ISSN: 0973-7510
E-ISSN: 2581-690X
Indiscriminate use of antibiotics has exerted selective pressure on the gut microbiota, increasing the prevalence of antibiotic-resistant bacteria among the commensal population. However, in a state of intestinal dysbiosis, the patient’s flora contributes to the spreading of genes bearing determinants for antibiotic-resistance via horizontal transfer of drug-resistant plasmid. Although the in vivo spread of antibiotic resistance through this mechanism is well-known, limited studies demonstrate evidence of it occurring between commensal and pathogenic Enterobacteriaceae in patients. This study investigated the possibility of horizontal transfer of plasmids bearing blaCTX-M, from a gut E. coli to pathogenic Enterobacteriaceae and vice versa. Clinical specimens from twelve patients were screened for beta-lactamase producing Enterobacteriaceae, followed by isolating corresponding gut Enterobacteriaceae from stool samples. Standard bacteriological procedures and antibiotic sensitivity testing were performed to identify and confirm ESBL, AmpC beta-lactamase, and carbapenemase producers. PCR to confirm beta-lactamase genes, ERIC PCR to assess clonal similarity, PCR-based replicon typing for plasmid profiling, conjugation by broth mating assay for evaluating the horizontal transfer of plasmids, were performed to study the possibility of horizontal transfer of blaCTX-M gene between gut and pathogenic E. coli. In two patients, we demonstrated the potential for horizontal transfer of blaCTX-M carrying IncFIA and IncFIB plasmids between uropathogenic E. coli and gut E. coli, in vivo. This study confirms in vivo horizontal transfer of plasmids bearing blaCTX-M types IncFIA and IncFIB between gut commensal and uropathogenic E. coli, highlighting the need for stringent antibiotic stewardship to curb multidrug-resistant pathogen spread.
HGT, Uropathogenic E. coli, IncFIA, IncFIB, Plasmids
Members of the Enterobacteriaceae family, a group of Gram-negative rod-shaped bacteria, are commonly associated with a wide range of clinical infections, including those affecting the urinary tract, surgical wounds, bloodstream, and respiratory system. Management of these infections frequently involves the use of antimicrobial therapy. Among the drugs most effective against these organisms are cephalosporins, carbapenems, aminoglycosides, and fluoroquinolones.1,2
In pathogenic Enterobacteriaceae obtained from patient specimens, carbapenem and cephalosporin resistance is mainly mediated by the enzymes-carbapenemases, extended spectrum beta-lactamases (ESBLs), and AmpC beta-lactamases. Often, the genes that encode these beta-lactamases are present in plasmids.3-5 It is well known that horizontal transfer of plasmids bearing carbapenemase, ESBL, and AmpC beta-lactamases genes results in rapid dissemination of resistance to carbapenems and cephalosporins.6 Also, these plasmids frequently bear resistance determinants for other antibiotics such as aminoglycosides and fluoroquinolones, thereby spreading multidrug-resistance. Another mode of drug-resistance is through efflux pumps observed among clinical isolates of Enterobacteriaceae. The genetic determinants for efflux pumps are found on chromosomal DNA as well as on plasmids.7
Overuse and misuse of antibiotics to treat infections give rise to selective antibiotic pressure, resulting in intestinal opportunistic pathogens exhibiting resistance to multiple drugs. Consequently, the gastrointestinal tract acts as a reservoir pool for multidrug-resistant opportunistic pathogens, which can lead to infections in immunocompromised individuals and pose a significant risk to hospitalized patients. Salyers et al. hypothesized that antibiotic resistance genes present in the human gut can be shared with both native gut bacteria and transient microbial populations.8 The human intestine is host to a large microbial community.9 Regardless of the infection type community or hospital-acquired the digestive tract remains the main reservoir of Enterobacteriaceae. Intestinal microbiota contribute substantially to the dissemination of antibiotic resistance genes.8 The gastrointestinal tract acts as a hotspot for resistance gene transfer, with antibiotic pressure driving the dominance of resistant bacteria. In the human intestine, microbial dysbiosis creates conditions conducive to the horizontal transmission of antibiotic resistance genes between commensals and pathogens.10
Although limited, there are reports on fecal carriage rates of beta-lactamase-producing Enterobacteriaceae among hospitalized as well as healthy individuals from our country. However, horizontal transfer of resistance genes, though a long-known mechanism of spreading antibiotic resistance in vivo, has not been demonstrated in Enterobacteriaceae between a commensal and pathogen in a patient in our country. Therefore, this study aimed to demonstrate the possibility of horizontal transfer of genes encoding beta-lactamase in Enterobacteriaceae between pathogenic and gut isolates.
