A major cause of respiratory tract infections in infants, adults, the elderly, and people with impaired immune systems is the human metapneumovirus (HMPV). The Paramyxoviridae family was replaced by the Pneumoviridae family in 2016. The genetic groups A and B that make up this virus are further subdivided into subclasses, with A1, A2, B1, and B2 varying from year to year. Originally identified in the Netherlands in 2001, HMPV has since spread throughout the world. Droplets from infected people’s respiratory systems are the main way it is transmitted. Although HMPV infections are often mild and self-limiting, they can have a complex clinical course in immunocompromised patients and the elderly. The diagnosis is primarily relied on a nucleic acid amplification test, such as reverse transcriptase polymerase chain reaction (RT-PCR) which is the gold standard for modern molecular diagnosis because of its higher sensitivity and specificity. However, because it requires specialized laboratory equipment that not all healthcare facilities have, RT-PCR is not as commonly used. While promising, other diagnostic techniques including next-generation sequencing and antigen detection assays are not yet widely used in clinical settings. All of the current HMPV therapy modalities offer a limited range of choices. Preclinical tests of novel techniques to monoclonal antibody creation have showed promise, but human testing is necessary to determine their safety and efficacy. There is currently no vaccination, and the available treatment is supportive. Nonetheless, current study yields positive findings. In this review, we highlight recent advancements in treatment, adult infections, and the structural features of known antigenic sites on the HMPV proteins.
Etiology, Treatment, Acute Respiratory Infection, Clinical Characteristics, Epidemiology, Human Metapneumovirus (HMPV)
Human Metapneumovirus (HMPV)
In 2001, human metapneumovirus was discovered in the Netherlands in nasopharyngeal specimens from children with acute respiratory tract illness, as detected by direct electron microscopy.1 Since the discovery of HMPV, numerous studies have looked at its prevalence, clinical characteristics, prevention, transmission, and therapy.2 Despite this, no specific treatment or vaccine exists to prevent or treat HMPV infection.3 With the advancement of molecular biology and sequencing capabilities, there are only a few entire or almost complete HMPV sequences accessible, making viral evolutionary research difficult.4 Researchers are increasingly interested in studying viral gene characteristics such as transmission, enhanced virulence, in vivo replication, immune response, therapeutic research, and development.5 In vivo animal models, accessible full-length sequences, and the absence of effective HMPV treatment suggest that researchers should focus more on HMPV, create efficient medications and vaccines, and standardize and harmonize case reporting to facilitate better case-to-case consultation.6 Within the Paramyxoviridae family, HMPV belongs to the Metapneumovirus (MPV) genus.7 Bovine MPV, avian MPV, and other members of the Metapneumovirus genus are included in MPV. The lipid envelope of the myxovirus is accompanied by an elongated, pleomorphic nucleocapsid that ranges in diameter from 100 to 700 nm. 9.5-10.5 multicistronic mRNAs based on the virus are produced by complexing the viral genome nucleocapsid, which is composed of a single serotype of negative-sense single-stranded RNA, following transcription and replication.8 Class I viral fusion proteins include the haemagglutinin-like (HN) protein, which is the primary structural protein in the viral envelope. Encasing single-stranded negative-sense RNA, HMPV is a member of the Pneumovirinae subfamily. Between 150 nm and 800 nm, HMPV virions vary in size and shape. Different features in the gene sequences of the phosphoprotein (P) and glycoprotein (G) that encode genes distinguish four groups of HMPV genotypes. Because P is the most conserved gene, HMPV genotypes can be further divided into subgroups A and B, just as other respiratory viruses. Between 7% and 15% of the population in the US is affected by HMPV each year. The respiratory system is its primary site of infection, and numerous investigations have shown that it is especially adept in co-infecting other respiratory diseases. Human metapneumovirus (HMPV) is more common among immunocompromised patients, the elderly, and young children, particularly those under six months of age.9 The genome of HMPV is shown in Table 1.
