ISSN: 0973-7510
E-ISSN: 2581-690X
Since its sudden outbreak in December 2019 in Wuhan, A pandemic of SARS-CoV-2 has been announced. Vitamin C is a water-soluble vitamin with anti-oxidant and immunity-boosting properties. Vitamin C acts as a nutritional supplement profoundly impacting the immune response to the second or third wave of the coronavirus disease (COVID-19). Vitamin C efficacy as an adjuvant treatment for inflammation and symptoms associated with COVID-19 infection should be investigated further. This report sheds light on the available information on the current clinical trials and pharmacotherapy related to COVID-19. Information available on Pubmed, EMBASE, Scopus, Web of Science databases and EU clinical trials regarding the use of therapeutic agents in patients with COVID-19 was used to perform analysis. Data was taken from 18 clinical trials available in the U.S. National Library of Medicine. All trials that are active, completed, or in the process of recruiting are included in data. Because of majority of clinical trials are still ongoing, specific results and high-quality clinical evidence are lacking. Before being standardised for use, the protocol must undergo large randomised clinical studies using a variety of existing medications and potential therapies. The pivotal role played by vitamins C in maintaining our immune system, is quite apparent. This review is an attempt to summarize the available information regarding the use of vitamin C as an adjuvant therapy in COVID-19 patients.
Coronavirus Infection, Vitamin C (Ascorbic acid), Antiviral Agents, Severe Acute Respiratory Syndrome
The latest COVID-19 epidemic has required emergency treatment for a large number of patients. COVID-19 pandemic is disseminating like a storm, necessitating effective preventive and precautionary measures in addition to the development of a vaccine to fight the disease. Vitamin C is used to treat respiratory infections and is now being increasingly used as a therapeutic agent. It’s main role is that of an antioxidant and it improves the functioning of the immune system, similar to the phagocytes and lymphocytes that improve the response of T lymphocytes by augmenting interferon levels.1 Dr Fowler and colleagues, in 2014 concluded that administration of intravenous vitamin C to patients with sepsis could decrease the levels of C-reactive protein and thrombomodulin.2 The patients with acute respiratory distress syndrome (ARDS) who had sepsis were given intravenous administration of 200 mg/kg/day of vitamin C for 4 days. The results of this randomized controlled trial showed that in 28 days, the mortality was 30% relative to 46% in the placebo group (p = 0.03) and a hazard ratio (HR) of 0.55 (95% CI, 0.33–0.90, p = 0.01) was also found.3 This review concluded that the intervention of vitamin C, which has a high efficacy, low cost, and a potential for rapid healing, can be used to treat severe respiratory infections as seen in COVID-19.4 The spectrum of the disease ranges from asymptomatic individuals to severe respiratory tract infection leading to system failure and finally death. This is partially due to a lack of an effective antiviral therapy and therefore there is only symptomatic treatment. Currently, vitamin C has been of interest to the physician to fight against coronavirus as it a play a pivotal role in maintaining immunity and has anti-inflammatory properties.5-7 When analyzing the current COVID-19 situation in India and worldwide, it seemed quite obvious to address some aspects of the ways to boost up immunity via vitamin C supplementation, which is an effective immune-modulator in severe acute respiratory syndrome associated with coronavirus 2 (SARS-CoV-2) or second wave COVID-19.8 Several animal studies conducted on vitamin C and infection, concluded potential protective role of vitamin C in infection.9 Various reports available in literature including increase in resistance generation noticed in chick embryo tracheal organ cultures to avian coronavirus infection,10,11 protection of broiler chicks against the avian coronavirus and vitamin C induced downregulation of proinflammatory genes, enhanced epithelial barrier and increased rate of alveolar fluid clearance among septic mice suffering from acute respiratory distress syndrome(ARDS),12 etc. An increase in mice lung pathology was also observed due to deficiency of vitamin C. Outcomes of various human trials reported shortening in rate of infection caused by virus with regular administration of nearly 1 gm/day of vitamin C.9,13 The effect of vitamin C on different new strains of respiratory viruses seems not very specific as the types of viruses varied during trials. It is likely that there may be some impact of the vitamin on various new strains of virus as well.
