Phage therapy is a treatment method that uses bacteriophages, which are viruses that infect bacteria, to treat bacterial infections. Phages are natural adversaries, helping to restrict their proliferation in the natural environment. Phages are made up of DNA or RNA in a protein capsid and cannot multiply independently, relying on bacterial hosts to live. The use of antibiotics in people and animals is a key contributor to antimicrobial resistance (AMR), a serious public health concern in the twenty-first century. Compared to traditional antibiotic treatments, phage therapy has several advantages, including automatic dosing, low inherent toxicity, and the ability to use low doses. Phages infect bacteria, reprogramming the cell to become a phage factory, and producing new phage particles that lyse the cell and release more phages. Some phages have a temperate life cycle in which infected cells carry the phage genome indefinitely in a dormant state. Since 1919, phage treatment has been used to treat diseases such as Shigella dysenteries and has the potential to be utilized to treat antibiotic-resistant bacteria.
Phage Therapy, Antimicrobial Resistance, Thermophage, Multidrug Resistance
Bacteriophages or phages are the most abundant organisms in the biosphere and they are a ubiquitous feature of prokaryotic existence. Phages are generally specific, and their specificity is determined by the surface of the phage–host receptor, the physiological defense mechanisms of the phage and the host, the phage’s nature, and their co-evolution.1 Felix d’Herelle discovered the potential of bacteriophage in 1917, creating a phage mixture to cure World War I soldiers with dysentery.2 Scientists have been interested in phages as tools for understanding fundamental molecular biology, vectors of horizontal gene transfer and drivers of bacterial evolution, suppliers of diagnostic and genetic tools, and potential therapeutic agents.3 The rise of antibiotic-resistant microorganisms has rekindled interest in bacteriophage treatment in recent years. Antibiotic resistance is an increasing worldwide health concern, and phage therapy may give an alternative therapeutic option for bacterial infections.4-6
The emergence of antibiotic-resistant bacteria has become a major public health threat worldwide. The overuse and misuse of antibiotics have led to the development of bacterial strains that are resistant to multiple antibiotics, making the treatment of infections increasingly difficult.7 It is estimated that 50% of all antibiotics used today will become ineffective in a few years as a result of bacterial resistance.8 Antibiotic abuse in humans and animals is one of the main causes of AMR.9 The excessive use of antibiotics in animals has contributed to the rapid spread of antibiotic-resistant genes and the emergence of antimicrobial-resistant pathogens. The overuse of antibiotics in humans is an important factor in the development of multidrug-resistant pathogens (MDRs), which causes high morbidity and mortality rates in patients with serious infections.10 Some of the examples of MDRs are MRSA (Methicillin-Resistant Staphylococcus aureus) is a strain of the common bacterium Staphylococcus aureus that is resistant to multiple antibiotics, including methicillin and other beta-lactam antibiotics. It can cause a wide range of infections, including skin infections, pneumonia, and bloodstream infections.11 ESBL-Producing Enterobacteriaceae includes bacteria such as Escherichia coli and Klebsiella pneumoniae that produce extended-spectrum beta-lactamases (ESBLs), making them resistant to a broad range of beta-lactam antibiotics, including cephalosporins and penicillins.12 Thermophages, as the name suggests, are bacteriophages that are adapted to thrive in high-temperature environments, typically above 50°C (122°F). These extremophiles have evolved mechanisms to not only withstand the extreme heat but also to utilize it for their reproductive cycle.13 By studying thermophages, researchers have gained insights into the molecular adaptations that allow these viruses to maintain their structural integrity and function even at elevated temperatures (Figure 1).
In the 21st century, an important public health issue is antimicrobial resistance (AMR), which threatens the efficient prevention and treatment of an increasing number of diseases caused by bacteria, parasites, viruses, and fungi that are no longer treatable with traditional treatments. AMR is a major concern, especially in light of bacterial antibiotic resistance.14 Bacteriophage therapy has the potential to be a safe and effective therapeutic option for bacterial infections, particularly those caused by antibiotic-resistant strains.
Use
Treats antibiotic-resistant infections
Bacteriophages, or phages, offer a unique solution to the growing problem of antibiotic resistance. Unlike antibiotics, which target a broad spectrum of bacteria, phages are highly specific in their ability to infect and kill particular strains of bacteria. This specificity means that even bacteria resistant to antibiotics can be vulnerable to phage attack. Phage therapy thus provides a promising avenue for treating infections that have become refractory to traditional antibiotic treatments.
