ISSN: 0973-7510
E-ISSN: 2581-690X
Thiamethoxam (THIA) degrading bacterial colonies were isolated from soil obtained from different ecosystems such as cotton, cabbage and paddy tolerating high concentrations of the insecticide followed by enrichment technique. Eight colonies were isolated and were morphologically and biochemically tested. Among eight bacterial isolates, three isolates named as THIA-3, THIA-4 and THIA-7 were found to be superior and recorded maximum growth in the medium supplemented with thiamethoxam as sole carbon source. Highest dissipation of thiamethoxam was recorded in the artificial medium inoculated with THIA-3 (94.72 %) followed by THIA-4 (90.78 %) and THIA-7 (82.06 %) compared to 53.85 percent in the control. In the thiamethoxam bioremediation study by using sterilized soil, half-life of thiamethoxam was reduced to 7.60 days in THIA-3 inoculation followed by THIA-4 (8.75 days) and THIA-7 (10.52 days) compared to 17.91 days in the control. The bacterial isolates viz., THIA-3, THIA-4 and THIA-7 were later identified as Acinetobacter sp., Enterobacter sp. and Bacillus sp., respectively through molecular characterization by 16S rRNA gene sequencing. These results highlight the potential of bacterial cultures to be used in the cleanup of pesticide contaminated sites in the environment.
Enrichment, Dissipation, 16S rRNA, Acinetobacter sp., Enterobacter sp. and Bacillus sp.
Microorganisms play an important role in degrading synthetic chemicals in soil1. Their unique ability to completely mineralize many aliphatic, aromatic, and heterocyclic compounds providing an environment friendly method of in situ detoxification. Bacteria and other organisms by the process of biodegradation alter and breakdown organic molecules into substances, eventually producing carbon dioxide and water or methane. The ability of these organisms to reduce the concentration of xenobiotics is directly linked to their long-term adaptation to environments where these compounds exist. They have the capacity to utilize virtually all naturally and synthetically occurring compounds as their sole carbon and energy source.
Neonicotinoid insecticides are one of the fastest growing classes of insecticides used in modern crop protection. They are systemic in nature which readily gets absorbed and translocated by plants. They are primarily used to control sucking and chewing pests such as aphids, whiteflies, leafhoppers and planthoppers, as well as thrips, microlepidoptera and coleopteran insect species2. Thiamethoxam [(EZ)-3-(2-chloro-1, 3-thiazol-5- ylmethyl)- 5-methyl-1,3,5-oxadiazinane-4-ylidene(nitro)amine] (THIA) is a second generation neonicotinoid insecticide that belongs to the thianicotinyl subclass and to date it holds registration for 115 crop uses in at least 64 countries3-4. Low use rates, flexible application methods, excellent efficacy, long-lasting residual activity and favorable safety profile make this insecticide well-suited for modern integrated pest management programmes in many cropping systems5.
It was proposed that the metabolism of thiamethoxam in the soil is mainly due to microbial degradation6. Among microbes, bacterial degradation of thiamethoxam was found to be more evident. Plant growth promoting rhizobacteria (PGPR) viz., Bacillus amyloliquefaciens IN937a, B. pumilus SE34, B. subtilis FZB24 and Ensifer adhaerens TMX23 have the capability to degrade the thiamethoxam compound in the soil7-8. Soil bacterium Pseudomonas sp. 1 G was found to degrade 70 per cent of thiamethoxam after 14 days of incubation wherein thiamethoxam was transformed to the nitroimino moiety to form nitrosoimino, imino and urea metabolites9. Therefore, an attempt was made to explore diverse group of soil bacteria which are capable of degrading thiamethoxam with an idea that they may be further exploited for bioremediation studies in the future.
Collection of soil samples and enrichment technique – Soil samples of 2.5kg were collected randomly from crop fields such as cabbage, cotton and paddy in Coimbatore, Tamil Nadu with prehistory of application of thiamethoxam. Physico-chemical properties were tested and later the samples were subjected to enrichment with thiamethoxam @ 50 µg/g five times at an interval of seven days10. In case of broth enrichment technique11, 10 g of sieved samples were suspended in a flask containing 50ml of modified nutrient broth supplemented with thiamethoxam (50 mg/l) as sole source of carbon.