Biochemistry of Vitamin C
Professor (Dr.) Albert Szent Goyrgi was awarded the Nobel Prize for his work in separating vitamin C molecule from red peppers and declaring its role in the gum disease- Scurvy.9 Vitamin C serves as an essential micronutrient in our body, is a potent antioxidant and acts as a coenzyme in various enzyme regulated biochemical reactions.6
The plethora of functions performed by vitamin C is due to its ability to donate electrons. Apart from contributing to the enhancement of immune system and support to various cellular activities, it also interferes with the entry of pathogens by destroying the oxidant species on the skin.14 The proliferation of T and B cells is also enhanced by vitamin C by its gene-regulating effects.14 It is also involved in phagocytosis, chemotaxis and production of antioxidant species to check the entry of microbes into the body.15,16
The vitamin is abundantly present in phagocytes such as neutrophils, macrophages and it potentiates their bactericidal role.15 It is also reported to have a role in apoptosis and removal of dead immune cells and debris from the site of immune response.14 It is a co-factor in collagen formation and catecholamine synthesis, and is a modulator of the immune system.15 It has been documented that vitamin C administration improves haemodynamics, organ work, and may also improve endurance in physically sick patients. As stated earlier, apart from its anti-inflammatory properties, vitamin C inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB), which are responsible for the activation of several pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), interleukin 6 (IL-6) and interleukin 8 (IL-8).14 Therefore, vitamin C has a potential anti-inflammatory activity and can be used in conditions like Acne Vulgaris and Rosacea.17 It can promote wound healing and prevent post-inflammatory hyperpigmentation. As expressed before, vitamin C hinders NFkB, which is responsible for the initiation of the various cytokines, for example, TNF-a, IL-1, IL-6 and IL-8. Therefore, vitamin C has a potential calming action and can be utilized in conditions like skin inflammation and Rosacea. It can advance recuperation of injury and forestall post-fiery hyperpigmentation.18,19 Excessive production of oxidative stress is the key to severe lung injury among COVID-19 infected patients. Disturbances in the immune system generate huge amount of free radicals responsible for wounding patients.20,21 Vitamin C being antioxidant works by scavenging free radicals from the site protecting the organ. Vitamin is very abundantly available in white blood cells and are well reported to improve phagocytosis, T cell maturation, cytokine production acting as safeguards against virus menace. While recently Deborah et al using Docking & molecular simulation procedures reported the binding of various other vitamins and steroid ligands to the newly discovered fatty acid binding sites in COVID-19 spike proteins.18 Simulations studies have depicted that dexamethasone and vitamin D as ligand facilitate the locked conformation of spike proteins thereby inhibiting the ace receptor binding. Docking experiments also suggest fat soluble vitamins (vitamin D, K and A) as very potential ligands for fatty acid binding sites on spike proteins.22 Vitamin D 3 metabolite calcitriol has been found to diminish viral titres in animal models and nasal epithelium cell line of humans. Obesity is one well known risk factors for COVID-19 infection developing the severity to the level of death. Vitamin D level is known to be sequestered in adipose tissue mass among obese subjects leading to deficiency.22 In comparison to these fat soluble vitamins, entirely different approach has been reported by vitamin C as discussed above. Preventive role of vitamin C is less clear, being reported to pose its T and B cell differentiation and proliferation effect through its gene regulatory property.17
Vitamin C and COVID-19
There is a wealth of literature available showing the role of quality nutrition in maintaining the immune system. The important role played by vitamin C in boosting immunity is well known and more effective in SARS-COV-2 or COVID-19.23 To minimize infections, correction of nutritional regimen has been of utmost importance. A nutritional focus on the immune system could help minimize the impact of many kinds of infections including COVID-19. The prevailing global situation with COVID-19 and the number of people dying due to the respiratory complications is forcing all the public health officials to develop some strategy focusing on nutrition. In the current pandemic caused by coronavirus, vitamin C has been of interest to the research stalwarts in the fight against the disease. All over the world, several clinical trials are going on vitamin C to assess its impact on COVID-19.17-19 In one such study conducted in China, COVID-19 critical patients on ventilators were tried with intravenous large doses of vitamin C.23 The use of high dose of Vitamin C has numerous pharmacological characteristics: antiviral, anti-oxidant, anti-inflammatory and immunomodulatory effects, and potential therapeutically good management of COVID-19. There were no reported adverse reactions with the short-term use of high dose of vitamin C. The current ongoing clinical trials testing the effect of vitamin C in management of COVID-19 show positive results.24