Table (1):
The genome of HMPV is organized into eight genes, encoding nine proteins
Gene |
Protein(s) Encoded |
Function |
|---|---|---|
Nucleoprotein |
N |
Binds and protects viral RNA, forming the nucleocapsid. |
Phosphoprotein |
P |
Acts as a polymerase cofactor, assisting in viral replication. |
Matrix Protein |
M |
Plays a structural role in virion assembly and budding. |
Fusion Protein |
F |
Facilitates viral entry by mediating membrane fusion. |
Transcription factor |
M2-1, M2-2 |
M2-1 regulates viral transcription; M2-2 controls RNA synthesis balance. |
Small Hydrophobic Protein |
SH |
Possible role in immune evasion and virus stability (not essential for replication). |
Glycoprotein |
G |
Mediates viral attachment to host cells (non-essential for replication). |
Large Polymerase |
L |
RNA-dependent RNA polymerase responsible for genome replication and transcription. |
Epidemiology
HMPV was first discovered in 2001, and the virus has subsequently been reported in numerous places around the world.10 Data from several countries show that a considerable proportion of the population possesses anti-HMPV antibodies, indicating a high prevalence of virus transmission in both temperate and tropical climates, and that the virus infects humans all over the world, indicating a global spread. The mean seroprevalence of HMPV was 36.9% across all trials. In general population studies, weighted averages were 27.1% in children aged 0-7 years and 61.2% in children aged 0-10 years.11 In young children, the elderly, and individuals with weakened immune systems, HMPV typically leads to tracheobronchitis, bronchitis, bronchiolitis, pneumonia, and mild, self-limiting upper and lower respiratory tract infections. Infections caused by the related respiratory syncytial virus often present with a broad spectrum of symptoms, ranging from mild cold-like signs to severe conditions such as bronchiolitis, croup, bronchitis, and viral pneumonia, frequently requiring medical attention, including hospitalization. More severe symptoms are generally observed in cases of co-infection or among immunocompromised individuals12 More severe symptoms are usually experienced in cases of co-infection events or in people who are immunocompromised. The severity of the infection usually varies on the age and pre-existing health state of the infected individual. Clinical manifestations pose serious diagnostic challenges because many respiratory viruses, including human metapneumovirus (HMPV) and respiratory syncytial virus (RSV), share overlapping symptoms. This similarity makes it difficult to differentiate between infections based on clinical presentation alone. HMPV shedding can last for up to three weeks, just like that of other viral pathogens, and in a small percentage of instances, co-infections between HMPV and other respiratory pathogens have been documented.13 In temperate regions, HMPV exhibits a distinct seasonality from winter to early spring, usually reaching its peak in the United States between January and April. A season of heightened circulation suggests some seasonality, but most years detect the virus again throughout the year, according to numerous studies documenting a sub-seasonal circulation in tropical regions.
Molecular epidemiology and global burden
Human metapneumovirus (HMPV) is a significant contributor to the global burden of acute respiratory infections, particularly affecting young children, the elderly, and immunocompromised individuals. In 2018 alone, HMPV was responsible for an estimated 14.2 million cases of acute lower respiratory infections (ALRI) and 16,100 deaths among children under five years of age worldwide. Notably, approximately 58% of hospital admissions and 64% of in-hospital deaths occurred in infants younger than 12 months and 6 months, respectively. The majority of these deaths (79%) were reported in low- and lower-middle-income countries.14,15
Genetic diversity and evolution
HMPV is classified into two major genetic lineages, A and B, each further subdivided into sublineages (A1, A2, B1, and B2). Multiple genotypes often co-circulate during the same respiratory season across different regions. Shifts in dominant genotypes and genetic variations over time have been documented, reflecting the virus’s dynamic evolution and continuous global transmission.16
Coinfections and disease severity
Coinfection with other respiratory pathogens is common among individuals infected with HMPV. Frequently associated viruses include respiratory syncytial virus (RSV), influenza viruses, parainfluenza viruses, adenoviruses, rhinoviruses, and human bocavirus. Additionally, bacterial coinfections, especially with Streptococcus pneumoniae and Haemophilus influenzae, have been reported and are thought to contribute to increased disease severity and complications.17
Mutation dynamics and implications
Similar to other RNA viruses, HMPV undergoes frequent mutations due to the absence of proofreading mechanisms in its RNA-dependent RNA polymerase. These mutations occur throughout the viral genome, particularly in genes encoding surface glycoproteins such as the fusion (F) and attachment (G) proteins. The G protein exhibits a high degree of variability and is considered a key driver of genetic drift, enabling immune evasion and contributing to antigenic diversity and potential reinfections.