Study isolates
Patients with clinical specimens testing positive for Enterobacteriaceae harboring beta-lactamase genes were identified. Stool samples were obtained from these patients to isolate intestinal Enterobacteriaceae and investigate the horizontal transfer of beta-lactamase genes between pathogenic and commensal strains. A total of 12 paired Enterobacteriaceae isolates one from clinical specimens and the other from stool samples were obtained from patients at a tertiary care hospital in Chennai, India. This study was approved by the Institutional Human Ethics Committee (IHEC). IHEC Approval No.: UM/IHEC/01-2014-II dated 18.02.2015 and IHEC Approval No.: UM/IHEC/01-2017-I dated 26.04.2017.
Isolation and identification of bacterial isolates
Standard microbiological techniques were employed to isolate and characterize the bacterial strains. Both the pathogenic isolate and the gut isolate were then subjected to the following tests individually:
Antibiotic Sensitivity Testing (AST) by disc placement method
Detection of carbapenemase, ESBL, AmpC, inducible AmpC beta-lactamases, and combined resistance mechanisms was carried out using the one-plate method outlined by Rodrigues et al.11 Antibiotic discs used were (imipenem 10 µg, cefotaxime 30 µg, cefoxitin 10 µg, ceftazidime 30 µg, ceftazidime + clavulanic acid 30 µg + 10 µg, aztreonam 30 µg, ceftriaxone 30 µg Hi Media Labs, Mumbai, India) were placed on the agar surface. Interpretation of the susceptibility data was performed following Clinical & Laboratory Standards Institute (CLSI) standards, with E. coli ATCC 25922 serving as the quality control reference strain.
PCR to detect beta-lactamase encoding genes
Genomic DNA from the bacterial isolates was extracted using the heat lysis protocol. Briefly, 1-2 bacterial colonies were suspended in 100 µL of sterile double autoclaved water in a 1.5 mL microcentrifuge tube. The suspension was boiled in a dry bath at 100 °C for 10 minutes. The suspension was then immediately kept at -20 °C for 10 minutes. It was then centrifuged, and the supernatant was used as DNA template for polymerase chain reaction (PCR) reactions. PCR was performed, targeting the following beta-lactamase genes: blaCTX-M grp 1, blaCTX-M grp 2, blaCTX-M grp 8, blaCTX-M grp 9, blaCTX-M grp 25, blaNDM, blaOXA-48, blaKPC-1, blaIMP, blaVIM using primers and annealing conditions as described.12,13
Enterobacterial Repetitive Intergenic Consensus sequence-based PCR (ERIC PCR)
ERIC PCR is a quick and easy technique to divide the strains with the same serotypes into the different sub-types. Here, ERIC PCR was performed to detect clonal similarity between the isolates tested. This was done with the primers and PCR conditions as described earlier.14
PCR based replicon typing (PBRT)
PBRT is the most commonly used method of classifying plasmids based on the incompatibility type of the plasmid. PBRT consists of 5 multiplex PCR and 3 simplex PCR reactions. This was performed as described by Carattoli et al.15
Conjugation assay16
To evaluate the potential horizontal transfer of β-lactamase genes, a broth mating conjugation assay was performed using sodium azide-resistant E. coli J53 as the recipient and either a clinical pathogenic or gut-associated enterobacterial isolate as the donor. In brief, 500 µL of overnight cultures from both the donor and recipient strains were combined and incubated together at 37 °C (to mimic the human body’s core temperature) for 6-7 hours, after which a loopful of the mixture was plated onto an antibiotic-azide selective plate (Mac Conkey’s agar incorporated with 1 mg/L cefotaxime and 100 mg/L sodium azide) and incubated at 37 °C for 18-24 hours. Transconjugants that grew on the selective plates were subjected to AST and PCR to confirm the acquisition and horizontal transfer of beta-lactamase genes.