Vitamin C Related to COVID-19 Infections
Researchers around the globe are recommending large doses of this vitamin C in routine lifestyle. An Australian study was done in order to assess the effect of vitamin C in COVID-19 patients, but they did not find any correlation of vitamin C among SARS-COV-2 patients.24 The United States are in the process of trying a combination of various nutrients including vitamin D, zinc and hydroxychloroquine along with vitamin C for COVID-19 prevention.25 Current health strategies include: social distancing, hand washing, other hygienic practices, and vaccination along with a complementary strategy in the form of nutritional supplement to boost immunity. In one non-randomized clinical trials among 252 students, 85% students experienced reduction of influenza like symptoms with high doses of vitamin C.26 Another interventional randomized prospective clinical trial is going on among 140 participants in Wuhan, China to see the effect of vitamin C infusion for treating severe COVID-19 infected pneumonia. Estimated completion time of the study is September 2020 and the results have not been published yet.27 It is reported in the literature that severity and duration of common cold symptoms have been reduced by taking large doses of vitamin C.26 Various studies conducted on critical COVID-19 patients with vitamin c supplementation have given mixed results on the mortality rate, their intensive care unit (ICU) stay and on ventilator stay. Stature of vitamin C as an adjuvant healing agent is still in dilemma therefore large clinical studies are needed to be done in the direction so as to establish its therapeutic role.28,29 Total 18 clinical trials have used adjuvant therapy of vitamin C in the treatment of COVID-19 that were conducted in New York, Australia, United States, Canada, Italy, Saudi Arabia, Iran and Portland.
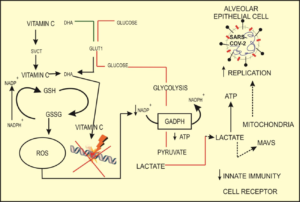
Intravenous administration of high doses of vitamin C in sepsis and septic shock acts as a pro-oxidant for immune cells, dependent on glycolysis for bioenergetic function, but as an antioxidant for lung epithelial cells, which produce ATP by oxidative phosphorylation in the mitochondria. Furthermore, vitamin C can inhibit the production of lactate protecting alveolar type II epithelial cells. Intravenous administration of high doses of vitamin C may be beneficial for the patient with COVID-19 if the decision is made at the right time i.e. as early as possible after the report of respiratory distress, based on clinical scores and biomarkers of inflammation and oxidative stress. Activated macrophages lead to an elevated level of lactate, which is moved to alveolar epithelial cells of type II, where it is utilized to produce ATP and inhibits the mitochondrial signaling loci of viral proteins. Macrophage-discharged lactate diminishes the production of type I Interferons (IFN), decreasing viral clearance as shown in Figure 1.
Figure 1. Mechanism of action of high-dose Vitamin C in immune effector cells (Figure modified from Adnan Erol et al.)26
Search Strategy and Selected Criteria
This integrative literature review was conducted in Medical Literature Analysis and Retrieval System, National Library of Medicine (PubMed), Cochrane Library, Elsevier and China National Knowledge Infrastructure (CNKI). The databases were searched from their earliest records through August 2020 using the following keywords: COVID-19 [Coronavirus disease 2019], Second wave COVID-19 [second wave Coronavirus disease 2019], SARS-CoV-2 [severe acute respiratory syndrome coronavirus 2], vitamin C, and ascorbic acid. All the records were selected and screened by two independent reviewers, and a third reviewer was consulted when there was any disagreement. This search included articles published up until 11th February 2021. The review was conducted according to the guidelines for Preferred Reporting, items for Systematic Reviews and Meta-Analyses (PRISMA).30,31
Search Results
The 3 Systemic reviews (SRs) and 18 clinical trials (CT) included assessed the effects of therapeutics interventions (vitamin C) for participants with positive COVID-19. After the selection process, CT & SRs were found to fulfil our inclusion criteria and were included in the analysis.
