ISSN: 0973-7510
E-ISSN: 2581-690X
The effect of crude extracted and essential oils was compared for controlling anthracnose disease in Dendrobium ‘Earsakul’. Dendrobium ‘Earsakul’ was tested to determine the efficiency of five Thai medicinal herbs: galangal, garlic, lemongrass, turmeric and ginger on the growth inhibition of Colletotrichum sp. as the pathogens of anthracnose or leaf blight disease. Each medicinal plant was blended and macerated in 95% ethyl alcohol and sterile distilled water at ratio 200g:400mL for seven days. Crude extracts and essential oil were tested for inhibition of their antifungal potentials against Colletotrichum sp. by food poison technique. Results in the laboratory and under greenhouse condition showed that 8,000 ppm essential oil of ginger inhibited the growth of Colletotrichum sp. both before and after disease infection at 100% similar with the chemical compound Mancozeb.
Dendrobium ‘Earsakul’, plant extract, essential oil, Thai medicinal herb, Colletotrichum sp.
Anthracnose or leaf blight disease is caused by fungi in the genus Colletotrichum, a common group of pathogens that are responsible for diseases on many plant species. Anthracnose infects the aerial portion of the orchid and leaves are most often attacked. Leaf tips turn brown, beginning at the apex and proceeding toward the base. Dark brown or light gray patches develop, sometimes as concentric rings or as numerous dark bands across the leaf. The affected area is usually sharply defined and somewhat sunken, while the remainder of the leaf appears normal. Sporing bodies develop in the infected area. Flowers develop watery, black or brown pustules which are usually raised and occur on the underside of older sepals and petals. The spots may merge and cover the entire flower1,2. The control of this pathogen remains a challenge and is still based upon multiple applications of fungicides. Chemical control is effective and efficient but can lead to the development of pathogen resistance, chemical residues in fruit, phytotoxicity to other organisms or environmental and public health problems, as well as the occurrence of fungicide resistant pathogen strains. This has stimulated research on alternative methods to control diseases which are needed because of the negative effects of synthetic chemicals which increase the risk of high levels of toxic residues3,4,5,6. Natural plant extracts are important sources of new agrochemicals with large antimicrobial spectrum properties for the control of plant diseases7,8. However, the potential toxic effect of applying pesticides to plants on soil beneficial organisms needs to be addressed. Another method is to use plant pathogen control substances which are environmentally friendly. Both these methods leave toxic residues in the environment but do not harm the ecosystem. Disease management using plant essential oils has been applied as an eco-friendly control method9,10. Many plant essential oils showed different levels of antimicrobial efficacies to various ranges of plant fungal and bacterial pathogens, and efficiently reduced the major diseases in crops. Colletotrichum sp. which infect diverse economically important crops have been successfully managed by plant essential oils and their individual components11. The present work therefore aimed to evaluate plant crude extracts and essential oil for their antifungal activities against Colletotrichum sp. that could possibly lead to their use to control anthracnose disease in vitro and in the greenhouse
Disease protection activity of the crude plant extracts and essential oils were tested on detached pseudobulbs or leaves of orchid. Experiments were conducted in the science laboratory at the Department of Agricultural Technology, Faculty of Technology, Mahasarakham University, Thailand.
Plant material
To prepare the experimental Dendrobium ‘Earsakul’ orchid plants, the first step involved inducing protocorm-like bodies of orchid to seedlings on MS media12 with 0.5 mg/L benzylaminopurine (BAP) and 0.5 mg/L dichlorophenoxy acetic acid (2,4-D) for 8 weeks. Seedling growth was then induced on VW media13 with 15 mg/L chitosan for 8 weeks with subculture once a month. Finally, the seedlings were transplanted in the greenhouse after one year.
Isolation of target pathogen
Colletotrichum sp. was isolated from pseudobulbs or leaves of orchid showing anthracnose lesions. An isolate of the pathogen grown as a pure culture was maintained in PDA (potato dextrose agar) medium as a stock culture.
Inoculum disc: Seven days old culture of the test fungus was used for the preparation of inoculum discs 5 mm in diameter.