Targeted therapy
Phage therapy’s hallmark is its precision. Bacteriophages have evolved to recognize and bind to specific receptors on the surfaces of their bacterial hosts (Table). This high degree of specificity means that when phages are introduced into the body, they selectively target the disease-causing bacteria while sparing beneficial bacteria and human cells. This precision significantly reduces the risk of side effects commonly associated with broad-spectrum antibiotics, such as disruptions to the body’s microbiome.
Table:
Timeline of Bacteriophage therapy
Year |
Event |
|---|---|
1896 |
Ernest Hankin discovered antimicrobial activity in the Ganges River water in India.15 |
1915 |
Bacteriophages were discovered independently by Frederick Twort and Felix d’Herelle.16 |
1917-1919 |
Felix d’Herelle first uses bacteriophages to cure human bacterial illnesses.2 |
1923 |
Eli Lilly and Company began manufacturing and marketing phage preparations.17 |
1928 |
The discovery of penicillin reduces interest in phage treatment in the West.18 |
1930s-1940s |
Phage therapy continues to be used in Eastern Europe and the Soviet Union.19 |
1080s-1990s |
The advent of antibiotic-resistant microorganisms has reignited Western interest in phage treatment.5 |
2006 |
The FDA has approved the first phage treatment clinical study in the United States.20 |
2019 |
The FDA has approved the first phage treatment clinical study in the United States.21 |
Potential for personalized treatment
One of the most remarkable aspects of phage therapy is its adaptability to individual patients. When facing bacterial infections that are resistant to multiple antibiotics or exhibit unique characteristics, phage therapy can be customized. By isolating and identifying the infecting bacteria, healthcare providers can select the most appropriate phages to combat the specific strain causing the infection. This personalized approach enhances the chances of successful treatment, especially in cases where traditional antibiotics fail.
Low toxicity
Bacteriophages are generally regarded as safe for human use. They are naturally occurring viruses that have evolved alongside bacteria for eons. Clinical studies and historical use have demonstrated that phages have low toxicity to humans. This characteristic minimizes the risk of adverse side effects commonly associated with certain antibiotics. While some concerns exist about potential immune responses to phages, these reactions are typically mild and manageable, further establishing phage therapy’s safety profile.
Environmental benefits
Unlike antibiotics, which can accumulate in the environment and contribute to the development of antibiotic-resistant bacteria in various ecosystems, bacteriophages have minimal environmental impact. Phages are naturally occurring entities found in soil, water, and other environments. They do not introduce synthetic chemicals into ecosystems and are biodegradable. This environmentally friendly aspect aligns with the growing awareness of the need for sustainable and responsible medical treatments.
How phage therapy works
Phages are infectious particles with at least two of each of the following: nucleic acid and protein, much like viruses. A cycle of phage generation begins when a phage plunges into a bacterial cell that is open to it.22 In this cycle, cells are reprogrammed to become phage factories, and the components of biosynthetic devices are removed from the usual roles in bacterial growth.23
Phage-specific proteins are translated from phage mRNA produced after infection and initiate numerous reprogramming processes. The temporal schedule is structured and governed. Replication of nucleic acids typically occurs first, and then the production of the phage particle’s structural proteins. The creation of fresh phage particles is followed by their discharge from the cell.24 Cell envelope ruptures and cell lysis, resulting in cell contents, including new phage particles, being released into the media, occur in most Coliphages studied in classical methods. The entire process can take up to 40 minutes and result in 100 new phages. For many phages, the lytic cycle is the only means of reproduction (Figure 2).25
Other TEMPERATE phages have a different life cycle in which some infected cells enter the lytic phase of infection, while other cells survive the infection and carry the phage genome in a quiescent state indefinitely.26 Prophage-containing cells are called LYSOGENIC cells. Lysogenic cells can be induced by specific substances to reenter the lytic cycle.
The simple manner in which phages can synchronize the cell cycle and track the progress of molecular events throughout the culture is one of the reasons why phages are important in research.18 Simultaneous infection or activation of phage growth of phages in lysogenic cells can cause synchronization. The second factor is the simplicity of isolation and study of mutations in specific stages of the cycle.27
Advantages
Phage characteristics can be used to explain why phage therapy is preferable to the use of prescription antibiotics.