Isolation and purification of the isolates from the enrichment cultures –Serial dilution and plating technique was used to isolate bacterial colonies from both soil and broth enrichment cultures which were plated on mineral salt medium (MSM) supplemented with thiamethoxam (50 µg/ml).
Morphological and biochemical characterization of the isolates and the estimation of the growth of the isolates in the medium – The purified colonies were morphologically identified for their shape, size, gram staining ability etc., and biochemical tests viz., utilization of carbon sources, extracellular enzymatic activities, IMViC tests etc., were conducted12-13. Growth of the isolates in the MSM was estimated by measuring the increase in turbidity of the medium using UV-VIS spectrometer at 600 nm.
Testing the degradation potential of the isolates in the MSM and estimation of residue using HPLC – The isolates were inoculated into flasks containing 50ml of the MSM broth supplemented with thiamethoxam besides maintaining a control (without bacterial inoculums). Thiamethoxam residues were extracted from the media using the liquid-liquid extraction method by making slight modification14. A quantity of 50ml of dichloromethane and 10ml of 10 per cent sodium chloride solution was added to the sampled culture medium in a separating funnel. The mixture was vigorously shaken for two minutes and pressure was released intermittently. The solvent mixtures were left to stand until phase separation took place.
The dichloromethane layer (bottom layer) was collected in a rotary flask by passing through anhydrous sodium sulphate and then the aqueous sample was reextracted twice with dichloromethane (25ml x 2). The organic layer (dichloromethane) was combined and concentrated to near dryness in a rotary vacuum evaporator (Roteva # 8763RD, M/s Media Instrument, Mfg., Co., Mumbai) at 40°C. The residue was redissolved in 5ml of HPLC grade acetonitrile and diluted to 50 times (200 µl of the sample to 10ml with acetonitrile) before injecting into HPLC (High Performance Liquid Chromatography, Shimadzu 20 AT). The quantification of residue was carried out by HPLC using Photo Diode Array (PDA) detector. The recovery studies were conducted in triplicate at three different concentration level (0.1, 0.5 and 1.0 ppm) to evaluate extraction efficiency of the analytical method.
Testing bioremediation potential of the bacterial isolates in sterilized soil – Soil sample (2.5kg) were collected, shade dried and sieved through 2 mm sieve. Samples were sterilized by autoclaving at 15 psi pressure and at 121°C. Thiamethoxam was added into the soil samples as granules (50 µg/g of soil) separately into the respective containers and soil samples were mixed well. In each case, a control was also maintained without the inoculums. At the time of inoculation, inoculum density was quantified by plate count technique (1ml of the inoculum to 100 g of soil). Extraction of thiamethoxam residues from the soil was done as per the procedure15. The recovery test was followed and the quantification of residue was carried out by HPLC using PDA (Photo Diode Array) detector.
Molecular Characterization of screened isolates – Genomic DNA was extracted, PCR was done with 16S rRNA gene specific primers (Forward primer, 5’ à 3’: CGAATTCGTCGACAACAGAG TTTGATCCTGGCTCAG and Reverse primer, 5’ à 3’: CCCGGGATCCAAGCTTACG GCTACCTTG TTACGACTT) and the PCR products were sequenced (Rajeev Gandhi Centre for Biotechnology, Trivendrum). The obtained sequences were compared with already available sequences in NCBI sequence database by BLAST analysis.
Statistical analysis – Statistical parameters like confidence intervals for intercept (a), slope of regression line (b) and coefficient of determination (r2) were determined16. The different functions were worked out for the residue data to fit the dissipation curves.