Preparation of plant crude extract and essential oils
Sample collection: Potential plant crude extracts and essential oils were selected by screening the efficiency of five Thai medicinal herbs (galangal, garlic, lemongrass, turmeric and ginger) on the growth inhibition of Colletotrichum sp.
The method for the preparation of plant crude extracts and essential oils from five Thai medicinal herbs (galangal, garlic, lemongrass, turmeric and ginger) was as follows. For plant crude extracts, firstly, fresh plant bulbs or rhizomes were selected and washed thoroughly 2-3 times with running tap water followed by distilled water. They were then artificially heated by air drying in a hot air oven at 50°C for 72hrs or until stable dry weight. Secondly, each plant sample was mixed in a blender and the powder was soaked in ethanol. An aqueous extract was prepared by blending 200 g of each plant bulb or rhizome in 400 mL 95% ethanol for seven days. The macerate was filtered through double-layered muslin cloth and centrifuged at 8,000 rpm at 10°C for 30 minutes. The supernatant was filtered through Whatman No. 1 filter paper followed by evaporation using an R-205 Buchi rotary evaporator to remove the ethanol to obtain concentrates. The crude extracts were kept at 4°C in sterile universal bottles until required for use. Essential oils were separated from the crude extracts by the water distillation process.
The inhibitory effects of plant extracts and their antifungal potentials against Colletotrichum sp. which cause anthracnose disease in Dendrobium ‘Earsakul’ orchid were tested by food poison technique14. Each of the plant crude extracts were dissolved in 1%DMSO and a volume of 5.5 mL of each concentrate was aseptically poured into a Petri dish followed by the addition of 9.5 mL of melted PDA and then agitated gently to achieve a thorough mixing of the contents. For the control set, no extract was used. After solidification of the media, one inoculum disc of the test fungus was aseptically inoculated upside down at the center of the Petri dish and incubated at 25°C. Average radial growths of the fungal colonies were measured on the seventh day of incubation. The treatments were as follows:
Experiment 1
To screen the efficiency of plant crude extracts and essential oils for the control of Colletotrichum sp. in vitro by PDA standard medium with plant crude extracts and essential oils for seven days. This experiment was conducted in a CRD (completely randomized block design) with four replications. Four discs were prepared for repeated experiments per each replication. Four individual experiments were performed in vitro.
T1 |
Control (distilled water) |
T2 |
1,500 ppm Mancozeb |
T3 |
10,000 ppm DMSO (1% DMSO) |
T4 |
10,000 ppm Galangal extract |
T5 |
80,000 ppm Garlic extract |
T6 |
7,500 ppm Lemongrass extract |
T7 |
20,000 ppm Turmeric extract |
T8 |
8,530 ppm Ginger extract |
T9 |
3,000 ppm Galangal essential oil |
T10 |
100 ppm Garlic essential oil |
T11 |
500 ppm Lemongrass essential oil |
T12 |
2,500 ppm Turmeric essential oil |
T13 |
8,000 ppm Ginger essential oil |
Following observations, the percentage inhibition of diameter growth (PIDG) values was determined according to the equation below:
Experiment 2
To study the efficiency of plant crude extracts and essential oils for the control of Colletotrichum sp. by the inoculation modified detached leaf technique in vitro. This experiment was conducted at 7×2 factorials in CRD for 14 treatments. Seven factor A substances were selected from Experiment 1 (Control, Mancozeb, DMSO, 10,000 ppm Galangal extract, 80,000 ppm Garlic extract, 500 ppm Lemongrass essential oil and 8,000 ppm Ginger essential oil), and the two factor B time periods were the time of usage (before and after pathogen infection) with four replications and 10 leaves per replication. Following observations, the PIDG values were determined according to the equation below:
Inhibition percentage =100-[(Diameter of sample)/(Diameter of control)×100]
Experiment 3
To study the efficiency of plant crude extracts and essential oils for the control of Colletotrichum sp. by the inoculation modified detached leaf technique in vivo (greenhouse). This experiment was conducted at 6×2 factorials in CRD for 14 treatments. Six factor A substances were selected from Experiment 2 (Control, Mancozeb, DMSO, 10,000 ppm Galangal extract, 80,000 ppm Garlic extract, and 8,000 ppm Ginger essential oil), and the two factor B time periods were the time of usage (before and after pathogen infection) with four replications and 10 plants per replication.