A total of 12 paired sets of Enterobacteriaceae isolates comprising clinical isolates and gut-derived strains (from stool samples) were collected from patients attending a tertiary care hospital. All the clinical isolates of Enterobacteriaceae of the 12 patients produced either one or a combination of the beta-lactamases-carbapenemases, ESBL, and AmpC beta-lactamases, detected by AST by disc placement method and further confirmed by PCR targeting specific genes. Gut-derived enterobacterial isolates producing beta-lactamases were compared with their corresponding clinical Enterobacteriaceae isolates to investigate the potential horizontal transfer of β-lactamase genes between commensal and pathogenic strains. It was found that, out of the 12 patients, two harbored pathogenic and gut Enterobacteriaceae that had a similar antibiogram pattern and produced the same beta-lactamases and were consequently examined in greater detail to investigate horizontal gene transfer of beta-lactamase genes. The results are as follows:
Evidence of horizontal transfer in non-clonally related strains
Patient A
Urine and stool samples were collected from a 50-year-old female patient. E. coli was isolated and identified from urine as well as stool samples of the patient. The E. coli isolated from urine samples is subsequently referred to as UPEC (uropathogenic E. coli) and the E. coli isolated from stool sample is subsequently referred to as gut E. coli. AST by disc placement method revealed that both the UPEC and gut E. coli isolates showed a similar antibiogram pattern and also produced ESBL. PCR analysis confirmed the presence of blaCTX-M group-1 gene in both the isolates. PBRT demonstrated that UPEC was of replicon types IncI1 and IncF1B whereas gut E. coli was of replicon type IncF1B only (Figure 1A). ERIC PCR showed that there was no clonal relatedness between UPEC and gut E. coli establishing that the two isolates, although of the same species, were two different strains (Figure 1B). Both the isolates shared IncF1B replicon types only. Two independent conjugation assays were conducted using UPEC and gut E. coli isolates as donors, with sodium azide-resistant E. coli J53 serving as the recipient. The assays were performed at 37 °C to simulate human body temperature, and transconjugants were selected on MacConkey agar plates supplemented with cefotaxime (1 mg/L) and sodium azide (100 mg/L). Transconjugants of UPEC that were obtained were positive by PCR for blaCTX-M group-1 and were of replicon types IncI1 and IncF1B replicon types (Figure 1A). Transconjugants of gut E. coli were not obtained (Table 1).
Table (1):
Data to indicate the horizontal transfer of blaCTX-M from UPEC to gut E. coli to have occurred in the gut of the patient A
| Experiment | Clinical isolate | Gut isolates |
|---|---|---|
| Bacterial identification | UPEC | Gut E. coli |
| Phenotypic test for beta-lactamase | ESBL producer | ESBL producer |
| blaCTX-M PCR | Positive | Positive |
| PBRT | IncI1, IncF1B | IncF1B |
| Conjugation study | TC of UPEC obtained | Conjugation did not occur |
| Confirmation of HGT of blaCTX-M in TC of UPEC | ||
| blaCTX-M PCR | Positive | |
| PBRT of TC | IncI1, IncF1B | |
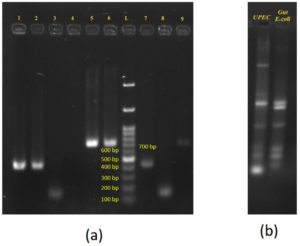
Figure 1. Gel electrophoresis demonstrating horizontal transfer of blaCTX-M from UPEC to gut E. coli in patient A:
(a): PCR amplification of resistance gene and plasmid replicons. Lanes 1 & 2 – blaCTX-M group 1 Positive for UPEC and gut E. coli respectively; Lanes 3 & 4 – IncI1 Positive in UPEC and – IncI1-negative in gut E. coli respectively; lanes 5 & 6 – IncF1B Positive for both UPEC and gut E. coli respectively; Lanes 7, 8 & 9 – TC UPEC positive for blaCTX-M group 1, IncI1 and IncF1B respectively. L – 100 bp – 1.5 Kb ladder (bands at 100, 200, 300, 400, 500, 600, 700 bp, etc). Amplicon sizes of: blaCTX-M group 1 – 415 bp; IncI1 – 139 b; IncFIB – 702 bp.