Automatic “dosing.”
Although the reliance on relatively high bacterial concentrations is a restriction, phages are capable of proliferating, particularly where hosts are present throughout the bacterial-killing process. Because the phages themselves help choose the phage dose, we refer to this as auto-dosing.28
The inherent toxicity is low
Phages are naturally harmless because nucleic acids and proteins make up most of their structure. However, there is little proof that doing so while treating phages creates a risk. Immune systems can interact with phages, leading to unfavorable immunological reactions.29 Some phage therapy regimens may require high-purity phage preparations to prevent allergic reactions to bacterial components such as endotoxins present in crude phage lysates. Phages can kill bacteria in place similarly to antibiotics that harm cell walls, but can also release parts of the bacterium as they do.30
Capacity for low-dose use
Since the phage’s density increases in situ, given the appropriate bacterial density, reducing the amount of phage needed to achieve its effectiveness can reduce treatment costs. The use of phages in low concentrations can help promote product safety because phages develop in density if they actively remove bacteria and otherwise do not stay for an extended period within the body.31 However, there is minimal evidence to show that the use of pure phage preparations or higher doses of phage may increase negative effects as compared to using lower phage doses. Avoiding phage applications at higher levels for safety concerns is only beneficial if doing so is risky.32
Low cost relatively
The two basic processes of phage growth and subsequent purification are host growth and purification. Although the costs of host growth vary depending on the type of bacteria, as technology develops, the cost of cleaning appears to be decreasing.28,33 The cost of discovery (isolation) and description is fairly low and the cost of phage manufacturing is equivalent to the cost of pharmaceutical products on average.34
Rapid discovery
Phages are resistant to many dangerous bacteria, usually found in high-density wastewater and other waste. However, if it is difficult to cultivate host bacteria and there are several types of bacteria to which they are susceptible, isolation may be more technical. Unlike dangerous antibiotics, phages can be isolated from most targeted microorganisms and show no toxicity or low toxicity.35,36
Disadvantages
In addition to its high ability to locate and eliminate pathogens, effective therapeutic phages should have a low tendency to harm the ecosystem they supply. As long as the phage is legally active, stable under normal storage conditions and temperatures, subject to appropriate safety and efficacy investigations, and preferably fully sequenced, it is recommended that undesirable genes, such as toxins, do not exist.37 These qualities are almost always present. It should be stressed that “obligatory lytic” phages lack lysogeny and can only be released from infected cells by lysis. Due to the combination of immune immunity to infection, the encoding of viral bacterial factors, including bacterial toxins, and the insensitivity of phages to parasites, it is difficult to treat phages with moderate phages.38
Phage characterization aims to exclude from therapy any phages that have a poor ability to kill the target bacteria, as well as any phages that are temperate or toxin-carrying. This low “virulence” can be caused by a lack of adsorption power, a limited capacity to overcome bacterial defenses, and weak replication power.39 Phages have poor pharmacokinetics, which refers to poor absorption, distribution, and in situ survival. This makes them less desirable for therapeutic use. The potential of phages to spread bacterial genes between bacteria should be minimal.40
Virion morphology, protein profiles, and genotype characterization can also be included in phage characterization in addition to full genome sequencing, although the expense of thorough phage characterization can be prohibitive before phage use.41 The general goal is to find phages with strong primary pharmacodynamics, minimal secondary pharmacodynamics, and good pharmacokinetics.42 If phages do not meet these requirements, it is not typically advisable to use them as treatments. The absolute least is to exclude temperate phages and the ideal is to use whole genome sequencing to rule out the carry of virulence factors.43
Phage cocktails
Due to the huge diversity of environmental phages, it is much more difficult to design a phage cocktail than to design a regimen of combined antibiotic therapy.44 The effectiveness depends on the composition of the phage cocktail. One of the main logistical problems is deciding whether to use standard or personalized cocktail therapy. Although the components of the phage cocktail were outside the scope of this study and were thoroughly investigated elsewhere, they were not investigated at this time.45 Although developing phage cocktails for each disease costs time and money, a “one-size-fits-all” approach may not provide the strain specificity required for successful therapeutic results.46
The development of therapeutic phage cocktails can be difficult due to the diversity of environmental phages. To ensure its effectiveness, it is essential to determine the composition of the phage cocktail. However, it is more complex to formulate a phage cocktail than to create a combination antibiotic therapy program.