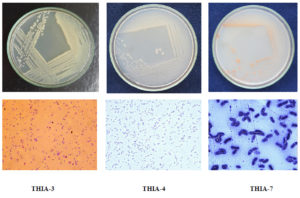
Altogether, eight bacterial colonies were isolated and purified viz., THIA-1 to THIA-8. Among eight isolates, three isolates were selected further, studied for their thiamethoxam degrading ability based on their growth response in the MSM containing thiamethoxam (50µg/ml) as sole source of carbon. THIA-3 recorded maximum growth with an optical density of 1.519 at 600 nm followed by THIA-4 (1.488) and THIA-7 (1.013). Initial studies pertaining to morphological characteristics and biochemical test analysis results are represented in Table 1. The enzymatic activity of the isolates THIA-3 and THIA-4 had shown positive results for lipid and casein hydrolysis and that of THIA-7 had shown positivity for starch hydrolysis. Bacterial colony plates and microscopic observations are represented in Fig.1.
Table (1):
Morphological and biochemical characteristics of thiamethoxam degrading bacterial isolates.
| Characteristics | Bacterial isolates | ||
|---|---|---|---|
| THIA-3 | THIA-4 | THIA-7 | |
| A. Morphology | |||
| 1. Grams Stain reaction and shape of the cell | – and tiny cocci | – and elliptical rods | + and Straight long rods |
| 2. Size of the cell (µm) | 0.8-1.0 | 0.7-0.9 | 4.0-4.2 |
| B. Biochemical tests | |||
| 1. Glucose utilization | – | + | – |
| 2. Sucrose utilization | + | – | – |
| 3. H2S production | + | + | – |
| 4. Indole production | – | + | + |
| 5. MR Reaction | – | + | – |
| 6. VP reaction | – | – | + |
| 7. Citrate utilization | + | + | + |
| 8. Catalase activity | + | + | + |
| 9. Gelatin Liquefaction | – | – | – |
| 10. Starch Hydrolysis | – | – | + |
| 11. Lipid hydrolysis | + | + | – |
| 12. Casein hydrolysis | + | + | – |
| 13. Growth on Mackonkey Agar | + | + | + |
(+) Positive, ( – ) Negative, MR – Methyl Red, VP – Voges Proskauer,
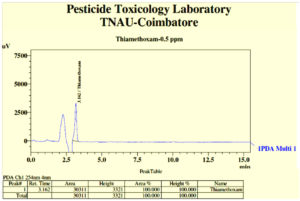
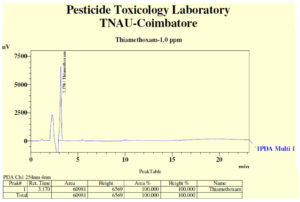
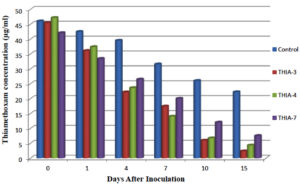
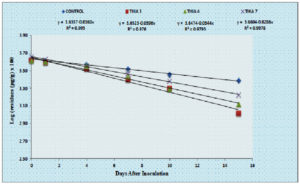
Degradation of thiamethoxam in the MSM medium – The mean recovery of thiamethoxam in the artificial medium was 83.14, 89.27 and 99.26 per cent for the three different fortification levels of 0.1, 0.5 and 1.0 ppm, respectively. The method was found to be satisfied with more than 80 percent recovery (Fig, 2a and 2b). The dissipation of thiamethoxam inoculated with the isolates was found to be farthest from the control. There was a greater reduction in half-life of the compound in the MSM medium from 13.93 days in control to 3.46 days with THIA-3 isolate. The half-life of thiamethoxam in other two isolates was 4.21 days and 6.15 days for THIA-4 and THIA-7, respectively (Table, 2). The half-life was calculated in 1st order kinetics which was observed to be best fit model based on regression coefficient (R2) of determination. Reduction in the concentration of thiamethoxam over the period of 15 days is given in Fig, 3.