While the F protein is more conserved than the G protein, it also undergoes mutations that can affect viral fusion efficiency, neutralization by host antibodies, and vaccine design. The accumulation of mutations facilitates the emergence of new genotypes or sublineages, helping the virus adapt to changing host immune environments. Overall, these genetic changes contribute to HMPV’s persistence in the human population, influencing its epidemiology, virulence, and challenges to vaccine development.18
Pathogenesis
The F protein of HMPV facilitates attachment to host cell receptors and plays a critical role in viral entry. Although fusion proteins in other viruses mainly mediate membrane fusion, in HMPV, the F protein also contributes to viral penetration. After binding to the host cell, the virus is internalized through endocytosis. Dendritic cells and T cells, which express the HRBm receptor, are prime targets for HMPV infection and replication.19,20
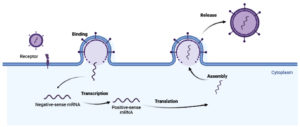
Two distinct replication mechanisms result in viral RNA and, eventually, proteins after HMPV RNA has been integrated and replicated in the cellular cytoplasm: Genomic RNA transcription and replication in cytoplasmic inclusions are shown in Figure, along with immediate translation and replication by the incoming RNA-dependent RNA polymerase. Necrosis of pulmonary epithelial cells and, occasionally, syncytial development are caused by HMPV infection.21,22
Figure. Pathogenesis of HMPV: The incoming RNA-dependent RNA polymerase’s instant translation and replication, as well as the transcription and replication of genomic RNA in cytoplasmic inclusions
A mild and delayed immune response may also be the cause of a prolonged HMPV infection, in addition to delayed cytotoxic T cell activity and inadequate viral clearance during the first infection, which inhibits the activation of T cells triggered by superantigens. Consequently, the development of long-term immunity is impeded, and the growth of antigen-specific CD4+ T cells is restricted.23,24 HMPV alters cytokine responses, producing IL-12, TNF-α, IL-6, IL-1β, IL-8, and IL-10. The response is usually weaker than RSV or influenza. Age , disease severity, and viral strain all influence how much inflammation occurs. A balanced cytokine response is key to avoid either persistent infection or lung damage. Inflammation and infiltration of the perivascular and peribronchiolar regions are further consequences of these changes.25,26 Smudge cells, alveolar damage, hyaline membrane disease, and intra-alveolar foamy and hemosiderin-loaded macrophages are observed in immunological and histological investigations.27
It is widely known that HMPV infection triggers cellular signaling that is dependent on toll-like receptors. However, it is unclear how toll-like receptor-mediated signaling contributes to the host’s defense against pulmonary HMPV infection and pathogenesis. According to a recent study, MyD88-deficient mice showed much lower levels of pulmonary inflammation and associated sickness following intranasal HMPV infection than wild-type C57BL/6 mice.28
There is some evidence that HMPV may spread beyond the respiratory tract, as studies have discovered HMPV in middle ear fluid and HMPV RNA in the brain tissue of a patient who passed away from encephalitis, though more research is needed.29-31
Diagnosis of HMPV
Among children in particular, human metapneumovirus induced respiratory tract infections are often common. Since it can aid in the prompt beginning of patient-specific treatment, early isolation in healthcare settings, and the required implementation of infection control techniques, an early and correct sampling and diagnosis is essential to the efficient management of respiratory infections related to HMPV.32 A number of conventional diagnostic techniques used by health centers to identify HMPV have serious drawbacks, such as lengthy analysis times, limited sensitivity, a lack of uniformity, and inadequate strain-specific characterization (Table 2 and 3). Over the past few decades, new approaches have been developed as a result of the diagnostic difficulties and information gaps that those antiquated procedures brought about.33 The introduction of rapid diagnostic tests in clinical settings appears to have significant possibilities due to its quick turnaround time and possible clinical impact. The diagnosis of HMPV is still limited, nonetheless, as evidenced by the necessity of differentiating it from other respiratory pathogens that have conditions on top of it.34 End users will have a clearer view of the usefulness of a determination or association with a patient’s progress thanks to the differential diagnosis of illnesses linked to HMPV infection.35 There are several limitations, Rapid antigen tests may cross-react with RSV or influenza and show lower sensitivity compared to RT-PCR, especially after day 5-7 of illness. Poor sampling technique can cause false negatives, and delayed testing reduces detection due to lower viral loads. PCR-based methods, while sensitive, are costly and not universally accessible. Coinfections are common, complicating interpretation without careful clinical assessment.
