37 identified similar functional groups in dry methanol extracts, including alkenes, aliphatic fluoro compounds, alcohols, ethers, and esters. Furthermore, the EA extract of A. niger of our study showed functional groups such as alcohols, alkanes, alkyl aryl ethers, and aliphatic ethers, corroborating the results38 observed similar functional groups in their FTIR analysis.
The present study successfully identified A. niger RAS 3 (PQ97245) and F. oxysporum RAS 2 (PQ97244) through 18S rRNA gene sequencing, confirming their identity with high accuracy. The antimicrobial activity in the crude extracts of these fungi was evaluated, revealing significant antibacterial and antifungal properties. Notably, the EA extract of A. niger confirmed remarkable activity in various pathogens, including a 38 ± 0.8 mm inhibition zone against B. subtilis and 35 ± 0.8 mm against Shigella sp. Similarly, the extracts of F. oxysporum also exhibited potent antimicrobial effects, particularly against E. coli and S. paratyphi. The FTIR spectrum also confirmed the availability of diverse chemical functional groups in the extracts, contributing to their antimicrobial efficacy. This study emphasizes the prospect of these fungal extracts as a source of bioactive compounds possessing comprehensive antimicrobial action against drug-resistant pathogens. These findings underscore the importance of exploring marine-derived fungi for novel antimicrobial agents, offering promising avenues for the development of new therapeutics. Further experiments may concern to isolate and characterize the explicit active metabolites obliged for the observed antimicrobial effects and evaluate their therapeutic potential.
ACKNOWLEDGMENTS
The authors thank the Dean and Director CAS in Marine Biology, Faculty of Marine Sciences and the Authorities of Annamalai University for providing facilities. Authors are also thankful the Head, Department of Chemistry, Annamalai University for FTIR analysis.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SA performed sample collection and isolation of the fungi. TR designed the study and performed compound analysis. VS identification and bioactive compound production. SN antimicorbial activity. MT supervised the study. SA wrote the manuscript. MT reviwed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Hassan N, Rafiq M, Hayat M, Shah AA, Hasan F. Psychrophilic and psychrotrophic fungi: a comprehensive review. Rev Env Sci Biotechnol. 2016;15:147-172.
Crossref - Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitas A. Hypersaline waters in salterns-natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol. 2000;32(3):235-240.
Crossref - Petrovic¡ U, Gunde-Cimerman N, Plemenitas¡ A. Cellular responses to environmental salinity in the halophilic black yeast Hortaea werneckii. Mol Microbiol. 2002;45(3):665-672.
Crossref - Ramesh T, Yamunadevi R, Sundaramanickam A, Thangaraj M, Kumaran R, Annadurai D. Biodiversity of fungi in Extreme marine environments. Fungi Bio-Prospects in Sustainable Agriculture Environment and Nano-Technology. Vol. 2. Extremophilic Fungi and Myco-mediated Environmental Management. 2020:P5-100.
Crossref - Koch AL. Genetic response of microbes to extreme challenges. J Theor Biol. 1993;160(1):1-21.
Crossref - Lu ZY, Lin ZJ, Wang WL, et al. Citrinin dimers from the halotolerant fungus Penicillium citrinum B-57. J Nat Pro. 2008;71(4):543-546.
Crossref - Mejanelle L, Lopez JF, Gunde-Cimerman N, Grimalt JO. Ergosterol biosynthesis in novel melanized fungi from hypersaline environments. J Lipid Res. 2001;42(3):352-358.
Crossref - Demain AL. Pharmaceutically active secondary metabolites of microorganisms. App Microbiol Biotechnol. 1999;52:455-463.
Crossref - Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep. 2001;18(4):448-459.
Crossref - Kalyanasundaram I, Nagamuthu J, Muthukumaraswamy S. Antimicrobial activity of endophytic fungi isolated and identified from salt marsh plant in Vellar Estuary. J Microbiol Antimicrobials. 2015;7(2):13-20.
Crossref - Lehmann L, Wagner J, Metzler M. Estrogenic and clastogenic potential of the mycotoxin alternariolin cultured mammalian cells. Food Chem Toxicol. 2006;44(3):398-408.
Crossref - Wang W, Wang Y, Tao H, Peng X, Liu P, Zhu W. Cerebrosides of the halotolerant fungus Alternaria raphani isolated from a sea salt field. J Nat Pro. 2009;72(9):1695-1698.
Crossref - Dewi RT, Minarti, Darmawan A, Mulyani H. Emodin, an anthraquinone from ethyl acetate extract of Aspergillus terreus koji. Proc Int Semin Chem. 2008:731-734.
- Jadulco R, Proksch P, Wray V, Sudarsono, Berg A, Grafe U. New macrolides and furan carboxylic acid derivative from the sponge-derived fungus Cladosporium herbarum. J Nat Pro. 2001;64(4):527-530.
Crossref - Shigemori H, Kasai Y, Komatsu K, Tsuda M, Mikami Y, Kobayashi JI. Sporiolides A and B, new cytotoxic twelve-membered macrolides from a marine-derived fungus Cladosporium species. Mar Drugs. 2004;2(4):164-169.
