ISSN: 0973-7510
E-ISSN: 2581-690X
The complex interplay between H. pylori and autophagy was elucidated in gastritis in term of host defense and survival of microbe. However, H. pylori frequently succeed in their survival and develops more aggressive tissue damage by different ways. A total of 80 gastritis patients undergoing gastroscopy during September, 2013 to August, 2014. Formalin fixed paraffin embedded gastric tissue were prepared for immunohistochemistry evaluation of ATG16L1, LC3C and vacA protein expression. Rapid urease test was used to discriminate between H. pylori or none H. pylori gastritis. Xenophagy index was elevated among both urease and IgG positive cases (29.86% and 27.13%) compared with negative cases (13.43% and 17.6% respectively (p<0.001). vacA have been found in 62.5% of urease positive cases and 55.88% of IgG positive cases. The higher xenophagy index score found to be associated with vacA positive cases (p=0.002). Xenophagy index to be associated with chronic H. pylori infection and this might be related to vacA positivity.
H. pylori, Xenophagy, gastritis
The Gram-negative bacteria “Helicobacter pylori” being one of the most common bacterial infections worldwide1. H. pylori possess wide range of tissue pathology like: atrophic gastritis, peptic ulcer and neoplasia2. Chronic gastritis represents the most frequent gastropathy depending on the intensity and persistence of bacterium3. H. pylori infection activates an inflammatory response in its host which leads to the recruitment of macrophages, neutrophils, and lymphocytes to the gastric tissue4. H. pylori can efficiently inhibit its own uptake by these professional phagocytes. This antiphagocytic phenotype which constitute the majority of these bacterium can reside within the mucosa or with the epithelium5-8, macrophages9, and dendritic cells (DCs)10.
Xenophagy is a specific form of autophagy that targets intracellular pathogens as cargo and makes critical contributions to both the innate and adaptive immune response to infection11. In general, the induction of autophagy by pathogenic bacteria is triggered by virulence factors or bacterial components12. Vacuolating cytotoxin-A, VacA, one of the most important virulence factors produced by H. pylori causing massive cytoplasmic vacuolation in cultured cells13. In vitro studies have been reported that vacA can modulate host cell activities including autophagy14. To the best of our knowledge, no clinical data have reported an association between the presence of vacA and xenophagy markers. This study aims to investigate the possible association between the presence of vacA toxin and “xenophagy index” LC3C/ATG16L1 ratio determined by immunohistochemistry.
This cross-sectional analytical study conducted in the department Microbiology & Immunology and Pathology Department, College of Medicine, AL-Nahrain University during the period of September, 2013 to August, 2014. A total of 80 patients attending gastroenterology units in Gastroenterology and Hepatology Teaching Hospital and Al-Emamain Al-Kadhemain Medical City suffering from dyspepsia and requiring upper gastrointestinal endoscopy were included. The study was approved by College of Medicine – AL-Nahrain University institutional review board, and written informed consent was obtained from each patient.
From each patient, blood sample was taken and serum was separated and stored in – 20°C for serology (anti H. pylori IgM and IgG antibodies) according manufacturer instruction. Two endoscopic biopsies were taken from the lesion of stomach, one of these biopsies was processed as soon as possible for rapid urease test and the second one preserved in neutral buffered formalin for routine Hematoxylin and eosin stain and immunohistochemistry.
The tissue sections were stained by ATG16L1 (bs-4007R) and LC3C (bs-20416R) polyclonal antibodies purchased from Bioss ® and visualized by LSAB System-HRP Dako (K0679). The immunoreactivity was evaluated by calculating the percentage of positive protein expression. The relative xenophagy index were calculated by dividing LC3C over ATG16L1 percentage of expression, then scoring system were expressed according to decimal intervals as the follow; score 1: £0.1, score 2: 0.11-0.2, score 3: 0.21-0.3, score 4: 0.31-0.4 and so on.