(b): ERIC PCR profile of UPEC and Gut E. coli – showing dissimilar banding patterns
Evidence of horizontal transfer in clonally related strains
Patient B
Urine and stool samples were collected from a 70-year-old male patient with urinary tract infection (UTI). Isolation and identification of E. coli from both urine and stool samples revealed UPEC and gut strains, respectively. The colony morphology of UPEC was: lactose fermenting, circular, elevated center with smooth edge and gut E. coli was lactose fermenting, circular, flat, dry with smooth edge. AST by disc placement method showed that while UPEC was an ESBL and AmpC beta-lactamase producer, gut E. coli was a carbapenemase, ESBL and AmpC beta-lactamase producer. PCR confirmed that both isolates carried blaCTX-M group-1 gene. ERIC PCR showed that there was clonal relatedness between UPEC and gut E. coli. Both UPEC and gut E. coli harbored one plasmid each of similar size and was of replicon types IncFIA and IncFIB, indicating that the same plasmid was present in both the isolates. Two independent conjugation assays were conducted using UPEC and gut E. coli isolates as donors, with sodium azide-resistant E. coli J53 as the recipient strain. The assays were performed at 37 °C to simulate human body temperature, and transconjugants were selected on MacConkey agar plates supplemented with cefotaxime (1 mg/L) and sodium azide (100 mg/L). Transconjugants were obtained for both the UPEC and gut E. coli strains thus demonstrating the ability to horizontally transfer blaCTX-M bearing IncFIA and IncFIB plasmid to recipient E. coli J53 (Figure 2 and Table 2).
Table (2):
Data to indicate the horizontal transfer of blaCTX-M between UPEC and gut E. coli to have occurred in the gut of the patient B
| Experiment | Clinical isolate | Gut isolates |
|---|---|---|
| Bacterial identification | UPEC | Gut E. coli |
| Phenotypic test for | ESBL and AmpC beta- | Carbapenemase, ESBL and AmpC |
| beta-lactamase | lactamase producer | beta-lactamase producer |
| blaCTX-M PCR | Positive | Positive |
| PBRT | IncFIA and IncFIB | IncFIA and IncFIB |
| Conjugation study | TC of UPEC obtained | TC of UPEC obtained |
| Confirmation of HGT of blaCTX-M in TC of UPEC | ||
| blaCTX-M PCR | Positive | |
| PBRT of TC | IncFIA and IncFIB | |
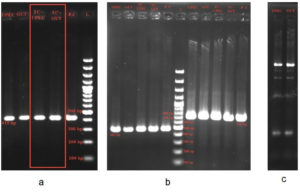
Figure 2. Gel electrophoresis demonstrating horizontal transfer of blaCTX-M from UPEC to gut E. coli in patient B:
(a): blaCTX-M group 1 – 415 bp present in: UPEC, Gut E. coli, TC-UPEC, TC Gut- Transconjugant Gut E. coli, PC – Positive control, L- ladder 100 bp – 1.5 Kb
(b): FI A – 462 bp present in UPEC, Gut E. coli, TC-UPEC, TC Gut, PC – Positive control, L- ladder 100 bp – 1.5 Kb FI B – 702 bp present in UPEC, Gut E. coli, TC-UPEC, TC Gut, PC – Positive control.