Table (2):
Diagnostic Tests for the detection of HMPV
Test Type |
Method |
Remark |
|---|---|---|
RT-PCR (Reverse Transcriptase PCR) |
Detects HMPV RNA |
Gold standard; high sensitivity and specificity. |
Rapid Antigen Detection Tests (RADTs) |
Lateral flow assays |
Available but limited sensitivity, especially in adults. Mostly used in pediatrics. |
Direct Fluorescent Antibody (DFA) Testing |
Fluorescent-labeled antibodies to HMPV antigens |
Quick (hours), but needs trained personnel and good-quality specimens. Lower sensitivity than PCR. |
Viral Culture |
Growth of HMPV in cell lines |
Rarely used clinically now; slow (days to weeks), requires specialized lab. |
Serology (Antibody Detection) |
IgM, IgG assays |
Useful in retrospective diagnosis or epidemiological studies; not helpful for acute management. |
Multiplex PCR Panels |
Detects multiple respiratory pathogens simultaneously (including HMPV) |
Useful for differentiating HMPV from RSV, influenza, adenovirus, etc. Very efficient in clinical settings. |
Table (3):
Respiratory Samples for HMPV Diagnosis
Sample Type |
Details |
Recommended for |
|---|---|---|
Nasopharyngeal Swab (NPS) |
Deep nasal swab reaching nasopharynx |
First-line in children; ideal for PCR/antigen tests. |
Nasopharyngeal Aspirate (NPA) |
Suction of nasal secretions |
Higher viral yield than swabs; common in infants/young children. |
Throat (Oropharyngeal) Swab |
Swab back of throat |
Less sensitive than NPS for HMPV. |
Sputum (expectorated or induced) |
Especially if productive cough |
More for older children or adults; rare in small kids. |
Endotracheal Aspirate |
Collected from intubated patients |
In critically ill children (ICU). |
Bronchoalveolar Lavage Fluid (BALF) |
From bronchoscopy |
Severe/deep lung infection; invasive, not routine. |
Combined Nasal + Throat Swabs |
Collected together |
Improves diagnostic yield without needing invasive methods. |
Molecular methods of detection of HMPV
Real Time RT-PCR
Real time Reverse Transcription Polymerase Chain Reaction is regarded as the gold standard for diagnosing HMPV. In Pune, India, there is a one-step real-time RT-PCR assay that can identify the HMPV lineages that are circulating and detect all four HMPV lineages. A probe and real-time primers were designed using conserved sections of the nucleoprotein gene. This molecular technique detects HMPV RNA in respiratory secretions, such as bronchoalveolar lavage specimens (BAL) or nasopharyngeal swabs.36
Antigen detection tests
Rapid tests for HMPV infection diagnosis enable the use of suitable infection control measures, thereby limiting nosocomial transmission, and are helpful in the evaluation of prompt antiviral medication treatment. There have been reports of quick antigen assays using HMPV-specific monoclonal antibodies for HMPV detection. Diagnostic Hybrids produced a direct fluorescent-antibody assay (DFA) for HMPV (Bartels; Trinity Biotech).37
Cell culture
Human metapneumovirus (HMPV), a member of the Paramyxoviridae family, represents a significant etiological agent of acute respiratory tract infections. The isolation and cultivation of HMPV in vitro remain challenging, and available data regarding its replication kinetics and cellular tropism are limited. Moreover, the stability of HMPV under varying environmental conditions, including temperature fluctuations, has not been comprehensively characterized. Among human-derived cell lines, the BEAS-2B airway epithelial cells demonstrated the highest permissiveness to HMPV replication. In contrast, among non-human primate-derived cell lines, LLC-MK2 and Vero cells exhibited superior permissiveness. Notably, due to their tolerance to trypsin treatment, LLC-MK2 cells remain the preferred substrate for efficient propagation of HMPV. Furthermore, the infectivity of HMPV was markedly enhanced when the virus was inoculated into cells cultured as monolayers.38
Serology
Serological assays, including enzyme-linked immunosorbent assays (ELISA), play an important role in the retrospective detection of human metapneumovirus (HMPV) infection and in seroepidemiological investigations. However, their application in the acute clinical diagnosis of respiratory infections is limited. Antibody responses against HMPV, particularly IgM and IgG, typically emerge only several days to weeks following initial infection, rendering serological methods suboptimal for early diagnosis and acute patient management. Consequently, molecular techniques such as RT-PCR are preferred for the timely identification of HMPV during the early stages of illness.