Statistical analysis
The statistical analysis was done by using Graphpad PRISM ® version 6. Crosstab model used to estimate association of allelic variant among study groups and relative risk (RR) and corresponding 95% CIs were estimated. ANOVA test were used to compare means of numerical variables between more than two groups.
The study subjects comprise 80 patients their mean age was 40.31 and ranged from 15-69 years old. Female were 48 patients, 21 were smokers, only 5 were alcohol drinkers, 64 patients presented with abdominal pain, 44 with nausea, 37 with vomiting. According to rapid urease test, 40 patients were H. pylori positive and 40 patients were considered as negative.
The serological evaluation of serum anti-H. pylori IgM antibody were detected in 6 patients, while, 34 patients were IgG positive.
LC3C (Xenophagy marker) is differentially expressed in gastritis
The gastric epithelial cells were evaluated for expression of LC3C among gastritis patients. According to rapid urease results, LC3C WAS highly expressed in tissue biopsies with positive rapid urease 23.78±7.49 than those negative group 8.55±3.8 (p<0.001), while, none significant difference of LC3C molecule between patients whom IgM positive 16.83±10.55 and those with IgM negative 16.11±9.67 (p=0.861). interestingly, IgG positive cases have had statistically significant (p<0.001) LC3C expression in gastric epithelium (20.62±9.16) compared with those IgG negative (12.87±8.75). Autophagy related protein (ATG16L1) were significantly higher in expressed in epithelial cells with positive urease test (79.47±5.94) compared with urease negative group (64.73±11.35).
While, none significant difference in the mean of ATG16L1 expression between IgM positive group (69.33±16.65) and IgM negative group (68.57±11.2), also, between IgG positive group (73.32±10.71) and IgG negative group (69.46±12.18).
Table (1):
Patients characteristics included in the study.
Value |
|
|---|---|
Age (years) mean±SD Median (Min-Max) |
40.31±13.85 39 (15-69) |
Gender male (%) |
32 (40) |
Smoker (%) |
21 (26.2) |
Drinker (%) |
5 (6.2) |
Abdominal pain (%) |
64 (80) |
Nausea (%) |
44 (55) |
Vomiting (%) |
37 (46.2) |
Rapid urease test |
40 (50) |
IgM |
6 (7.5) |
IgG |
34 (42.5) |
Total number= 80.
These results might indicate an increased expression of xenophagy related molecules in gastric epithelial cells in patients with an evidence of presence of H. pylori with active urease test in their tissue or who had been previously infected with the bacterium as indicated by positive serum IgG. This suggestion has been noted when we divided LC3C over ATG16L1 percentage of expression to suggest a relative xenophagy index, accordingly, this index was higher among rapid urease tissue biopsies (29.86±8.94) and IgG positive group (27.13±10.81) compared with negative urease group (13.43±5.96) and IgG negative group (17.6±9.74) p value <0.001) (Table 2).
Table (2):
Immunohistochemical evaluation of autophagy related proteins and xenophagy index.