(c): ERIC PCR of UPEC and Gut E. coli
The beta-lactam group of drugs are most diverse and widely used among all the groups of antimicrobial agents. Resistance to these drugs is often mediated by beta-lactamase enzymes, the genes for which are commonly borne on plasmids. Rapid and disseminated spread of resistance to beta-lactam drugs is known to occur by horizontal transfer of beta-lactamases borne on plasmids. Acquisition and diffusion of beta-lactam resistance immensely limits treatment options.17 Several investigations have demonstrated the exchange of blaCTX-M genes among diverse bacterial strains and species. Cho et al. reported horizontal blaCTX-M-14 gene transfer from Shigella sonnei to a commensal E. coli strain.18 Whole-genome sequencing by Knudsen et al. revealed the horizontal transfer of a blaCTX-M-1 encoding plasmid between various E. coli strains residing in the human gut.19 Plasmid-mediated transfer of ISEcp1-blaCTX-M-15 and aac(6′)-Ib-cr genes by conjugation to a recipient strain was observed in 49% of ESBL-producing isolates in a Lebanese study.20 In Israel, horizontal interspecies transfer of a plasmid bearing the KPC-3 gene from a carbapenem-resistant KPC-3 producing K. pneumoniae to a carbapenem-susceptible E. coli was suggested to occur in the gut of a patient from whom the strains were isolated.21 There is scarce documentation of in vitro bacterial resistance gene transfer in studies involving both human and animal hosts.22-24 Despite its clinical significance, in vivo evidence for horizontal transfer of the blaCTX-M gene between two E. coli strains in the human gut remains scarce.25-28 Our findings contribute to this body of knowledge by providing in vivo evidence of such horizontal transfer, particularly highlighting the potential for plasmid-mediated gene transfer within the human gut microbiota.
The current study aimed to investigate the potential horizontal transfer of beta-lactamase genes between gut and clinical Enterobacteriaceae isolates in hospitalized patients. A total of 12 patients were sampled, and two cases exhibited noteworthy findings. In the first case (patient A), both UPEC and gut E. coli isolates from a 50-year-old female patient produced ESBL and harbored the blaCTX-M group-1 gene. Despite their identical resistance profiles, ERIC PCR demonstrated no clonal relatedness, indicating they were distinct strains. UPEC had two replicon types IncI1 and IncF1B but the gut E. coli had only IncF1B. To understand if the horizontal transfer of blaCTX-M group-1 gene had occurred between UPEC and gut E. coli, conjugation experiment was performed. Transconjugants were observed only for UPEC and not for gut E. coli. These findings suggest that the gut E. coli may have acquired the blaCTX-M bearing IncF1B plasmid from UPEC through horizontal gene transfer. The absence of clonal relatedness supports the hypothesis that horizontal transfer, rather than clonal expansion, facilitated the spread of the resistance gene. This demonstrates the potential for plasmid-mediated transfer of resistance genes between different E. coli strains within the same host.
In the second case (patient B), involving a 70-year-old male patient, both UPEC and gut E. coli were positive for the blaCTX-M group-1 gene, and ERIC PCR analysis showed clonal relatedness between the two isolates. Despite the clonal relatedness, there were marked differences between the two strains. Both UPEC and gut E. coli had different colony morphologies and dissimilar antibiogram patterns. Gut E. coli was resistant to imipenem by AST, but PCR targeting carbapenemase genes was negative, suggesting that imipenem resistance was not enzyme-mediated. In contrast, UPEC was sensitive to imipenem. These findings suggest that although UPEC and gut E. coli were clonally related, as demonstrated by ERIC PCR, they were different E. coli strains. Cremet et al. showed that in spite of clonal relatedness, E. coli can undergo phenotypic and genotypic changes.29 Both UPEC and gut E. coli isolates carried a single plasmid of similar size, identified as IncFIA and IncFIB types. Conjugation assays confirmed the transferability of the blaCTX-M bearing IncFIA and IncFIB plasmid to recipient E. coli J53 from both UPEC and gut E. coli. The findings from this case strongly support the in vivo transfer of a plasmid harboring the blaCTX-M gene within the patient’s gut. This case provides strong evidence of in vivo horizontal transfer of the blaCTX-M bearing plasmid within the patient’s intestine. The clonal relatedness between the isolates suggests that the plasmid-mediated resistance gene transfer could have occurred after the initial colonization by a single strain, facilitating the dissemination of resistance within the gut microbiota.