A novel ELISA platform has been developed utilizing a recombinant vesicular stomatitis virus (VSV) vector engineered to express the HMPV fusion (F) protein. Expression of the HMPV F protein was validated by Western blot analysis using HMPV F-specific antisera. This recombinant antigen-based ELISA exhibits high specificity for HMPV, with no detectable cross-reactivity with respiratory syncytial virus (RSV)-specific antibodies, despite the close phylogenetic relationship between RSV and HMPV within the Paramyxoviridae family. While this assay represents an advancement in serological tools for HMPV, it remains primarily suited for retrospective diagnosis, vaccine evaluation, and epidemiological studies rather than routine clinical practice. The lack of immediate clinical applicability stems from the inherent delay in antibody generation, variability in host immune responses, and the limited utility of serology in differentiating acute co-infections with other respiratory pathogens.39
Next-Generation Sequencing (NGS)
The fusion (F) and attachment (G) glycoprotein genes, which are directly impacted by antibody-mediated immunological pressure, provide the basis for HMPV genotyping. Whole genome sequencing is a powerful technique for investigating virus evolution and disease epidemiology, as well as for identifying transmission events and nosocomial outbreaks, because it yields more information than subgenomic fragment sequencing.40
Treatment of HMPV
Monoclonal antibodies
Recently, it was demonstrated that the human monoclonal antibody MPE8 had in vitro neutralizing activity against both RSV and HMPV. When used as passive prophylactics to prevent HMPV infection in hamsters, two murine MAbs, MAb 234 and MAb 338, demonstrated high-affinity binding to the F protein. When given prophylactically or therapeutically, intraperitoneal injection of MAb 338 into BALB/c mice resulted in a substantial reduction of lung virus titers following HMPV exposure. When administered therapeutically, Fab DS7, a recombinant human MAb fragment specific to the HMPV F protein, decreased viral titers in cotton rats’ lungs and slightly decreased titers in nasal tissues.41-43
Corticosteroids
The two main treatments are intravenous hydration and additional oxygen. Despite their empirical use, bronchodilators and corticosteroids have not been the subject of controlled trials for HMPV, and there is no evidence to support or contradict their effectiveness. A study conducted on cotton rats revealed that bronchodilators and corticosteroids were beneficial in treating experimental HMPV infection.44 For a similar virus, such as RSV, the sole antiviral medication currently on the market is ribavirin, an aerosolized nucleoside analogue. The in vitro neutralizing activity of polyclonal human IgG and ribavirin against HMPV is comparable to that of both substances against RSV.45
Ribavirin
There are presently no antivirals for the treatment of HMPV that are widely used. Although no controlled studies or data from extensive retrospective reviews support the use of ribavirin to treat HMPV pneumonia in humans, and no medication has yet shown therapeutic efficacy in humans, it is active against HMPV both in vitro and in vivo.46 Potentially effective treatment methods include intravenous, oral, or inhalation ribavirin either by itself or in conjunction with IVIG. In a retrospective review, the results of ten patients who received no treatment and thirteen immunocompromised individuals with HMPV pneumonia who received ribavirin ± IVIG were compared. Perhaps as a result of the delayed start of treatment, ribavirin medication was linked to similar mortality and greater hypoxemia.47
Palivizumab
A vaccination to prevent HMPV or HRSV has not been licensed. Furthermore, there is currently no approved therapeutic drug to treat HMPV. Palivizumab, a humanized monoclonal antibody (mAb), is given prophylactically to high-risk children, including preterm newborns and children with pre-existing comorbidities such congenital heart disease, bronchopulmonary dysplasia, and chronic lung sickness.48 According to estimates, palivizumab has a 50% success rate in averting hospitalization.49 As a result, new therapies to fight HRSV have been developed. Two of these therapeutic antibodies, MK-1654 and Nisevimab, are currently in clinical development.50 On the other hand, the development of existing HMPV therapies is still in the pre-clinical phase. The high cost and known adverse effects of ribavirin have hindered its widespread usage, despite the fact that aerosol delivery of this nucleotide analog may be effective against HRSV and HMPV.51,52
Motavizumab
MedImmune is developing motavizumab, a second-generation humanized monoclonal antibody, to prevent HMPV infection in high-risk individuals; Abbott Laboratories is also investigating the medication for the same purpose. Motavizumab targets a highly conserved epitope in the HMPV fusion (F) protein’s A antigenic site, which is crucial for HMPV’s cell-to-cell invasion.46 When compared to the first-generation mAb, motavizumab, which is only 13 amino acids different from palivizumab, has demonstrated a 70-fold increase in binding to the RSV F protein, with an 11-fold faster association rate and a 6-fold slower disassociation rate. In vitro, motavizumab was around 20 times more potent than palivizumab, and in vivo, it worked better at lower dosages.53,54
Clesrovimab (MK-1654-004)