Statistical analyses
ANOVA (analysis of variance) was used to determine the effects of anthracnose treatments at both the laboratory and greenhouse. Means were compared using Duncan’s multiple range tests. Statistical analyses were performed using SPSS version 22 (IBM SPSS Statistics 22.Ink).
(1) The efficiency of plant crude extracts and essential oils for the control of Colletotrichum sp. in vitro by PDA standard medium with plant crude extracts and essential oils for seven days. Laboratory results showed that plant crude extracts of galangal (10,000ppm), garlic (80,000ppm), and essential oils from lemongrass (500ppm) and ginger (8,000ppm) inhibited the growth of Colletotrichum sp. at 100% compared with the control and the chemical compound Mancozeb (Table 1). Other treatments were not effective in inhibiting the growth of Colletotrichum sp. Under a compound microscope, the mycelium showed growth and abnormalities such as bent, knotted, twisted and kinked hypha (Figure 1).
Table (1):
Efficiency of plant crude extracts and essential oils for control of Colletotrichum sp. in vitro by PDA medium for seven days.
| Treatments | Inhibition percentage (%) for 7 days | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Control (distilled water) | 0.00b | 0.00b | 0.00f | 0.00f | 0.00d | 0.00d | 0.00e |
| Mancozeb | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| DMSO | 100.00a | 100.00a | 65.02d | 39.15d | 17.06c | 0.00d | 0.00e |
| 10,000 ppm Galangal extract | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| 80,000 ppm Garlic extract | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| 7,500 ppm Lemongrass extract | 100.00a | 100.00a | 68.65c | 45.33c | 18.48c | 2.19d | 0.00e |
| 20,000 ppm Turmeric extract | 100.00a | 100.00a | 71.21b | 50.05b | 31.98b | 10.77c | 0.00e |
| 8,530 ppm Ginger extract | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 85.67b |
| 3,000 ppm Galangal essential oil | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 83.64b | 69.12d |
| 100 ppm Garlic essential oil | 100.00a | 100.00a | 62.64e | 24.46e | 0.00d | 0.00d | 0.00e |
| 500 ppm Lemongrass essential oil | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| 2,500 ppm Turmeric essential oil | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 84.72b | 71.17c |
| 8,000 ppm Ginger essential oil | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| F-test | ** | ** | ** | ** | ** | ** | ** |
| CV (%) | 8.62 | 28.26 | 4.06 | 1.81 | 1.34 | 1.86 | 1.76 |
** = significant difference at p =0.01. Means within a column followed by the same letter do not differ significantly according to DMRT.
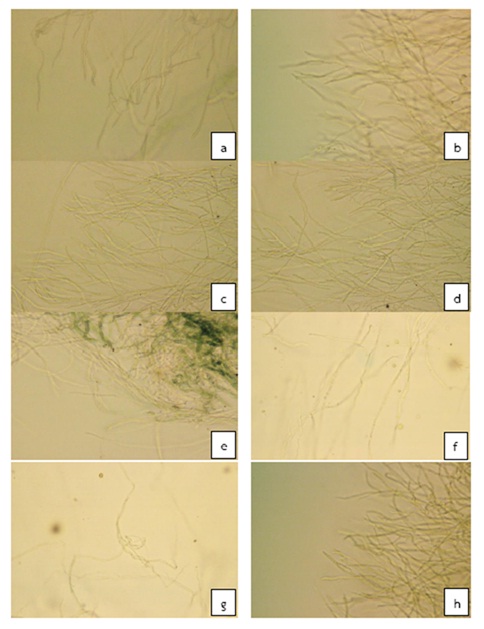
Fig. 1. Characteristics of Colletotrichum sp. mycelium causing anthracnose disease tested with different treatment for seven days. (a) Control (PDA), (b) DMSO, (c) 7,500 ppm Lemon grass extract, (d) 20,000 ppm Turmeric extract, (e) 8,530 ppm Ginger extract, (f) 100 ppm Garlic essential oil, (g) 3,000 ppm Galangal essential oil, (h) 2,500 ppm Turmeric essential oil
(2) The efficiency of plant crude extracts and essential oils for the control of Colletotrichum sp. by the inoculation modified detached leaf technique in vitro. Laboratory results for the main study, factor A substances and time factor interaction B were significant at 99%. Ginger essential oil (8,000 ppm) inhibited the growth of Colletotrichum sp. at 100% similar with the chemical compound Mancozeb both before and after disease infection (Table 2).