Table (2):
Persistence and dissipation behaviour of thiamethoxam in the MSM medium.
| Days after inoculation | Control | Bacterial isolates | ||||||
|---|---|---|---|---|---|---|---|---|
| THIA-3 | THIA-4 | THIA-7 | ||||||
| Residues (µg/ml) | Dissipation (%) | Residues (µg/ml) | Dissipation (%) | Residues (µg/ml) | Dissipation (%) | Residues (µg/ml) | Dissipation (%) | |
| 0 (2 h) | 43.43 | – | 41.9 | – | 41.26 | – | 45.37 | – |
| 1 | 41.26 | 4.99 | 39.38 | 6.01 | 40.09 | 2.83 | 42.17 | 7.05 |
| 4 | 37.03 | 14.73 | 33.16 | 20.85 | 35.78 | 13.28 | 35.67 | 21.37 |
| 7 | 32.82 | 24.43 | 25.03 | 40.26 | 27.06 | 34.41 | 29.17 | 34.53 |
| 10 | 28.44 | 34.51 | 19.87 | 52.57 | 19.64 | 52.39 | 24.18 | 46.70 |
| 15 | 24.78 | 42.94 | 10.3 | 75.41 | 13.11 | 68.22 | 16.68 | 63.23 |
| R2 | 0.9804 | 0.9753 | 0.9819 | 0.9816 | ||||
| T1/2 (days) | 13.93 | 3.46 | 4.21 | 6.15 | ||||
Bioremediation studies of thiamethoxam in the sterilized soil – The average recovery of thiamethoxam in soil was ranged from 89.54 to 99.23 per cent for the three different fortification levels of 0.1, 0.5 and 1.0 ppm, respectively. As the recovery percentage is more than 89 per cent, method was found to be desirable for residue and dissipation study of thiamethoxam in soil.
On comparison of the isolates and the control over the period of 15 days, the dissipation of thiamethoxam was in the order of THIA-3 (75.41%) > THIA-4 (68.22%) > THIA-7 (63.23%) isolates and control (42.94%). Persistence and dissipation of thiamethoxam are shown in Table, 3. The dissipation rate of thiamethoxam for all the three bacterial isolates along with control was followed linear pattern and reflect first order kinetics (Fig, 4).
Table (3):
Persistence and dissipation behaviour of thiamethoxam in the sterilized soil.
| Days after inoculation | Control | Bacterial isolates | ||||||
|---|---|---|---|---|---|---|---|---|
| THIA-3 | THIA-4 | THIA-7 | ||||||
| Residues (µg/ml) | Dissipation (%) | Residues (µg/ml) | Dissipation (%) | Residues (µg/ml) | Dissipation (%) | Residues (µg/ml) | Dissipation (%) | |
| 0 (2 h) | 43.43 | – | 41.9 | – | 41.26 | – | 45.37 | – |
| 1 | 41.26 | 4.99 | 39.38 | 6.01 | 40.09 | 2.83 | 42.17 | 7.05 |
| 4 | 37.03 | 14.73 | 33.16 | 20.85 | 35.78 | 13.28 | 35.67 | 21.37 |
| 7 | 32.82 | 24.43 | 25.03 | 40.26 | 27.06 | 34.41 | 29.17 | 34.53 |
| 10 | 28.44 | 34.51 | 19.87 | 52.57 | 19.64 | 52.39 | 24.18 | 46.70 |
| 15 | 24.78 | 42.94 | 10.3 | 75.41 | 13.11 | 68.22 | 16.68 | 63.23 |
| R2 | 0.9950 | 0.9760 | 0.9793 | 0.9978 | ||||
| T1/2 (days) | 17.91 | 7.60 | 8.75 | 10.52 | ||||
16S rRNA analysis for identification – NCBI sequence data bank by BLAST analysis revealed that the isolates THIA-3, THIA-4 and THIA-7 were Acinetobacter sp., Enterobacter sp. and Bacillus sp., respectively.
Pesticides when added to the environment will be governed by the activities of microbes. Some microbes have the capability to withstand higher concentration of pesticides in the environment. Adaptation of microflora for the chemicals in the soil after their repeated application leads to the evolution of the microbial population responsible for the mineralization of xenobiotic pesticides in the soil17. Hence, enrichment culturing is proved to be highly essential to isolate the microbial population.
Microbes with appropriate degradative enzymes catalyses the degradation of the pesticides. In the present study, the purified bacterial isolates developed clear zone on solid synthetic medium provided with thiamethoxam as the sole source of carbon. These clear zones can be explained by the liberation of extracellular enzymes produced by the microbial cells18. This phenomenon indicates the degradative ability of the isolated strains in the present study.