The findings underscore the complexity of resistance gene dynamics within the human host, where both clonal expansion and horizontal gene transfer can contribute to the spread of resistance. The study highlights the significance of the gut microbiota as a reservoir for antimicrobial resistance genes and the potential for horizontal gene transfer to pathogenic strains. These findings have important clinical implications, particularly in the context of infection control and antimicrobial stewardship. Understanding the mechanisms of resistance gene transfer can help in forming strategies to mitigate the spread of resistance within healthcare settings. Further studies are needed to explore the prevalence and mechanisms of horizontal gene transfer in diverse patient populations and settings to develop effective interventions to curb the dissemination of antimicrobial resistance.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PS conceptualized the study. JM and PK collected resources. PK supervised the study and funding acquisition. JM and PS applied methodology. JM, PS and PK performed investigation. PS and PK wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Human Ethics Committee (IHEC), Dr. ALM PG IBMS, University of Madras, Chennai, India. (IHEC Approval No.: UM/IHEC/01-2014-II dated 18.02.2015 and IHEC Approval No.: UM/IHEC/01-2017-I dated 26.04.2017)
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. Am J Med. 2006;119(6 Suppl 1):S20-S70.
Crossref - Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;29(6):630-636.
Crossref - Banerjee K, Sekar P, Krishnan P, et al. Whole genome sequence analysis of NDM-1, CMY-4, and SHV-12 coproducing Salmonella enterica serovar Typhimurium isolated from a case of fatal burn wound infection. Infect Drug Resist. 2018;11:2491-2495.
Crossref - Menezes GA, Menezes PS. New Delhi metallo-β-lactamase (NDM-1): how real is the threat? Inter J Med Upd. 2013;8(1):1-3.
- Mohammed MM, Sekar P, Al Jamal J, Abu Taha L, Bachir A, Al Kawas S. Comparative analysis of salivary antimicrobial resistance genes in dental students: A PCR and questionnaire study. PLoS One. 2025;20(1):e0315450.
Crossref - Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol. 2010;300(6):371-379.
Crossref - Sekar P, Mamtora D, Bhalekar P, Krishnan P. AcrAB-TolC Efflux Pump Mediated Resistance to Carbapenems among Clinical Isolates of Enterobacteriaceae. J Pure Appl Microbiol. 2022;16(3):1982-1989.
Crossref - Salyers AA, Gupta A, Wang Y. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 2004;12(9):412-416.
Crossref - Huddleston JR. Horizontal gene transfer in the human gastrointestinal tract: potential spread of antibiotic resistance genes. Infect Drug Resist. 2014;7:167-176.
Crossref - Stecher B, Maier L, Hardt WD. ‘Blooming’ in the gut: how dysbiosis might contribute to pathogen evolution. Nat Rev Microbiol. 2013;11(4):277-284.
Crossref - Rodrigues C, Joshi P, Jani SH, Alphonse M, Radhakrishnan R, Mehta A. Detection of b-lactamases in nosocomial gram negative clinical isolates. Indian J Med Microbiol. 2004;22(4):247-250.
Crossref - Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119-123.
Crossref - Woodford N, Fagan EJ, Ellington MJ. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J Antimicrob Chemother. 2006;57(1):154-155.
Crossref - Dalla-Costa LM, Irino K, Rodrigues J, Rivera IN, Trabulsi LR. Characterisation of diarrhoeagenic Escherichia coli clones by ribotyping and ERIC-PCR. J Med Microbiol. 1998;47(3):227-234.