Study Selection
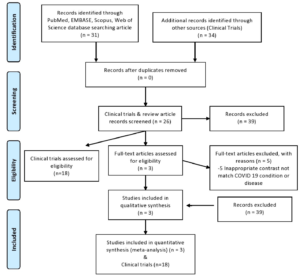
Total 30 articles and 39 clinical trials were retrieved. We identified and screened 18 clinical trials & 25 articles, we excluded 39 unqualified trials on the basis of titles and abstracts, and then 18 trials were excluded because of inappropriate content, while 30 were excluded for the assessment of inappropriate content because it did not match with COVID-19 disease. Finally, 3 articles,23-25,29 fulfilled our eligibility criteria after manual search and a review of full text manuscripts. The study selection procedure is outlined in [Figure 2].
Trials Search Outcomes
Up to 11 February 2021, 18 clinical trials from the National Institutes of Health, the U.S.A Food and Drug Administration (FDA), and Industry (Clinical Trials.gov) clinical trials data were retrieved from a total of 18 clinical trials of first wave & second wave of COVID-19. COVID-19 disease (SARS-COV-2), Pneumonia, acute lung injury are included in clinical trials. Subsequent screening of headings, abstracts and suspended or discontinued clinical trials were removed; a total of 17 used vitamin C or ascorbic acid alone or with other combinations; 7 used hydroxychloroquine, 4 used zinc, 3 used azithromycin and vitamin D3 single one used vitamin B12, methylene blue, vitamin C, N-acetyl cysteine; folic acid; zinc gluconate; oral nutrition supplement (ONS) enriched in eicosapentaenoic acid, gamma-linolenic acid and antioxidants. The remaining studies used different types of molecules or interventions.
Above all, most of the trials have cleared ethical approval and total 18 trials of these studies are still in the recruitment phase, 2 trials are not recruiting yet, 1 trial has shown status of suspended enrolling by invitation and 1 withdrawal. None of the clinical trials had yet been completed. In the present study, we included 18 trials, in terms of clinical trial phases, 3 trials are in the post marketing surveillance (phase-4), 4 trial are is in the testing of the drug on patients to assess efficacy, effectiveness and safety (Phase-3), 10 are testing of the drug on patients to assess efficacy and side effects (Phase-2), 2 are in testing of the drug on healthy volunteers for safety, involves testing multiple doses (dose ranging) in Phase-1, 2 are in not applicable’ status and there are trials in phase-4 drug with supplements vitamin C, hydroxychloroquine and oral nutrition supplement (ONS) enriched with eicosapentaenoic acid, gamma-linolenic acid and antioxidants. Post-marketing studies, which are conducted after a treatment is approved for use by the Food and Drug Administration, provide additional information including the treatment or drug’s risks, benefits, and best use. Summaries of the various clinical trials studies are presented in Table.
Table (1):
Summary of different combinational approaches adjuvant therapy of Vitamin C used for ongoing clinical trials in the Treatment of COVID-19 Searched on https://clinicaltrials.gov/ As of February 11, 2021.
S.No |
NCT Number |
Title |
Recruitment Status |
Interventions |
Sponsor |
Age |
Phase |
Condition of disease |
Enrollment |
Study type |
Start date |
Primary completion date |
Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1. |
NCT 04363216 |
Pharmacologic Ascorbic Acid as an Activator of Lymphocyte Signaling for COVID-19 Treatment |
Not yet recruiting |
Ascorbic Acid |
Thomas Jefferson University |
18 Years and older |
Phase 2 |
COVID-19 |
66 |
Interventional |
May 2020 |
May 2021 |
United States |
2. |
NCT04347889 |
Use of Ascorbic Acid in Patients With COVID-19 |
Not yet recruiting |
Vitamin C |
University of Palermo |
Child, Adult, Older Adult |
Not Applicable |
COVID-19 |
500 |
Interventional |
March
2020 |
March
2021 |
Italy |
3. |