Persistence of thiamethoxam in the soil is quite evident. It is known to persist in a sandy loam soil with half life of 16.9 days under laboratory condition19 and in the light condition it was 19.2 days20. In the current study, dissipation of thiamethoxam in the modified nutrient broth inoculated with different thiamethoxam degrading isolates recorded variable rates. The study notably shows that percentage of dissipation of thiamethoxam in the medium varied from 82.06 to 94.72 per cent after fifteen days of incubation. The dissipation of thiamethoxam in the sterilized soil also quite significant and it is varied from 63.23 to 75.41 per cent. Hence, the bacteria cultures are quite effective in reducing the insecticide (thiamethoxam) load in the environment. This study was in agreement with the report that soil enrichment culture rapidly degraded 96 percent of 200 mg/L thiamethoxam in mineral salt medium broth within 30 days21.
One of the identified isolate, Acinetobacter sp. is also known to degrade the insecticides such as chlorpyrifos and methyl parathion22 and it is also active on triazine weedicides viz., simazine, terbutryn, cyanazine, and prometon23. Enterobacter sp. named in the study as THIA-4 also reported to degrade chlorpyrifos by 86 per cent after 18 days of inoculation24. The Enterobacter aerogenes found highly efficient in degradation of bifenthrin, fenpropathrin and cypermethrin25. One more bacterium, Bacillus sp. was found to have larger adaptations to pesticide degradation ability as mentioned earlier. Hence, the isolates proved to be involved in the degradation of other xenobiotic compounds also.
Thus, we can conclude that the bacterial isolates such as Acinetobacter sp., Enterobacter sp. and Bacillus sp. are involved in the degradation of thiamethoxam both in the medium as well as in soil. Hence, the study signifies their usage and focuses on further research concentrating upon the enzyme involved in the degradation is of great importance to formulate an effective consortium for remediation of affected soils.
ACKNOWLEDGMENTS
The research paper is a part of M.Sc. dissertation work of corresponding author. The authors are thankful to Indian Council of Agricultural Research and Tamil Nadu Agricultural University for financial and technical support for the research work.
- Alexander, M. Non-biodegradable and other recalcitrant molecules. Biotechnol. Bioeng., 1981; 15: 611-47.
- Vadodaria, M.P., Patel, U.G., Patel, C.J., Patel, R.B., Maisuria, I.M. Thiamethoxam (Cruiser) 70 WS: a new seed dresser against sucking pests of cotton. Pestology, 2001; 25: 13-18.
- Jeschke, P., Nauen, R., Schindler, M., Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food. Chem., 2011; 59: 2897-08.
- Elbert, A., Haas, M., Springer, B., Thielert, W. Nauen, R. Applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci., 2008; 64: 1099-105.
- Maienfisch, P., Angst, M., Brandl, F., Fischer, W., Hofer, D., Kayser, H., Kobel, W., Rindlissbacher, A., Senn, R., Stienemann, A. Chemistry and biology of thiamethoxam: a second generation neonicotinoid. Pest Manag. Sci., 2001; 57: 906-13.
- Gupta, S., Gajbhiye, V.T., Gupta, R.K. Soil dissipation and leaching behavior of a neonicotinoid insecticide thiamethoxam. Bull. Environ. Contam. Toxicol., 2008; 80: 431-7.
- Myresiotis, C., Vryzas, Z., Papadopoulou-Mourkidou, E. Biodegradation of soil-applied pesticides by selected strains of plant growth-promoting rhizobacteria (PGPR) and their effects on bacterial growth. Biodegradation, 2011; 23: 297-10.
- Zhou., G.C., Wang, Y., Zhai, S., Ge, F., Liu, Z.H., Dai, Y.J., Yuan, S., Hou, J.Y. Biodegradation of the neonicotinoid insecticide thiamethoxam by the nitrogen-fixing and plant-growth-promoting rhizobacterium Ensifer adhaerens strain TMX-23. Appl. Microbiol. Biotecnol., 2013; 97: 4065-74.