One of the most difficult decisions is whether to use a standard or personalized phage cocktail. Although the components of a phage cocktail are beyond the scope of this investigation, extensive research has been conducted in this area. Developing a phage cocktail for each disease takes time and money, but a one-size-fits-all strategy may be ineffective due to the strain specificity necessary for excellent treatment effects.
In summary, creating a therapeutic phage cocktail is a difficult procedure that requires careful study of the phage makeup. Although a standard phage cocktail is more convenient, it may lack the strain specificity required for successful therapeutic outcomes.
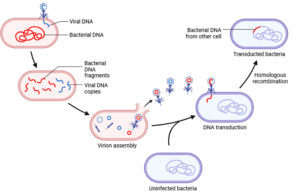
Bacteria evolve resistance to phages through genetic mutations, altering cell surface receptors, producing proteins that block phage attachment, releasing decoy molecules, and enhancing extracellular matrix production. Additionally, reversible gene expression changes and epigenetic modifications reduce receptor availability. While protecting against phages, these adaptations may impact the host’s fitness, necessitating specific defense mechanisms within complex microbial communities.47
Recent developments
- Clinical Trials: Phage therapy was advancing through various stages of clinical trials for the treatment of bacterial infections. Researchers were evaluating its effectiveness, safety, and optimal dosing regimens in humans.48
- Phage Cocktails: The use of phage cocktails, which consist of multiple phage strains targeting a specific bacterial pathogen, gained traction. This approach aimed to overcome the potential for bacterial resistance to single phages.45
- Phage Discovery: Ongoing efforts in phage discovery and isolation were expanding the repertoire of phages available for therapy. Researchers were looking for phages that target a wide range of bacterial pathogens.49
- Phage Engineering: Some research focused on phage engineering techniques to enhance their therapeutic potential. This included genetic modifications to improve phage stability, host range, and efficacy.50
- Regulatory Framework: Regulatory agencies in various countries were working to establish clear guidelines and regulations for the use of phage therapy in clinical settings. This was important for ensuring safety and efficacy standards.51
- Combination Therapies: Combining phage therapy with antibiotics or other antimicrobial agents was being explored to improve treatment outcomes, especially in cases of multidrug-resistant infections.5
- Phage Banks: The establishment of phage banks, similar to antibiotic libraries, was in progress. These repositories would store a diverse collection of phages for potential clinical use.52
- Personalized Medicine: Tailoring phage therapy to individual patients based on the specific bacterial strain causing their infection was under investigation. This personalized approach aimed to maximize treatment efficacy.53
- Education and Awareness: Efforts were underway to educate healthcare professionals about phage therapy, its potential benefits, and its limitations.54
Bacteriophages hold immense promise as medicinal agents in the ongoing battle against bacterial infections. Their safety and remarkable efficacy in lysing dangerous bacteria, as demonstrated in clinical usage, underscore their potential as valuable therapeutic tools. The dynamic landscape of phage therapy research, marked by clinical trials, phage cocktails, genetic enhancements, regulatory advancements, and personalized treatment approaches, suggests a bright future for this field. To stay up-to-date on the latest developments, it is essential to follow ongoing research and regulatory updates, as phage therapy continues to evolve as a promising avenue in the fight against antibiotic-resistant infections.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kasman LM, Porter LD. Bacteriophages. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. http://www.ncbi.nlm.nih.gov/books/NBK493185/
- Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage. 2011;1(2):66-85.
Crossref - Carlton RM. Phage therapy: past history and future prospects. Arch Immunol Ther Exp (Warsz). 1999;47(5):267-74.
- Merabishvili M, Pirnay JP, Verbeken G, et al. Quality-Controlled Small-Scale Production of a Well-Defined Bacteriophage Cocktail for Use in Human Clinical Trials. Ojcius DM, editor. PLoS ONE. 2009;4(3):e4944.
Crossref - Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8(3):162.
Crossref - Summers WC. Bacteriophage therapy. Annu Rev Microbiol. 2001;55(1):437-451.