NCT04347889 |
Preventing COVID-19 in Healthcare Workers With HCQ: A RCT |
Not yet recruiting |
Hydroxychloroquine Vitamin C |
Stony Brook University |
18 Years and older (Adult, Older Adult) |
Phase 2 |
COVID-19 Pneumonia |
1212 |
Interventional |
April 20, 2020 |
December 30, 2020 |
New York |
4. |
NCT04395768 |
International ALLIANCE Study of Therapies to Prevent Progression of COVID-19 |
Not yet recruiting |
Vitamin C Hydroxychloroquine Azithromycin Zinc Vitamin D3 Vitamin B12 |
National Institute of Integrative Medicine, |
18 Years and older (Adult, Older Adult) |
Phase 2 |
COVID-19 |
200 |
Interventional |
May 25, 2020 |
May 31, 2021 |
Australia |
5. |
NCT04357782 |
Administration of Intravenous Vitamin Cin Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation (AVoCaDO) |
Recruiting |
L-ascorbic acid |
Hunter Holmes Mcguire Veteran Affairs Medical Center |
18 Years to 99 Years (Adult, Older Adult) |
Phase 1 |
COVID-19
Hypoxia |
20 |
Interventional |
April 16, 2020 |
June 1, 2020 |
Virginia |
6. |
NCT04344184 |
Early Infusion of Vitamin C for Treatment of Novel COVID-19 Acute Lung Injury (EVICT-CORONA-ALI) |
Not yet recruiting |
L-ascorbic acid |
Virginia Commonwealth University |
18 Years and older (Adult, Older Adult) |
Phase 2 |
COVID-19
Acute Lung Injury |
200 |
Interventional |
June 2020 |
May 2021 |
United States |
7. |
NCT04401150 |
Lessening Organ Dysfunction With Vitamin C- COVID-19 (LOVIT-COVID) |
Not yet recruiting |
Vitamin acid |
Université de Sherbrooke |
18 Years and older (Adult, Older Adult) |
Phase 3 |
COVID-19 |
800 |
Interventional |
June 2020 |
November 2020 |
Canada |
8. a |
NCT04335084 |
A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection (HELPCOVID-19) |
Not yet recruiting |
Vitamin C Hydroxychloroquine Vitamin D3 |
ProgenaBiome |
18 Years and older (Adult, Older Adult) |
Phase 2 |
COVID-19
SARS-CoV-2 |
600 |
Interventional |
May 2020 |
May 2021 |
United States |
9. |
NCT04370288 |
Clinical Application of Methylene Blue for Treatment of COVID-19 Patients (COVID-19) |
Recruiting |
Methylene blue, vitamin C, N-acetyl cysteine |
Mashhad University of Medical Sciences |
18 Years to 90 Years (Adult, Older Adult) |
Phase 1 |
COVID-19 |
20 |
Interventional |
April 19, 2020 |
September 20, 2020 |
Iran |
10. |
NCT04334967 |
Hydroxychloroquine in Patients With Newly Diagnosed COVID-19 Compared to Standard of Care |
Suspended |
Vitamin C Hydroxychloroquine |
Providence Cancer Center, Earle A. Chiles Research Institute |
45 Years and older (Adult, Older Adult) |
Phase 4 |
COVID-19
SARS-CoV-2 |
13 |
Interventional |
March 30, 2020 |
May 27, 2020 |
Portland |
11. |
NCT04328961 |
Hydroxychloroquine for COVID-19 Post-exposure Prophylaxis (PEP) |
Recruiting |
Vitamin C Hydroxychloroquine |
University of Washington |
18 Years to 80 Years (Adult, Older Adult) |
Phase 3
Phase 2 |
COVID-19
SARS-CoV-2 |
2000 |
Interventional |
March 31, 2020 |
September 30, 2020 |
United States |
12. |
NCT04264533 |
Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia |
Recruiting |
Vitamin C |
ZhiYong Peng |
18 Years and older (Adult, Older Adult) |
Phase 2 |
Pneumonia, Viral |
140 |
Interventional |
February 14, 2020 |
September 30, 2020 |
China |
13. |
NCT04354428 |
Treatment for COVID-19 in High-Risk Adult Outpatients |
Recruiting |
Ascorbic Acid Hydroxychloroquine Sulfate Azithromycin Folic Acid |
University of Washington |
18 Years to 80 Years (Adult, Older Adult) |
Phase 2
Phase 3 |
COVID-19
SARS-CoV-2 |
630 |
Interventional |
April 16, 2020 |
July 2020 |
United States |
14. |
NCT04334512 |
A Study of Quintuple Therapy to Treat COVID-19 Infection (HAZDpaC) |
Not yet recruiting |
Hydroxychloroquine Azithromycin Vitamin C Vitamin D Zinc |
ProgenaBiome |
18 Years and older (Adult, Older Adult) |
Phase 2 |
COVID-19