| Rapid urease test | IgM | IgG | |||||
|---|---|---|---|---|---|---|---|
| No (n=40) | Yes (n=40) | No (n=74) | Yes (n=6) | No (n=46) | Yes (n=34) | ||
| LC3C | 8.55±3.8 | 23.78±7.49 | 16.11±9.67 | 16.83±10.55 | 12.87±8.75 | 20.62±9.16 | |
| P value | <0.001** | 0.861 NS | <0.001** | ||||
| ATG16L1 | 64.73±11.35 | 79.47±5.94 | 68.57±11.2 | 69.33±16.65 | 69.46±12.18 | 73.32±10.71 | |
| P value | <0.001** | 0.210 NS | 0.144 NS | ||||
| LC3C/ATG16L1 ratio (Xenophagy Index) |
13.43±5.96 | 29.86±8.94 | 21.48±11.29 | 23.67±10.77 | 17.6±9.74 | 27.13±10.81 | |
| P value | <0.001** | 0.649NS | <0.001** | ||||
| Xenophagy
index |
Score 1 | 14 (35) | 0 (0) | 14 (18.9) | 0 (0) | 12 (26.1) | 2 (5.9) |
| Score 2 | 22 (55) | 3 (7.5) | 22 (29.7) | 3 (50) | 19 (41.3) | 6 (17.6) | |
| Score 3 | 4 (10) | 20 (50) | 22 (29.7) | 2 (33.30) | 11 (23.9) | 13 (38.2) | |
| Score 4 | 0 (0) | 12 (30) | 12 (16.2) | 0 (0) | 3 (6.5) | 9 (26.5) | |
| Score 5 | 0 (0) | 5 (12.5) | 4 (5.4) | 1 (16.7) | 1 (2.2) | 4 (11.8) | |
| P value | <0.001** | 0.407NS | <0.001* | ||||
| vacA | Negative | 39 (97.5) | 15 (37.5) | 52 (70.27) | 2 (33.33) | 39 (84.78) | 15 (44.12) |
| Positive | 1 (2.5) | 25 (62.5) | 22 (29.73) | 4 (66.67) | 7 (15.22) | 19 (55.88) | |
| P value | <0.001 | 0.084 | <0.001 | ||||
NS: none statistical significance (p>0.05)
*: Statistical significance (p£0.05).
**: Highly statistical significance (p£0.001).
Xenophagy index is correlated with vacuolating cytotoxin expression
Furthermore, this study investigates the expression of vacA protein in gastritis cases using immunohistochemistry staining method. vacA positive cases were 32.5% (26 out of 80 patients). Furthermore, its expression found to be associated with active urease positive cases 62.5% were vacA positive among 40 urease positive cases (p<0.001) and IgG positive cases in which 55.88% were vacA positive (p<0.001). however, none significant association had been found with IgM positive cases p=0.084 (Table 2).
Further analysis of results of vacA protein expression and xenophagy score, the results showed that a statistically significant association (p=0.002). in which, higher percentage 30%, 34% of vacA positive were score 3 and 4 respectively while, in vac A negative cases a higher percentage found in were score 2 and 3 in which 38.89% and 29.63% respectively (Table 3).
Table (3):
Association between vacA expression and xenophagy index in gastric epithelial cells.
| vacA | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| Xenophagy index | Score 1 | 2 (7.69) | 12 (22.22) | 14 |
| Score 2 | 4 (7.38) | 21 (38.89) | 25 | |
| Score 3 | 8 (30.77) | 16 (29.63) | 24 | |
| Score 4 | 9 (34.62) | 3 (5.56) | 12 | |
| Score 5 | 3 (11.54) | 2 (3.7) | 5 | |
| Total | 26 | 54 | ||
| P value | 0.002* | |||
*: Statistical significance (p£0.05).
The chronicity of H. pylori infection, until last decade had been attributed to the complex interaction between virulence factors produced by H. pylori like: cagA and vacA and host innate immune defense mechanisms5,12,15. The current study provides an index for xenophagy process based on relative protein expression of LC3C/ATG16L1. The measurement of antimicrobial phagocytosis process is essential to describe an innate immune mechanism against microbe (s) in pathological cases16. Here in this study we try to describe the cross-talk between Helicobacter pylori and gastric epithelial expression of both LC3C and ATG16L1 molecules which are essential for complete phagosome assembly against microbe17,18. The results showed that patients with higher expression of xenophagy index were
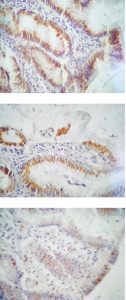
In this study, it’s a first time that reports an association between the presence of vacA protein and higher index of LC3C/ATG16L1 proteins (xenophagy index) in gastric epithelial cells. The localization of these markers in gastric tissue biopsies (Fig. 1) provide an evidence for this relationship. At experimental level, studies have been reported that vacA producing H. pylori have the ability to invade AGS cell line, forming large vacuoles, which enhance long term survival of bacteria while vacA negative did not5. Furthermore, vacA have the ability to reduce cellular activities like: impaired production of cathepsin D that results in inability to eliminate bacteria5,19, inhibition of T cell activation20,21, proliferation22 and induction of apoptosis in gastric epithelial cells23.