Crossref - Hicks LA, Bartoces MG, Roberts RM, et al. US Outpatient Antibiotic Prescribing Variation According to Geography, Patient Population, and Provider Specialty in 2011. Clin Infect Dis. 2015;60(9):1308-1316.
Crossref - Gupta R, Prasad Y. Efficacy of Polyvalent Bacteriophage P-27/HP to Control Multidrug Resistant Staphylococcus aureus Associated with Human Infections. Curr Microbiol. 2011;62(1):255-260.
Crossref - Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057-1098.
Crossref - Ackermann HW, Ackermann HW. The first phage electron micrographs. Bacteriophage. 2011;1(4):225-227.
Crossref - Shelke YP, Bankar NJ, Bandre GR, Hawale DV, Dawande P. An Overview of Preventive Strategies and the Role of Various Organizations in Combating Antimicrobial Resistance. Cureus. 2023;15(9):e44666.
Crossref - Rawat D, Nair D. Extended-spectrum b-lactamases in gram negative bacteria. J Glob Infect Dis. 2010;2(3):263-274.
Crossref - Liu H, Kheirvari M, Tumban E. Potential Applications of Thermophilic Bacteriophages in One Health. Int J Mol Sci. 20234;24(9):8222.
Crossref - Kazmierczak Z, Gorski A, Dabrowska K. Facing Antibiotic Resistance: Staphylococcus aureus Phages as a Medical Tool. Viruses. 2014;6(7):2551-2570.
Crossref - Deresinski S. Bacteriophage Therapy: Exploiting Smaller Fleas. Clin Infect Dis. 2009;48(8):1096-1101.
Crossref - Summers WC. The strange history of phage therapy. Bacteriophage. 2012;2(2):130-133.
Crossref - Pirnay JP. Phage Therapy in the Year 2035. Front Microbiol. 2020;11:1171.
Crossref - Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5(1):226-235.
Crossref - Myelnikov D. An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922-1955. J Hist Med Allied Sci. 2018;73(4):385-411.
Crossref - Parracho HM, Burrowes BH, Enright MC, McConville ML, Harper DR. The role of regulated clinical trials in the development of bacteriophage therapeutics. J Mol Genet Med. 2012;6:279-286.
Crossref - McCallin S, Sacher J, Zheng J, Chan BK. Current State of Compassionate Phage Therapy. Viruses. 2019;11(4):343.
Crossref - Sunagar R, Patil SA, Chandrakanth RK. Bacteriophage therapy for Staphylococcus aureus bacteremia in streptozotocin-induced diabetic mice. Res Microbiol. 2010;161(10):854-860.
Crossref - Ventola CL. The Antibiotic Resistance Crisis. Pharm Ther. 2015;40(4):277-283.
- Penziner S, Schooley RT, Pride DT. Animal Models of Phage Therapy. Front Microbiol. 2021;12:631794.
Crossref - Zurabov FM, Chernevskaya EA, Beloborodova NV, et al. Bacteriophage Cocktails in the Post-COVID Rehabilitation. Viruses. 2022;14(12):2614.
Crossref - Caflisch KM, Suh GA, Patel R. Biological challenges of phage therapy and proposed solutions: a literature review. Expert Rev Anti Infect Ther. 2019;17(12):1011-1041.
Crossref - Brix A, Cafora M, Aureli M, Pistocchi A. Animal Models to Translate Phage Therapy to Human Medicine. Int J Mol Sci. 2020;21(10):3715.
Crossref - Skurnik M, Pajunen M, Kiljunen S. Biotechnological challenges of phage therapy. Biotechnol Lett. 2007;29(7):995-1003.
Crossref - Altamirano FLG, Barr JJ. Phage Therapy in the Postantibiotic Era. Clin Microbiol Rev. 2019;32(2):e00066-18.
Crossref - Malik DJ, Sokolov IJ, Vinner GK, et al. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv Colloid Interface Sci. 2017;249:100-133.
Crossref - Maciejewska B, Olszak T, Drulis-Kawa Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application? Appl Microbiol Biotechnol. 2018;102(6):2563-2581.
Crossref - Bull JJ, Levin BR, Molineux IJ. Promises and Pitfalls of In Vivo Evolution to Improve Phage Therapy. Viruses. 2019;11(12):1083.