SARS-CoV-2 |
600 |
Interventional |
May 2020 |
May 2021 |
United States |
15. |
NCT04342728 |
Coronavirus 2019 (COVID-19)- Using Ascorbic Acid and Zinc Supplementation (COVIDAtoZ) |
Enrolling by invitation |
Ascorbic Acid Zinc Gluconate Zinc |
The Cleveland Clinic |
18 Years and older (Adult, Older Adult) |
Not Applicable |
COVID-19 |
520 |
Interventional |
April 8, 2020 |
December 30, 2020 |
United States |
16. |
NCT04323228 |
Anti-inflammatory/Antioxidant Oral Nutrition Supplementation in COVID-19 (ONSCOVID19) |
Not yet recruiting |
Oral nutrition supplement (ONS) enriched in eicosapentaenoic acid, gamma-linolenic acid and antioxidants |
King Saud University |
18 Years to 65 Years (Adult, Older Adult) |
Phase 4 |
COVID-19 |
30 |
Interventional |
July 1, 2020 |
October 1, 2020 |
Saudi Arabia |
17. |
NCT03680274 |
Lessening Organ Dysfunction With Vitamin C (LOVIT) |
Recruiting |
Vitamin C |
Université de Sherbrooke,
Lotte & John Hecht Memorial Foundation |
i18 Years and older (Adult, Older Adult) |
Phase 3 |
COVID-19 |
800 |
Interventional |
November 8, 2018 |
December 31, 2020 |
Canada |
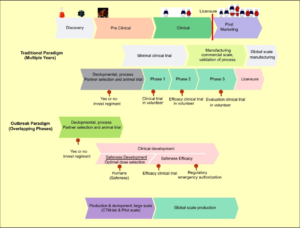
As a result of the critical circumstances in the treatment, anticipation and control of the COVID-19 infection, an inside-out exploration and development of effective methods of intervention for COVID-19 to facilitate disease control is fundamental. Since the COVID-19 pandemic, a few specialists worldwide have quickly completed vaccine development for A paradigm shift for Traditional Vaccine Development and Development of Outbreak paradigm COVID-19 [Figure 3].
The current enrolled clinical trials of this examination aim to provide an appropriate status to develop the Immunomodulator vitamin C as an adjuvant therapy SARS-COV-2 in the first and second wave of Coronavirus Diseases 2019 (COVID-19). During the time of an epidemic when human-to-human transmission is built up and announced case numbers are rising exponentially, presently forecasting are of urgent significance for general wellbeing and control.
Still poorly understood, there is no recognized effective treatment for SARS-CoV-2. The genetic material of SARS-CoV-2 virus comprise a segment of ribonucleic corrosive (RNA) inside a circular protein capsule that is covered in spikes. The viral spike (S) protein, has a important function in the viral infectivity, although, different examinations seriously focused around other viral proteins, for example, the nucleocapsid (N) protein, envelope (E) protein, and non-structural protein 16 (NSP16) has likewise been discussed. In any case, the utility of live attenuated vaccines is restricted right now of the risks of reversion or repair. Most of the reports demonstrate the intervention of clinical trials access on 11 February 2021, 18 clinical trials, out of which 8 are in current status are 4 are recruiting preliminaries; Davis., reported vitamin C 1000 mg orally daily is proven COVID-19 intervention.31-34 4 preliminary in stage 3 phase Ahmed Reported vitamin C 50 mg/kg of weight administered intravenously every 6 hours for 96 hours (16 doses), 7 preliminary in stage 2 phase, as shown in Table. Various investigations demonstrated that ascorbic acid (vitamin C) decidedly influences the turn of events and development of T-lymphocytes, specifically NK (natural Killer) cells engaged with the immune reaction to viral agents.35-37 It also contributes to the inhibition of ROS production and to the remodulation of the cytokine network typical of systemic inflammatory syndrome.38-40 Corrao et al.,41 refers the observer to a current continuing clinical trail have demonstrated the effectiveness of vitamin C administration in terms of reducing mortality, in patients with sepsis hospitalized in intensive care wards.