Targeting position IV of the F protein, Clesrovimab is a completely human neutralizing monoclonal antibody against HMPV or RSV that has YTE mutations to extend its half-life. Clesrovimab has excellent safety and immunogenicity characteristics in healthy individuals. Merck announced in July 2024 that the Phase 2b/3 clinical trial MK-1654-004 (NCT04767373), which examined the preventive potential of clesrovimab against RSV infection, had produced good results. The purpose of this randomized, double-blind, placebo-controlled study was to assess the safety and effectiveness of a single intramuscular injectable dose of clesrovimab in preventing RSV or HMPV infection in newborns, including healthy preterm and term neonates.55
Management of HMPV
The management places a strong emphasis on monitoring for possible problems, preventive interventions, and supportive care. The strategy is similar to that of other viral respiratory infections, including RSV, because there is no particular antiviral medication available. Initiatives in public health are crucial to reducing transmission. Keeping a watchful eye out for any serious respiratory issues is essential. It is critical that you or another person seek medical attention right away if symptoms increase, such as breathing difficulties or a persistently high fever.56 Acetaminophen (Tylenol) and ibuprofen are two over-the-counter drugs that can effectively reduce fever and its accompanying discomfort and for Cough and Nasal Congestion, Decongestants, including nasal saline drops or sprays, can help relieve congestion in the nose. Congestion can also be lessened by using a humidifier in the space. The majority of patients do recover fully. To reduce and stop spread, all HMPV patients should be put on droplet precautions. As of right now, there is no HMPV vaccination. Nonetheless, a number of vaccinations against distinct HMPV structures have been tested on non-human primates and rodents and show promise; none of these have been tested on human volunteers.57
Treatment approaches for HMPV
Treatment approaches for HMPV focus on antiviral drugs, antibodies, fusion inhibitors, and RNA interference (RNAi). These techniques are currently being implemented in several in vitro and in vivo models to establish treatment efficacy for this respiratory virus. Antiviral investigation has examined ribavirin, which has been shown to work with HMPV in vitro and lessen pneumonia inflammation and viral replication in BALB/c mice. While there are some case studies on its use in humans, more clinical research needs to be done to determine its efficacy. Antibody based treatment approaches such as polyclonal and monoclonal antibodies (MAbs) have demonstrated success as well. While MAb 234 and MAb 338 were successful in hamsters for lung viral titer mitigation and in mice for lung viral titer mitigation, intravenous immunoglobulin (IVIg) showed neutralizing activity in vitro. In preclinical models, MAb MPE8 demonstrated effectiveness against HMPV and respiratory syncytial virus (RSV). MAb 54G10 showed neutralizing activity against the four subtype HMPV and proved effective in mice. Synthetic HR-1 sequences, a fusion inhibitor aimed at the HMPV’s F protein, have ascertained viral infectivity in vitro and have successfully protected mice from infection. Additionally, RNAi strategies utilizing siRNA against HMPV’s genes demonstrated subnanomolar inhibitory activity in vitro, where N-targeting siRNA reduced viral titers in mice significantly. These strides in therapy development are crucial steps toward effective management of HMPV infections in order to alleviate the respiratory disease burden.
Human metapneumovirus (HMPV), first identified in 2001, is now recognized as a significant respiratory pathogen, particularly affecting young children, the elderly, and immunocompromised individuals. Despite its global prevalence and clinical impact, no specific antiviral therapies or vaccines have yet been approved. Moreover, limitations in genomic data-including incomplete HMPV sequences-have hindered comprehensive evolutionary and epidemiological studies, underscoring the urgent need for improved case reporting and viral surveillance.
Advances in experimental therapeutics offer promising avenues for HMPV management. In preclinical models, antiviral agents such as ribavirin have demonstrated the ability to reduce viral replication and inflammation. Monoclonal antibodies, including MPE8 and 54G10, have shown potent neutralizing activity and protective efficacy. Additionally, RNA interference strategies and fusion inhibitors targeting the viral F protein have substantially limited viral replication in laboratory settings.
To effectively mitigate HMPV disease burden, future efforts must prioritize the development of targeted antiviral therapies and vaccines, enhance genomic surveillance, and standardize clinical protocols. Continued research is essential not only to address this specific pathogen but also to strengthen broader public health preparedness against emerging respiratory viruses.
ACKNOWLEDGMENTS
The authors extend their sincere gratitude to healthcare professionals whose dedication and contributions have significantly advanced the understanding of human metapneumovirus. Authors also express their heartfelt thanks to their respective institutions for their continued support and encouragement throughout the course of this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
BSA, NNS, BAT, and AH wrote original draft. NNS, BAT, FS, and MH wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Anderson EJ, Simoes EAF, Buttery JP, et al. Prevalence and characteristics of human metapneumovirus infection among hospitalized children at high risk for severe lower respiratory tract infection. J Pediatric Infect Dis Soc. 2012;1(3):212-222.
Crossref - Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006 Jul;19(3):546-57.
- Afonso CL, Amarasinghe GK, Banyai K, et al. Taxonomy of the order Mononegavirales: update 2016. Arch Virol. 2016;161(8):2351-2360.