Crossref - Mdarhri HA, Benmessaoud R, Yacoubi H, et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics. 2022;11(12):1826.
Crossref - Anyaegbunam NJ, Anekpo CC, Anyaegbunam ZKG, et al. The resurgence of phage-based therapy in the era of increasing antibiotic resistance: From research progress to challenges and prospects. Microbiol Res. 2022;264:127155.
Crossref - Endersen L, O’Mahony J, Hill C, Ross RP, McAuliffe O, Coffey A. Phage Therapy in the Food Industry. Annu Rev Food Sci Technol. 2014;5(1):327-349.
Crossref - Chan HCS, Shan H, Dahoun T, Vogel H, Yuan S. Advancing Drug Discovery via Artificial Intelligence. Trends Pharmacol Sci. 2019;40(8):592-604.
Crossref - Monk AB, Rees CD, Barrow P, Hagens S, Harper DR. Bacteriophage applications: where are we now?: Bacteriophage applications. Lett Appl Microbiol. 2010;51(4):363-369.
Crossref - Yoshikawa TT. Antimicrobial Resistance and Aging: Beginning of the End of the Antibiotic Era? J Am Geriatr Soc. 2002;50(7 suppl):226-9.
Crossref - Valente L, Prazak J, Que YA, Cameron DR. Progress and Pitfalls of Bacteriophage Therapy in Critical Care: A Concise Definitive Review. Crit Care Explor. 2021;3(3):e0351.
Crossref - Danis-Wlodarczyk K, Dabrowska K, Abedon ST. Phage Therapy: The Pharmacology of Antibacterial Viruses. Curr Issues Mol Biol. 2021;81-164.
Crossref - Bentley R, Bennett JW. What Is an Antibiotic? Revisited. Adv Appl Microbiol. 2003;52:303-331.
Crossref - Dabrowska K, Abedon ST. Pharmacologically Aware Phage Therapy: Pharmacodynamic and Pharmacokinetic Obstacles to Phage Antibacterial Action in Animal and Human Bodies. Microbiol Mol Biol Rev. 2019;83(4):e00012-19.
Crossref - Makumi A, Mhone AL, Odaba J, Guantai L, Svitek N. Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa. Antibiotics. 2021;10(9):1085.
Crossref - Goodridge L. Designing Phage Therapeutics. Curr Pharm Biotechnol. 2010;11(1):15-27.
Crossref - Gill J, Hyman P. Phage Choice, Isolation, and Preparation for Phage Therapy. Curr Pharm Biotechnol. 2010;11(1):2-14.
Crossref - Kutter E, De Vos D, Gvasalia G, et al. Phage Therapy in Clinical Practice: Treatment of Human Infections. Curr Pharm Biotechnol. 2010;11(1):69-86.
Crossref - Egido JE, Costa AR, Aparicio-Maldonado C, Haas PJ, Brouns SJJ. Mechanisms and clinical importance of bacteriophage resistance. FEMS Microbiol Rev. 2022;46(1):fuab048.
Crossref - Brives C, Pourraz J. Phage therapy as a potential solution in the fight against AMR: obstacles and possible futures. Palgrave Commun. 2020;6(1):100.
Crossref - Strathdee SA, Hatfull GF, Mutalik VK, Schooley RT. Phage therapy: From biological mechanisms to future directions. Cell. 2023;186(1):17-31.
Crossref - Meile S, Du J, Dunne M, Kilcher S, Loessner MJ. Engineering therapeutic phages for enhanced antibacterial efficacy. Curr Opin Virol. 2022;52:182-191.
Crossref - Brussow H. What is needed for phage therapy to become a reality in Western medicine? Virology. 2012;434(2):138-142.
Crossref - Nagel T, Musila L, Muthoni M, Nikolich M, Nakavuma JL, Clokie MR. Phage banks as potential tools to rapidly and cost-effectively manage antimicrobial resistance in the developing world. Curr Opin Virol. 2022;53:101208.
Crossref - Hibstu Z, Belew H, Akelew Y, Mengist HM. Phage Therapy: A Different Approach to Fight Bacterial Infections. Biol Targets Ther. 2022;16:173-186.
Crossref - Hyman P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals. 2019;12(1):35.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.