Past examination has demonstrated that high doses of intravenous vitamin C (HDIVC) may help patients with sepsis, adverse lung injury (ALI), and the intense respiratory distress condition (ARDS). In any case, it isn’t known whether early intervention of HDIVC could forestall development to ARDS. Davis, Current examination, phase IV has reported HDIVC would diminish the danger of respiratory distress requiring mechanical ventilation and improvement of ARDS alongside decrease in supplemental oxygen demand and inflammatory markers.31 There is a Phase II interventional study testing whether treatment with hydroxychloroquine, Vitamin C, Vitamin D, and Zinc can prevent symptoms of COVID-19.36-40
Previous studies have reported is 41 patients with SARI at first demonstrated that 13 patients were moved into the ICU, of which 11 (85%) had ARDS and 3 (23%) had stun. Of these, 10 (77%) required mechanical ventilation backing, and 2 (15%) required ECMO support of the over 13 patients, 5 (38%) in the long run passed on and 7 (38%) were moved out of the ICU. Viral pneumonia is a dangerous condition with a poor clinical prognosis. For most viral infection, there is a lack of effective targeted antiviral medications, and symptomatic supportive treatment is as yet the current main treatment. Peng et al., has reported phase 2 stage, vitamin C infusion can help improve the prognosis of patients with treatment of severe 2019-nCoV infected pneumonia administered with an intervention of 2 gram Vitamin C with sterile water for injection; total volume: 50ml. 12ml/hour.30-33 Biome42 reported a randomized, double-blind, placebo-controlled phase II interventional study that tests the efficacy of quintuple therapy (Hydroxychloroquine, Azithromycin, Vitamin C, Vitamin D, and Zinc) in the treatment of patients with COVID-19 infection. Continuous detrimental changes in COVID-19 genome through vigorous mutations, understanding the severity, effectiveness of measures, immune responses, newer diagnosing ways and knowing other relevant characteristics of VOC have become big challenge. Enhancement of surveillance and sequencing techniques are fewer strategies available to better understand various variants. Definitely continuous revaluation of vitamin C potential as preventive immunomodulator is required against these VOCs through well designed clinical trials.
Keeping in mind the current situation of second wave of COVID-19 pandemic, Supplementation of vitamin C is required to strengthen immunity as adjuvant therapeutic substances for severely infected or asymptomatic subjects. Development of any new antiviral drug or an efficacious vaccine is almost done. Countries all over the world are working hard to explore some treatment or immunization. Vitamin C and other antioxidants are among the currently available agents to mitigate COVID-19 associated acute respiratory distress syndrome (ARDS). Although the findings of this trial will be too late for the many thousands of people currently infected with the virus. Though outcome of studies will nevertheless provide valuable information regarding its potential to mitigate a number of symptoms and will be of utmost concern in such types of future viral outbreaks. Obviously, well-designed clinical studies are absolutely needed to develop standard protocols for bedside use. Vitamin C intake appears to be able to both; prevent and treat respiratory and systemic infections. In many treatment plans, significantly higher doses of vitamin C have been suggested to treat established infections. Looking at the beneficial effects of vitamin C, the public health officials should also set a clear guidelines for nutritional recommendations along with disseminating knowledge to the masses about handwashing, use of masks and other covid-19 protocols.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PK, PS and MKV conceptualised the research and performed the supervision. PK, MKV and RS designed the research. MKV, RS, RA, SPS, AJ, NS and OP performed the literature survey. MKV, RS and AHS collected the data. MKV, RA, SPS, AJ, NS and OP collected the materials, analysed and interpreted the data. PK, MKV, RS, RA, SPS, AJ, OP, NS and PS wrote the manuscript. PK, MKV PV, AHS, SG, PS performed the critical review. PK, MKV, PV, AHS, SG and PS edited the article. PK and PS approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hemila H. Vitamin C intake and susceptibility to the common cold. Br J Nutr. 1997;77(1):59-72.
Crossref - Fowler 3rd AA, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32.
Crossref - Fowler AA 3rd, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322(13):1261-1270.
Crossref - Carr AC, Rowe S. The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients. 2020;12(11):3286.
Crossref - Najmeh Zare-Feizabadi, Amiri-Tehranizadeh Z, Sharifi-Rad A, et al Determining the Interaction Behavior of Calf Thymus DNA with Anastrozole in the Presence of Histone H1: Spectroscopies and Cell Viability of MCF-7 Cell Line Investigations. DNA Cell Biol. 2021;40(8):1039-1051.
Crossref - Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980.