Table (2):
Efficiency of plant crude extracts and essential oils for control of Colletotrichum sp. by inoculation modified detached leaf technique in vitro.
| Treatment | F-test inhibition percentage (%) for seven days | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Factor A (type of substance) | ** | ** | ** | ** | ** | ** | ** |
| Factor B (before-after infection) | ns | ns | ns | ns | ns | ns | ns |
| A×B | ** | ** | ** | ** | ** | ** | ** |
| Control (A1) | 0.00e | 0.00e | 0.00d | 0.00d | 0.00e | 0.00d | 0.00d |
| Mancozeb (A2) | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| DMSO (A3) | 0.00e | 0.00e | 0.00d | 0.00d | 0.00e | 0.00d | 0.00d |
| 10,000 ppm Galangal extract (A4) | 73.50b | 69.75b | 50.00b | 49.75b | 47.75b | 24.13b | 24.13b |
| 80,000 ppm Garlic extract (A5) | 50.00c | 49.75c | 49.75b | 24.69c | 24.44c | 24.00b | 24.13b |
| 500 ppm Lemongrass (A6) | 24.75d | 24.63d | 24.38c | 24.25c | 19.63d | 17.25c | 13.63c |
| 8,000 ppm Ginger essential oil (A7) | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| before pathogen infection (B1) | 50.000 | 50.000 | 46.429 | 42.857 | 41.857 | 38.286 | 37.714 |
| after pathogen infection (B2) | 49.500 | 48.321 | 46.179 | 42.482 | 41.518 | 37.536 | 37.107 |
| A1B1 | 0.00e | 0.00e | 0.00 | 0.00d | 0.00 | 0.00d | 0.00d |
| A2B1 | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| A3B1 | 0.00e | 0.00e | 0.00d | 0.00d | 0.00e | 0.00d | 0.00d |
| A4B1 | 75.00b | 75.00b | 50.00b | 50.00b | 48.00b | 25.00b | 25.00b |
| A5B1 | 50.00c | 50.00c | 50.00b | 25.00c | 25.00c | 25.00b | 25.00b |
| A6B1 | 25.00d | 25.00d | 25.00c | 25.00c | 20.00d | 18.00c | 14.00 |
| A7B1 | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| A1B2 | 0.00e | 0.00e | 0.00d | 0.00d | 0.00e | 0.00d | 0.00d |
| A2B2 | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| A3B2 | 0.00e | 0.00e | 0.00d | 0.00d | 0.00e | 0.00d | 0.00d |
| A4B2 | 72.00b | 64.50b | 50.00b | 49.50b | 47.50b | 23.25b | 23.25b |
| A5B2 | 50.00c | 49.50c | 49.50b | 24.38c | 23.88c | 23.00b | 23.25b |
| A6B2 | 24.50d | 24.25d | 23.75c | 23.50c | 19.25d | 16.50c | 13.25c |
| A7B2 | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a | 100.00a |
| CV (%) | 14.70 | 9.50 | 10.80 | 8.80 | 12.04 | 6.02 | 8.40 |
ns = not significant, ** = significant difference at p = 0.01. Means within a column
followed by the same letter do not differ significantly according to DMRT.
(3) The efficiency of plant crude extracts and essential oils for the control of Colletotrichum sp. was tested by the inoculation modified detached leaf technique in vivo (greenhouse). Results in the greenhouse for the main study, factor A substances and time factor interaction B were significant at 99%. Leaves treated with ginger essential oil (8,000 ppm) did not show symptoms of anthracnose disease compared with the control and the chemical compound Mancozeb when used both before and after disease infection. Essential oil of ginger (8,000 ppm) showed 100% inhibition of Colletotrichum sp. compared with the control and the chemical compound Mancozeb. (Table 3).