Fig. 1. Immunohistochemical localization of ATG16L1 (A), LC3C (B) and vacA (C), stained by specific primary and visualized by peroxidase staining system on gastritis tissue section. 400X original magnification
They found that vacA can modulate cellular autophagy process, Jones, et al., showed that vacA can promote survival in gastric epithelial cell line5 and macrophages24. Furthermore, H. pylori trigger phagosome formation within the cell containing bacterial components which is similar to vacA induced vacuoles that characterized by LC3B puncta formation25.
This study found an association between active urease secretion and IgG positive patients with higher expression of LC3C protein, higher scores of xenophagy index and vacA positive cases. The chronicity of gastritis was achieved by Gram negative bacterium that actively invade gastric epithelium and evasion of host defense mechanisms. The chronic vacA exposure can promote inhibition of autophagy rather than induction and elimination of bacteria(26) inducing accumulation of lysosomes and larger vacuole containing bacteria(27). Moreover, longer persistence of bacterium would results in more advanced gastro-duodenal ulceration(28) and gastric cancer(15).
Clinically, based on immunohistochemical localization of vacA and determination of xenophagy index (LC3C/ATG16L1 ratio) gives further evidence that this bacterium modulates autophagy process and can persist in gastric epithelial cells of chronic gastritis patients.
Acknowledgements
A special thanks to Dr. Ban J Qassim for her efforts in reviewing histopathology reports of patients.
Conflict of Interest
Authors declares no conflict of interest regarding this work.
Funding
This work is self-funded by authors.
- Moodley Y, Linz B, Bond RP, Nieuwoudt M, Soodyall H, Schlebusch CM, et al. Age of the association between Helicobacter pylori and man. PLoS Pathog. 2012;8(5).
- Alzahrani S, Lina TT, Gonzalez J, Pinchuk I V., Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20(36):12767–80.
- Ghasemi Basir HR, Ghobakhlou M, Akbari P, Dehghan A, Seif Rabiei MA. Correlation between the intensity of Helicobacter pylori colonization and severity of gastritis. Gastroenterol Res Pract. 2017;2017:10–5.
- Wang Y-H, Gorvel J-P, Chu Y-T, Wu J-J, Lei H-Y. Helicobacter pylori Impairs Murine Dendritic Cell Responses to Infection. PLoS One [Internet]. 2010;5(5):e10844. Available from: http://dx.plos.org/10.1371/journal.pone.0010844
- Terebiznik MR, Vazquez CL, Torbicki K, Banks D, Wang T, Hong W, et al. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect Immun. 2006;74(12):6599–614.
- Chu Y-T, Wang Y-H, Wu J-J, Lei H-Y. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78(10):4157–65.
- Ricci V, Romano M, Boquet P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol. 2011;17(11):1383–99.
- Tang B, Li N, Gu J, Zhuang Y, Li Q, Wang HG, et al. Compromised autophagy by MIR30B benefits the intracellular survival of Helicobacter pylori. Autophagy. 2012;8(7):1045–57.
- Wang Y-H, Wu J-J, Lei H-Y. The autophagic induction in Helicobacter pylori-infected macrophage. Exp Biol Med (Maywood). 2009;234(2):171–80.
- Wang Y-H, Gorvel J-P, Chu Y-T, Wu J-J, Lei H-Y. Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS One. 2010;5(5):e10844.