Crossref - Van den Hoogen BG, Bestebroer TM, Osterhaus ADME, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295(1):119-132.
Crossref - Freymuth F, Vabret A, Legrand L, et al. Presence of the new human metapneumovirus in French children with bronchiolitis. Ped Infect Dis J. 2003;22(1):92-94.
Crossref - Evans AS. Viral Infections of Humans. Epidemiology and Control. 1989. Epidemiologic concepts and methods. 1989:22-28.
Crossref - Caracciolo S, Minini C, Colombrita D, et al. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features. Pediatr Infect Dis J. 2008;27(5):406-412.
Crossref - Foulongne V, Guyon G, Rodiere M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25(4):354-359.
Crossref - van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23(1):S25-32.
Crossref - Wilczynski J, Litwinska B. Human metapneumovirus-new identified virus infecting human respiratory tract. Przegl Epidemiol. 2004;58(2):325-33.
- Tecu C, Orasanu D, Sima A, et al. First detection of human metapneumovirus in children with respiratory infections in Romania. Roum Arch Microbiol Immunol. 2007;66(1-2):37-40.
- Moe N, Krokstad S, Stenseng IH, et al. Comparing Human Metapneumovirus and Respiratory Syncytial Virus: Viral Co-Detections, Genotypes and Risk Factors for Severe Disease. PLoS One. 2017;12(1):e0170200.
Crossref - Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Eng J Med. 2013;368(7):633-643.
Crossref - Kulkarni D, Cong B, Ranjini MJK, et al. The global burden of human metapneumovirus-associated acute respiratory infections in older adults: a systematic review and meta-analysis. Lancet Healthy Longev. 2025;6(2):100679.
Crossref - Wang X, Li Y, Deloria-Knoll M, et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob Health. 2021;9(1):e33-e43
- Banerjee S, Sullender WM, Choudekar A, et al. Detection and genetic diversity of human metapneumovirus in hospitalized children with acute respiratory infections in India. J Med Virol. 2011;83(10):1799-1781.
Crossref - Babawale PI, Guerrero-Plata A. Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes. Pathogens. 2024;13(4):316.
Crossref - Pragathi P, Shetty U, Parida P, Varamballi P, Mukhopadhyay C, Sudheesh N. Molecular detection and genotyping of HMPV in patients with severe acute respiratory infection in India. Annals of Medicine. 2024;56(1):2398719.
Crossref - Hamelin ME, Yim K, Kuhn KH, et al. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J Virol. 2005;79(14):8894-8903.
Crossref - Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82(7):1282-1290.
Crossref - van den Hoogen BG, Herfst S, Sprong L, et al. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10(4):658-666.
Crossref - Papenburg J, Carbonneau J, Isabel S, et al. Genetic diversity and molecular evolution of the major human metapneumovirus surface glycoproteins over a decade. J Clin Virol. 2013;58(3):541-547.
Crossref - Hermens JM, Kesmir C. Role of T cells in severe COVID-19 disease, protection, and long term immunity. Immunogenetics. 2023;75(3):295-307.
Crossref - Betakova T, Kostrabova A, Lachova V, Turianova L. Cytokines Induced During Influenza Virus Infection. Curr Pharm Des. 2017;23(18):2616-2622.
Crossref - Zeng H, Belser JA, Goldsmith CS, et al. A(H7N9) virus results in early induction of proinflammatory cytokine responses in both human lung epithelial and endothelial cells and shows increased human adaptation compared with avian H5N1 virus. J Virol. 2015;89(8):4655-4667.
Crossref - Dey P, Ahuja A, Panwar J, et al. Immune Control of Avian Influenza Virus Infection and Its Vaccine Development. Vaccines. 2023;11(3):593.
Crossref - Malainou C, Abdin SM, Lachmann N, Matt U, Herold S. Alveolar macrophages in tissue homeostasis, inflammation, and infection: evolving concepts of therapeutic targeting. J Clin Invest. 2023;133(19):e170501.
Crossref - Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol. 2022;13:812774.
Crossref - Kinder JT, Moncman CL, Barrett C, Jin H, Kallewaard N, Dutch RE. Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies. J Virol. 2020;94(20):e01068-20.
Crossref - Schildgen O, Glatzel T, Geikowski T, et al. Human metapneumovirus RNA in encephalitis patient. Emerg Infect Dis. 2005;11(3):467-470.
Crossref - Sivertsen B, Christensen PB. Acute encephalitis. Acta Neurol Scand. 1996;93(2-3):156-159.
Crossref - Banerjee S, Bharaj P, Sullender W, Kabra SK, Broor S. Human metapneumovirus infections among children with acute respiratory infections seen in a large referral hospital in India. J Clin Virol. 2007;38(1):70-72.