Table (3):
The efficiency of plant crude extracts and essential oils for control anthracnose disease of Dendrobium ‘Earsakul’ in vitro.
| Treatment | F-test of disease index for 21 days | ||
|---|---|---|---|
| 7 | 14 | 21 | |
| Factor A (type of substance) | – | ** | ** |
| Factor B (before-after infection) | – | ns | ns |
| A×B | – | ** | ** |
| Control (A1) | 0.00 | 2.25a | 3.00a |
| Mancozeb (A2) | 0.00 | 0.00b | 0.00d |
| DMSO (A3) | 0.00 | 2.13a | 2.38b |
| 10,000 ppm Galangal extract (A4) | 0.00 | 0.13b | 1.00c |
| 80,000 ppm Garlic extract (A5) | 0.00 | 0.25b | 1.25c |
| 8,000 ppm Ginger essential oil (A7) | 0.00 | 0.00b | 0.00d |
| before pathogen infection (B1) | 0.00 | 0.67 | 1.17 |
| after pathogen infection (B2) | 0.00 | 0.92 | 1.38 |
| A1B1 | 0.00 | 2.00a | 3.00a |
| A1B2 | 0.00 | 2.50a | 3.00a |
| A2B1 | 0.00 | 0.00b | 0.00d |
| A2B2 | 0.00 | 0.00b | 0.00d |
| A3B1 | 0.00 | 2.00a | 2.00b |
| A3B2 | 0.00 | 2.25a | 2.75b |
| A4B1 | 0.00 | 0.00b | 1.00c |
| A4B2 | 0.00 | 0.25b | 1.00c |
| A5B1 | 0.00 | 0.00b | 1.00c |
| A5B2 | 0.00 | 0.50b | 1.50c |
| A6B1 | 0.00 | 0.00b | 0.00d |
| A6B2 | 0.00 | 0.00b | 0.00d |
| CV (%) | – | 10.08 | 12.70 |
ns = not significant, ** = significant difference at p < 0.01. Means within a column followed by the same letter do not differ significantly according to DMRT.
This experiment used the DMSO solvent to compare treatments. Results indicated that DMSO had no effect on anthracnose inhibition compared with the control. This finding was consistent with Pothikhawet et al. (2013)15 who tested alfalfa and radish seeds inoculated with Aspergillus parasiticus or A. niger and then soaked in distilled water, 0.5% DMSO, 1,000 ppm Chi-der and 1.2 ppm Nano-Pt for 6hrs before washing by distilled water. The 1,000 ppm Chi-der and 1.2 ppm Nano-Pt significantly reduced seed infection but enhanced seed germination compared to distilled water and 0.5% DMSO. Prasoetsang and Subtang (2012)16 studied the effect of solvent on the antimicrobial activity of medicinal plant extraction using 1% DMSO as the negative control. The result of broth microdilution assay showed that 1% DMSO did not inhibit the bacteria. Medicinal plant extraction and essential oil treatment for Colletotrichum sp. to control anthracnose disease of Dendrobium ‘Earsakul’ before or after disease infection showed that essential oil of ginger (8,000 ppm) inhibited the growth of Colletotrichum sp. by 100% similar to the chemical Mancozeb. Medicinal constituents in ginger essential oil are zingiberene, zingiberol, bisabolene and camphene in high quantities, and extract of ginger also contains phenolic compounds with antibacterial, rancid and preservative properties17,18,19. Jamkratoke et at. (1996)20 studied the effect of Boesenbergia rotunda, Curcuma longa and Zingiber officinalis extracts on postharvest disease fungi. Results demonstrated that the inhibition characteristics of the tested herbs were significant at 10,000 ppm for crude extracts and 1,000 ppm for volatile extracts composed in PDA. Radial growth of almost all the tested fungi was inhibited by the tested herb extracts with various sensitivity. An identical inhibition (83.67 – 87.60%) was determined on fungal colonies formed by Colletotrichum spp. isolated on PDA supplemented with Z. officinalis crude extract. Shovan et al. (2008)21 studied the effect of plant extracts on the growth of Colletotrichum dematium which causes anthracnose in soybean by fungicides, plant extracts and Trichoderma sp. in the laboratory. Results showed that the most effective material was garlic followed by onion, ginger and neem.
Results in the laboratory and under greenhouse conditions showed that essential oil of ginger at 8,000 ppm inhibited the growth of Colletotrichum sp. both before and after disease infection at 100% similar with the chemical compound Mancozeb.