Crossref - Wood GM, Mann PJ, Lewis DF, Reid WJ, Moss MO. Studies on a toxic metabolite from the mould Wallemia. Food Addit Contam. 1990;7(1):69-77.
Crossref - Takahashi I, Maruta R, Ando K, et al. UCA1064-B, A new antitumor anti-biotic isolated from Wallemia sebi: production, isolation and structural determination. J Antibiot. 1993;46(8):1312-1314.
Crossref - Evidente A, Conti L, Altomare C, et al. Fusapyrone and deoxyfusapyrone, two antifungal a-Pyrones from Fusarium semitectum. Nat Toxins. 1994;2(1):4-13.
Crossref - Kobayashi H, Sunaga R, Furihata K, Morisaki N, Iwasaki S. Isolation and structures of an antifungal antibiotic, fusarielin A, and related compounds produced by a Fusarium sp. J Antibiot. 1995;48(1):42-52.
Crossref - Overy D, Calati K, Kahn JN, et al. Isolation and structure elucidation of parnafungins C and D, isoxazolidinone-containing antifungal natural products. Bioorg Med Chem Lett. 2009;19(4):1224-1227.
Crossref - Meca G, Soriano JM,Gaspari A, Ritieni A, Moretti A, Maes J. Antifungal effects of the bioactive compounds enniatins A, A1, B, B1. Toxicon. 2010;56(3):480-485.
Crossref - Renner MK, Jensen PR, Fenical W. Mangicols: structures and biosynthesis of a new class of sesterterpene polyols from a marine fungus of the genus Fusarium. J Org Chem. 2000;65(16):4843-4852.
Crossref - Jiang Z, Barret MO, Boyd KG, Adams DR, Boyd ASF, Burgess JG. JM47, a cyclic tetrapeptide HC-toxin analogue from a marine Fusarium species. Phytochem. 2002;60(1):33-38.
Crossref - Yi W, Jinkai Z, Peipei L, Wei W, Weiming Z. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Mar Drugs. 2011;9(8):1368-1378.
Crossref - Hoog GD, Guarro J, Gene JFMJ, Figueras MJ. Atlas of Clinical Fungi, Centraalbureau voor Schimmelcultures (CBS), Utrecht, Netherlands. ISBN: 90-70351-43-9
- Hunt J, Boddy L, Randerson PF, Rogers HJ. An evaluation of 18S rDNA approaches for the study of fungal diversity in grassland soils. Microbial Ecol. 2004;47:385-395.
Crossref - Gontia-Mishra I, Deshmukh D, Tripathi N, Bardiya-Bhurat K , Tantwai K , Tiwari S. Isolation, morphological and molecular characterization of phytate-hydrolysing fungi by 18S rDNA sequence analysis. Braz J Microbiol. 2013;44(1):317-323.
Crossref - Yang X, Sun JY, Guo JL, Weng XY. Identification and proteomic analysis of a novel gossypol-degrading fungal strain. J Sci Food Agri. 2012;92(4):943-951.
Crossref - Guiraud P, Bourmeyster H, Galzy P. Isolation and characterization of halophilic and halotolerant fungi from hypersaline soils. Mycol Res. 1995;99(2):203-206.
- Cantrell S A, Casillas-Martםnez L, Molina M. Characterization of fungi from hypersaline environments of solar salterns using morphological and molecular techniques. Mycol Res. 2006;110(8):962-970.
Crossref - Mandeel QA. Biodiversity of the genus Fusarium in saline soil habitats. J Basic Microbio. 2006;46(6):480-494.
Crossref - Chauhan R, Abraham J. In vitro antimicrobial potential of the lichen Parmotrema sp. extracts against various pathogens. Iran J Basic Med Sci. 2013;16(7):882.
- Rani R, Sharma D, Chaturvedi M, Yadav JP. Antibacterial activity of twenty different endophytic fungi isolated from Calotropis procera and time kill assay. Clin Microbiol. 2017;6(3):280.
Crossref - Al-Shaibani ABA, Al-Shakarchi FI, Ameen RS. Extraction and characterization of antibacterial compound from Aspergillus niger. Al-Nahrain. J Sci. 2013;16(4):167-174.
Crossref - Natarajan K, Rabiya S S, Sathish R. Screening for antibacterial compound in broth extracts of Aspergillus niger MTCC 2208. Drug Invention Today. 2010;2(8):385-386.
- Takahashi JA, de Castro MCM, Souza GG, et al. Isolation and screening of fungal species isolated from Brazilian cerrado soil for antibacterial activity against Escherichia coli, Staphylococcus aureus, Salmonella typhimurium, Streptococcus pyogenes and Listeria monocytogenes. JDe Mycologie Medicale. 2008;18(4):198-204.
Crossref - Hameed IH, Hamza LF, Kamal SA. Analysis of bioactive chemical compounds of Aspergillus niger by using gas chromatography-mass spectrometry and fourier-transform infrared spectroscopy. J Pharmacogn Phyto. 2015;7(8):132-163.
Crossref - Niazi SK, Basavarajappa DS, Kumaraswamy SH, et al. GC-MS based characterization, antibacterial, antifungal and anti-oncogenic activity of ethyl acetate extract of Aspergillus niger strain AK-6 isolated from rhizospheric soil. Curr Issues Mol Biol. 2023;45(5):3733-3756.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.