Crossref - Nabeya D, Kinjo T, Ueno S, et al. Characteristics of patients with viral infections of the lower respiratory tract: A retrospective study. Medicine. 2022;101(38):e30819.
Crossref - Vemula SV, Zhao J, Liu J, Wang X, Biswas S, Hewlett I. Current approaches for diagnosis of influenza virus infections in humans. Viruses. 2016;8(4):96.
Crossref - Ramani S, Kang G. Viruses causing childhood diarrhoea in the developing world. Curr Opin Infect Dis. 2009;22(5):477-482.
Crossref - Choudhary ML, Anand SP, Sonawane NS, Chadha MS. Development of real-time RT-PCR for detection of human metapneumovirus and genetic analysis of circulating strains (2009-2011) in Pune, India. Arch Virol. 2014;159(2):217-225.
Crossref - Aslanzadeh J, Zheng X, Li H, et al. Prospective evaluation of rapid antigen tests for diagnosis of respiratory syncytial virus and human metapneumovirus infections. J Clin Microbiol. 2008;46(5):1682-165.
Crossref - Tollefson SJ, Cox RG, Williams JV. Studies of culture conditions and environmental stability of human metapneumovirus. Virus Res. 2010;151(1):54-59.
Crossref - Leung J, Esper F, Weibel C, Kahn JS. Seroepidemiology of human metapneumovirus (HMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing HMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol. 2005;43(3):1213-1219.
Crossref - Groen K, van Nieuwkoop S, Bestebroer TM, Fraaij PL, Fouchier RAM, van den Hoogen BG. Whole genome sequencing of human metapneumoviruses from clinical specimens using MinION nanopore technology. Virus Res. 2021;302:198490.
Crossref - Corti D, Bianchi S, Vanzetta F, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501:439-443.
Crossref - Ulbrandt ND, Ji H, Patel NK, et al. Isolation and characterization of monoclonal antibodies which neutralize human metapneumovirus in vitro and in vivo. J Virol. 2006;80(16):7799-7806.
Crossref - Hamelin ME, Gagnon C, Prince GA, et al. Prophylactic and therapeutic benefits of a monoclonal antibody against the fusion protein of human metapneumovirus in a mouse model. Antiviral Res. 2010;88(1):31-37.
Crossref - Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother. 2006;50(2):774-777.
Crossref - Wyde PR, Chetty SN, Jewell AM, Boivin G, Piedra PA. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2003;60(1):51-59.
Crossref - Haas LE, Thijsen SF, van Elden L, Heemstra KA. Human metapneumovirus in adults. Viruses. 2013;5(1):87-110.
- Renaud C, Xie H, Seo S, et al. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19(8):1220-1226.
Crossref - Kumar P, Srivastava M. Prophylactic and therapeutic approaches for human metapneumovirus. VirusDisease. 2018;29:434-444.
Crossref - Storch G.A. Humanized Monoclonal Antibody for Prevention of Respiratory Syncytial Virus Infection. Pediatrics. 1998;102(3):648-651.
Crossref - Griffin MP, Yuan Y, Takas T, et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N Engl J Med. 2020;383(5):415-425.
Crossref - Principi N, Esposito S. Paediatric human metapneumovirus infection: Epidemiology, prevention and therapy. J Clin Virol. 2014;59(3):141-147.
Crossref - Miller RJ, Mousa JJ. Structural basis for respiratory syncytial virus and human metapneumovirus neutralization. Curr Opin Virol. 2023;61:101337.
Crossref - Kinder JT, Moncman CL, Barrett C, Jin H, Kallewaard N, Dutch RE. Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies. J Virol. 2020;94(20):e01068-20.
Crossref - Weisman LE. Motavizumab, a second-generation humanized mAb for the prevention of respiratory syncytial virus infection in high-risk populations. Curr Opin Mol Ther. 2009;11(2):208-18. Erratum in: Curr Opin Mol Ther. 2009;11(3):346-347.
- Phuah JY, Maas BM, Tang A, et al. Quantification of clesrovimab, an investigational, half life extended, anti respiratory syncytial virus protein F human monoclonal antibody in the nasal epithelial lining fluid of healthy adults. Biomed Pharmacother. 2023;169:115851.
Crossref - Schildgen V, van den Hoogen B, Fouchier R, et al. Human Metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev. 2011;24(4):734-754.
Crossref - Haas LEM, Thijsen SFT, van Elden L, Heemstra KA. Human metapneumovirus in adults. Viruses. 2013;5(1):87-110.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.