ACKNOWLEDGMENTS
This research was financially supported by Mahasarakham University 2016.
- Yang, Y.L., Cai, L., Yu, Z.N., Liu, Z.Y., Hyde, K.D. Colletotrichum species on orchids in southwest China. Cryptogamie Mycologie, 2011; 32: 229-253.
- Cannon, P.F., Damm, U., Johnston, P.R., Weir, B.S. Colletotrichum – current status and future directions. Studies in Mycology, 2012; 73(1): 181-213.
- Vali, R.J., Moorman, G.W. Influence of selected fungicides regimes on frequency of dicarboximide-resistant and dicarboximide-sensitive strains of Botrytis cinerea. Plant Dis., 1992; 76(9): 919-924.
- Leroux, P., Fritz, R., Debieu, D., Albertini, C., Lanen, C., Bach, J., Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci., 2002; 58: 876-888.
- Yao, H., Tian, S. Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biology and Technology, 2005; 35: 253-262.
- Adebayo, O., Dang, T., Belanger, A., Khanizadeh, S. Antifungal studies of selected essential oils and a commercial formulation against Botrytis cinerea. J. Food Res., 2013; 2(1): 217-226.
- Gulter, H.G. Natural products and their potential in agriculture: A personal overview. In H.G. Gulter (Ed.), Biologically active natural products: Potential use in agriculture. Washington: American Chemical Society, 1998; (pp 1-2).
- Tripathi, P., Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biology and Technology, 2004; 32: 235-245.
- Isman, M.B. Plant essential oils for pest and disease management. Crop Protect., 2000; 19: 603-8.
- Koul, O., Walia, S., Dhaliwal, G.S. Essential oils as green pesticides: potential and constraints. Biopestic Int., 2008; 4: 63–84.
- Ranasinghe, L., Jayawardena, B., and Abeywickrama, K. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et LM Perry against crown rot and anthracnose pathogens isolated from banana. Letters in Applied Microbiology, 2002; 35(3): 208-211.
- Murashige, T., Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant., 1962; 15: 473-497.
- Vacin, E.F., Went, F.W. Some pH changes in nutrient solutions. Bot. Gaz., 1949; 110: 605-613.
- Nene, Y.L., Thapliyal, P.N. Fungicides in Plant Disease Control. Third Edition, Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, India, 1993.
- Pothikhawet, C., Jitareerat, P., Uthairatanakij, A., Photchanachai, S. Suppression of fungal growth by chitosan-derivatives and platinum nanoparticles. Agricultural Sci. J., 2013; 44(2)(Suppl.): 525-528.
- Prasoetsang, J., Subtang, S. The effect of solvent on antimicrobial activity of medicinal plant extraction. Bulletin of Applied Sciences., 2012; 1(1): 99-109.
- Wang, H. Tzi, B.N. An antifungal protein from ginger rhizomes. Biochemical and Biophysical Research Communications, 2009; 336: 100-4.
- Stangarlin, J.R., Kuhn, O.J., Assi, L., Schwan-Estrada, K.R.F. Control of plant diseases using extracts from medicinal plants and fungi. Science against microbial pathogens: communicating current research and technological advances. Méndez-Vilas A. (Ed.), 2011; pp 1033-1042.
- Nikoliæ, M., Vasiæ, S., Ðurðeviæ, J., Stefanoviæ, O., Èomiæ, L. Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujevac J. Sci., 2014; 36: 129-136.
- Jamkratoke, S., Rattanakreetakul, C., Leksomboon, C., Farungsang, N., Wanichkul, K., Farungsang, U. Effects of Boesenbergia rotunda, Curcuma longa and Zingiber officinalis extracts on postharvest disease fungi. Proceedings of 42nd Kasetsart University Annual Conference: Plants, Agricultural Extension and Communication, 1996.
- Shovan, L.R., Bhuiyan, M.K.A., Begum, J.A., Pervez, Z. In vitro control of Colletotrichum dematium causing anthracnose of soybean by fungicides, plant extracts and Trochoderma harzianum. Int. J. Sustain. Crop Prod., 2008; 3(3): 10-17.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