Crossref - Figueroa-Mendez R, Rivas-Arancibia S. Vitamin Cin Health and Disease: It’s Role in the Metabolism of Cells and Redox State in the Brain. Front Physiol. 2015;6:397.
- Hemila H. Vitamin Cand SARS coronavirus. J Antimicrob Chemother. 2003;52(6):1049-1050.
Crossref - Uddin MS, Millat MS, Baral PK, et al. The protective role of vitamin C in the management of COVID-19: A Review. J Egypt Public Health Assoc. 2021;96(1):33.
Crossref - Jones BV, Hennion RM. The Preparation of Chicken Tracheal Organ Cultures for Virus Isolation, Propagation, and Titration. SARS- and Other Coronaviruses. 2008;454:103-107.
Crossref - Holmes HC. The resistance to reinfection of tracheal organ cultures from chickens previously infected with avian infections bronchitis virus. Res Ven Sci. 1978;25(1):122-124.
Crossref - Holford P, Carr AC, Jovic TH, et al. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020;12(12):3760.
Crossref - Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009;157(7):1097-1110.
- Farris PK. Cosmetical Vitamins: Vitamin C. In: Draelos ZD, Dover JS, Alam M, editors. Cosmeceuticals. Procedures in Cosmetic Dermatology. 2nd ed. New York: Saunders Elsevier; 2009: 51-56.
- Sharifi-Rad A, Mehrzad J, Darroudi M, Saberi MR, Chamani J. Oil-in-water nanoemulsions comprising Berberine in olive oil: biological activities, binding mechanisms to human serum albumin or holo-transferrin and QMMD simulations. J Biomol Struct Dyn. 2021;39(3):1029-1043.
Crossref - Sadeghzadeh F, Entezari AA, Behzadian K, et al. Characterizing the binding of angiotensin convertin enzyme 1 inhibitory peptide to human hemoglobin: influence of electromagnetic fields. Protein Pept Lett. 2020;27(10):1007-1021.
Crossref - Bacharach Al. Biochemical role of vitamin C. Nature. 1952;169:1107-1108.
Crossref - Strohle A, Hahn A. Vitamin Cund Immunfunktion. Med Monatsschr Pharm. 2009;32(2):49-56.
- Maggini S, Pierre A, Calder PC. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients. 2018;10(10):1531.
Crossref - De Tullio MC. Beyond the antioxidant: the double life of vitamin C. Subcell Biochem. 2012;56:49-65.
Crossref - Dareini M, Tehranizadeh ZA, Marjani N, et al. A novel view of the separate and simultaneous binding effects of docetaxel and anastrozole with calf thymus DNA: Experimental and in silico approaches. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2020;228:117528.
Crossref - Shoemark DK, Colenso CK, Toelzer C, et al. Molecular Simulations suggest Vitamins, Retinoids and Steroids as Ligands of the Free Fatty Acid Pocket of the SARS-CoV-2 Spike Protein. Angew Chem Int Ed Engl. 2021;60(13):7098-7110.
Crossref - Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for Vitamin Cadministration in severe sepsis and septic shock? Crit Care. 2015;19:418.
Crossref - Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24(4):248-255.
Crossref - Traikovich SS. Use of topical ascorbic acid and its effects on photodamaged skin topography. Arch Otolaryngol Head Neck Surg. 1999;125(10):1091-1098.
Crossref - National Cancer Institute. High-dose Vitamin C PDQ® Health professional version. Available from: https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq. Accessed in 2020 (Aug 17).
- Carr AC. A new clinical trial to test high-dose Vitamin C in patients with COVID-19. Crit Care. 2020; 24(1):133.
Crossref - Boretti A, Banik BK. Intravenous Vitamin C for reduction of cytokines storm in Acute Respiratory Distress Syndrome. Pharma Nutrition. 2020;12:100190.
Crossref - Bharara A, Grossman C, Grinnan D, et al. Intravenous Vitamin C Administered as Adjunctive Therapy for Recurrent Acute Respiratory Distress Syndrome. Case Rep Crit Care. 2016;2016:8560871.
Crossref - Shanghai Medical Association. Expert consensus on comprehensive treatment of COVID-19 in Shanghai. https://mp.weixin.qq.com/s? biz=MzA3Nzk5Mzc5MQ== &mid=12
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.