- Pandey, G.S., Dorrian, J., Russell, R.J., Oakeshott, J.G. Biotransformation of the neonicotinoid insecticides imidacloprid and thiamethoxam by Pseudomonas sp. 1 G. Biochem. Biophys. Res. Commun., 2009; 380: 710.
- Singh, B.K., Walker, A., Morgan, J.A.W., Wright, D.J. Biodegradation of chlorpyriphos by Enterobacter strain B-14 and its use in bioremediation of contaminated soil. Appl. Environ. Microbiol., 2004; 70(8): 4855.
- Rani, M.S., Lakshmi, K.V., Devi, P.S., Madhuri, R.J., Aruna, S., Jyothi, K., Narasimha, G., Venkateswaralu, K. Isolation and characterization of chloropyrifos degrading bacterium from agricultural soil and its growth response. Afr. J. Microbiol. Res., 2008; 2: 26-.
- Gerhardt, P., Murray, G.E., Wood, W.A., Krieg. N.R., Phelleps, G.B. Methods for general and molecular biology. ASM, Washington, DC, 1994; pp 1-200.
- Cappucino, J.G. Sherman, N. Microbiology: A laboratory Manual. Pearson Education, New York, 2002; pp 1-485.
- Ghanem, I.M., Orfi, M., Shamma, M. Biodegradation of chlorpyrifos by Klebsiella sp. isolated from an activated sludge sample of waste water treatment plant in Damascus. Folia Microbiol., 2007; 54: 423-27.
- Ramasubramanian, T., Paramasivam, M., Jayanthi, R., Rapid and sensitive analytical method for simultaneous determination of imidacloprid and thiamethoxam residues in soils of sugarcane ecosystem by reversed-phase HPLC. Water, Air, & Soil Poll., 2012; 223: 6045-50.
- Timme, G., Frehse, H. Statistical interpretation and graphic representation of the degradation behaviour of pesticide residues. Pflanzenschutz Nachrichten Bayer, 1980; 33: 189-203.
- Smelt, J.H., Crum, S.J.H., Teunissen, W. Leistra, M. Accelerated transformation of aldicarb, oxamyl and ethoprophos after repeated soil treatment. Crop Prot., 1987; 6: 295-03.
- Slaoui, M., Uhssine, M., Berny, E., Elyachioui, M. Biodegradation of the carbofuran by a fungus isolated from treated soil. Afr. J. of Biotech., 2007; 6(4): 419-23.
- Karmakar, R., Singh, S.B., Kulshrestha, G. Persistence and transformation of thiamethoxam, a neonicotinoid insecticide, in soil of different agroclimatic zones of India. Bull. Environ. Contam. Toxicol., 2006; 76: 400-06.
- Gupta, S., Gajbhiye, V.T. Gupta, R.K. Effect of ultraviolet and sunlight on persistence of thiamethoxam in Soil. Pestic. Res. J., 2006; 18(2): 211-14.
- Zhou, G.C., Wang, Y., Ma, Y., Zhai, S., Zhou, L.Y., Dai, Y.J., Yuan, S., The metabolism of neonicotinoid insecticide thiamethoxam by soil enrichment cultures, and the bacterial diversity and plant growth promoting properties of the cultured isolates. J. Environ. Sci. Health Part B., 2014; 49(6): 381-90.
- Pino, N.G., Penuela, G. Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site. Int. Biodeter. Biodegr., 2011; 65(6): 827-31.
- Singh, P., Suri, C.R., Cameotra, S.S. Isolation of a member of Acinetobacter species involved in atrazine degradation. Biochem. Bioph. Res. Co.2004; 317(3): 697-02.
- Chishti, Z., Arshad, M. Growth linked biodegradation of chlorpyrifos by Agrobacterium and Enterobacter spp. Int. J. Agric. Biol.,2013; 15(1): 19-26.
- Liao, M., Zhang, H.J. Xie, X.M. Isolation and identification of degradation bacteria Enterobacter aerogenes for pyrethriods pesticide residues and its degradation characteristics. Huanjing Kexue / Chinese Journal of Environmental Science. 2009; 30(8) 2445-51.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.