Crossref - Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219-228.
Crossref - Inwezerua C, Mendonחa N, Calhau V, Domingues S, Adeleke OE, Da Silva GJ. Occurrence of extended-spectrum beta-lactamases in human and bovine isolates of Escherichia coli from Oyo state, Nigeria. J Infect Dev Ctries. 2014;8(6):774-779.
Crossref - Navarro F. Acquisition and horizontal diffusion of β-lactam resistance among clinically relevant microorganisms. Int Microbiol. 2006;9(2):79-81.
- Cho SH, Han SY, Kang YH. Possibility of CTX-M-14 Gene Transfer from Shigella sonnei to a Commensal Escherichia coli Strain of the Gastroenteritis Microbiome. Osong Public Health Res Perspect. 2014;5(3):156-160.
Crossref - Knudsen PK, Gammelsrud KW, Alfsnes K, et al. Transfer of a blaCTX-M-1-carrying plasmid between different Escherichia coli strains within the human gut explored by whole genome sequencing analyses. Sci Rep. 2018;8(1):280.
Crossref - Harajly M, Khairallah MT, Corkill JE, Araj GF, Matar GM. Frequency of conjugative transfer of plasmid-encoded ISEcp1 – blaCTX-M-15 and aac(6′)-lb-cr genes in Enterobacteriaceae at a tertiary care center in Lebanon – role of transferases. Ann Clin Microbiol Antimicrob. 2010;9:19.
Crossref - Goren MG, Carmeli Y, Schwaber MJ, Chmelnitsky I, Schechner V, Navon-Venezia S. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg Infect Dis. 2010;16(6):1014-1017.
Crossref - Duval-Iflah Y, Raibaud P, Tancrede C, Rousseau M. R-plasmic transfer from Serratia liquefaciens to Escherichia coli in vitro and in vivo in the digestive tract of gnotobiotic mice associated with human fecal flora. Infect Immun. 1980;28(3):981-990.
Crossref - Schjorring S, Struve C, Krogfelt KA. Transfer of antimicrobial resistance plasmids from Klebsiella pneumoniae to Escherichia coli in the mouse intestine. J Antimicrob Chemother. 2008;62(5):1086-1093.
Crossref - Faure S, Perrin-Guyomard A, Delmas JM, Chatre P, Laurentie M. Transfer of plasmid-mediated CTX-M-9 from Salmonella enterica serotype Virchow to Enterobacteriaceae in human flora-associated rats treated with cefixime. Antimicrob Agents Chemother. 2010;54(1):164-169.
Crossref - Gottig S, Gruber TM, Stecher B, Wichelhaus TA, Kempf VAJ. In vivo horizontal gene transfer of the carbapenemase OXA-48 during a nosocomial outbreak. Clin Infect Dis. 2015;60(12):1808-1815.
Crossref - Lester CH, Frimodt-Moller N, Sorensen TL, Monnet DL, Hammerum AM. In vivo transfer of the vanA resistance gene from an Enterococcus faecium isolate of animal origin to an E. faecium isolate of human origin in the intestines of human volunteers. Antimicrob Agents Chemother. 2006;50(2):596-599.
Crossref - Trobos M, Lester CH, Olsen JE, Frimodt-Moller N, Hammerum AM. Natural transfer of sulphonamide and ampicillin resistance between Escherichia coli residing in the human intestine. J Antimicrob Chemother. 2009;63(1):80-86.
Crossref - Karami N, Martner A, Enne VI, Swerkersson S, Adlerberth I, Wold AE. Transfer of an ampicillin resistance gene between two Escherichia coli strains in the bowel microbiota of an infant treated with antibiotics. J Antimicrob Chemother. 2007;60(5):1142-1145.
Crossref - Cremet L, Caroff N, Giraudeau C, Reynaud A, Caillon J, Corvec S. Detection of clonally related Escherichia coli isolates producing different CMY β-lactamases from a cystic fibrosis patient. J Antimicrob Chemother. 2013;68(5):1032-1035.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.