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature [Internet]. 2011 Jan 20 [cited 2018 May 7];469(7330):323–35. Available from: http://www.nature.com/articles/nature09782
- Cemma M, Brumell JHH. Interactions of Pathogenic Bacteria with Autophagy Systems. Curr Biol [Internet]. 2012;22(13):R540–5. Available from: http://dx.doi.org/10.1016/j.cub.2012.06.001
- Foegeding NJ, Caston RR, McClain MS, Ohi MD, Cover TL. An overview of Helicobacter pylori VacA toxin biology. Toxins (Basel). 2016;8(6):1–21.
- Terebiznik MR, Raju D, Vázquez CL, Torbricki K, Kulkarni R, Blanke SR, et al. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5(3):370–9.
- Ki MR, Hwang M, Kim AY, Lee EM, Lee EJ, Lee MM, et al. Role of vacuolating cytotoxin VacA and cytotoxin-associated antigen CagA of Helicobacter pylori in the progression of gastric cancer. Mol Cell Biochem. 2014;396(1–2):23–32.
- Kwon DH, Song HK. A Structural View of Xenophagy, A Battle between Host and Microbes. 2018;8(1):27–34.
- Mao K, Klionsky DJ. Xenophagy: A battlefield between host and microbe, and a possible avenue for cancer treatment. Autophagy [Internet]. 2017;13(2):223–4. Available from: http://dx.doi.org/10.1080/15548627.2016.1267075
- Shpilka T, Elazar Z. Essential Role for the Mammalian ATG8 Isoform LC3C in Xenophagy. Mol Cell [Internet]. 2012;48(3):325–6. Available from: http://dx.doi.org/10.1016/j.molcel.2012.10.020
- Necchi V, Sommi P, Vanoli A, Fiocca R, Ricci V, Solcia E. Natural history of Helicobacter pylori VacA toxin in human gastric epithelium in vivo: Vacuoles and beyond. Sci Rep [Internet]. 2017;7(1):1–11. Available from: http://dx.doi.org/10.1038/s41598-017-15204-z
- Gebert B, Fischer W, Weiss E, Hoffman R, Haas R. Helicobacter pylori Vacuolating Cytotoxin Inhibits T Lymphocyte Activation. Science (80- ) [Internet]. 2003;301(5636):1099–102. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.1086871
- Boncristiano M, Paccani SR, Barone S, Ulivieri C, Patrussi L, Ilver D, et al. The Helicobacter pylori Vacuolating Toxin Inhibits T Cell Activation by Two Independent Mechanisms. J Exp Med [Internet]. 2003;198(12):1887–97. Available from: http://www.jem.org/lookup/doi/10.1084/jem.20030621
- Torres VJ, VanCompernolle SE, Sundrud MS, Unutmaz D, Cover TL. Helicobacter pylori vacuolating cytotoxin inhibits activation-induced proliferation of human T and B lymphocyte subsets. J Immunol [Internet]. 2007;179(8):5433–40. Available from: http://www.jimmunol.org/cgi/content/abstract/179/8/5433
- Zhu P, Xue J, Zhang Z, Jia Y, Tong Y, Han D, et al. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death Dis [Internet]. 2017;8(12):3207. Available from: http://www.nature.com/articles/s41419-017-0011-x
- Zheng PY, Jones NL. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell Microbiol. 2003;5(1):25–40.
- Terebiznik MR, Raju D, Vázquez CL, Torbricki K, Kulkarni R, Blanke SR, et al. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5(3):370–9.
- Oldani A, Cormont M, Hofman V, Chiozzi V, Oregioni O, Canonici A, et al. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathog. 2009;5(10).
- Raju D, Hussey S, Ang M, Terebiznik MR, Sibony M, Galindo-Mata E, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142(5):1160–71.
- Wada A, Yamasaki E, Hirayama T. Helicobacter pylori vacuolating cytotoxin, VacA, is responsible for gastric ulceration. J Biochem. 2004;136(6):741–6.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.