ISSN: 0973-7510
E-ISSN: 2581-690X
The main aim of this research is to obtain endophytic fungi from graminiae which has the ability to promote the growth of rice plant. The research was conducted in laboratory of Mycology Research Center for BioSciences and Biotechnology Institut Teknologi Bandung. Isolation of endophytic fungi was done by direct plating of root samples on the potatoe dextrose agar media after surface sterilization steps. Identification was based on morphology characteristics and DNA marker. At least 124 isolates were obtained from elephant grass (Pennisetum purpureum) (27 isolates), alang-alang (Imperata cylindrica) (25 isolates), sugar cane (Saccharum officinarum) (14 isolates), maize (Zea mays) (9 isolates), rice plant (Oryza sativa) (23 isolates) and cyperus (Cyperus rotundus) (26 isolates). Among them, 12 isolates demosntrated the capability to promote the growth of rice seedlings in term of seed germination, plant height, root length and degree of root colonization. Isolate I-11 identified as Phialemonium dimorphosphorum was the best to promote rice plant growth in glass house trial.
Growth Promotion, Endophytic fungi, isolation,
Rice is the most important plant in Indonesia, because it is the staple food for many. Rice production is expected to increase to meet population demand. According to Central Statistics Agency5, rice production in 2015 In Java Island increased to 2.31 million tons whereas in other area outside Java to 2.21 million tons. The increase mainly occured in Central Java, East Java, South Sumatera and Aceh. On the other hand, there was a decrease of rice production in West Java, Jambi and West Kalimantan. The decrease may relate to the production technology, land degradation, land conversion, as well as climate changes. Therefore, efforts to increase rice production or to reduce the production cost are still needed in the future. Various approaches have been done to address the issues including the application of biological aspect.
At present, developments of biological agents to promote plant growth or to control pests and diseases are being explored worlwide. One of the approaches is the use of endophytic fungi. Endophytic fungi are type of fungi that live within plant tissues without causing apparent disease6. It is estimated that there are approximately at least one million species of endophytic fungi from 300,000 known plant species in our planet13. The potency of these endophytes is still not extensively explored yet. Some studies reported that endophytic fungi are potential to be used as biological control agent, because these fungi live within plant and can act directly to inhibit the proliferation of patogen within plant tissue22.
Endophytic fungi protect plants from pathogen by several mechanisms including resistance induction to the host plant or competition, antagonism and mycoparasitic action to pathtogens. This fungus can induce or trigger the production of secondary metabolites that increase the immunity of the host which ultimately can increase the growth and productivity26. The existence of endophytic fungi within plant tissue was reported can inhibit tissue damage by pathogen, Arnold et al. [2003]1. Furthermore, endophytic fungi existance in plant tissues was also reported can induce secondary metabolites that prevents the growth of other fungi25 and therefore increase productivity24. Endophytic fungi were reported potential as biological control agent against fungal pathogen on oil palm, Yurnaliza et al. [2014]35. Endophytic fungi were also confirmed to improve the ability of plant host physiologically to cope water stress (drought).
Endophytic fungi interactions with host can be in a stable and mutualistic or parasitic symbiosis, depending on the genotype, their density and ability to colonize the host and also resistivity of the host28,32. There has been an increase in attention to endophytic fungi in the past few years because of their beneficial properties to plants. In the case of positive interaction of endophytic fungi on rice plant, there is no much studies have been done up to recently especially in tropical regions such as Indonesia. A study in Malaysia regarding endophytic fungi on rice has been reported but still in limitted scope of isolation and identification, Zakaria et al. [2010]36. This study was more comprehensive covering the isolation of endophyetic fungi from several species of graminiae and exploring their potensial to promote rice plant growth as well as identification of potential isolates using DNA marker approach.
Sample collection
Root samples from six species of graminiae as the source of endophytic fungi were obtained from Sumedang and Majalengka regions of West Java with the elevation of 650 m above sea level. Six species of graminiae plants were chosen as the sources of isolates include: elephant grass (Pennisetum purpureum), alang-alang (Imperata cylindrica) , sugar cane (Saccharum officinarum), maize (Zea mays), rice plant (Oryza sativa) and cyperus (Cyperus rotundus).
Isolation
Fungal isolation was conducted based on the method introduced by16 with slight modifications. Healthy organs were firstly washed thoroughly using clean water, then cut into several pieces before going through surface sterilization and rinsing in sterile distilled water. Sterile root were blotted in steril tissue paper then cut into small pieces (approx 1 cm long) aseptically in a laminar air flow. Agar plates containing Potatoe Dextrose Agar (PDA) were planted with root samples and incubated at room temperature (26-28 °C) for several days. Mycellial growth on PDA from root samples were transfered into fresh PDA for further purification. Axenic cultures were kept in test tubes containing PDA as stock cultures.
Rice seedling preparation
Rice seeds of Ciherang variety were first soaked in distilled water for 20 minutes and in 1% sodium hypochlorite for 2 minutes. Afterwards, the seeds were rinsed thoroughly using sterile distilled water before incubation for germination, Evan et al. [2013]7.
Evaluation of Potential Isolates
Rice seeds prepared previousely were planted on fully colonized endophyte isolates (7-14 days old). Each disk was planted with 10 seeds and spreaded evenly over the surface of mycelial colony. Petri dishes were incubated for 12 days and were sprayed with approximately 2 ml of sterile distilled water every other day, Munif et al. [2012]21. The germination of the seeds, survival, and growth performance of the seedlings were then evaluated and recorded over incubation time. Positive isolates were then selected for further evaluation on seedling growth promotion and root colonization.
Identification of potential isolates
All isolates supported germination and survival of seedling were identified based on ITS DNA marker and morphological characteristics. ITS DNA isolated and purified using standard protocol and KIT provided by QIA. DNA amplification products by PCR using ITS 1f and ITS 4 primers were sequnced by Macrogen Inc. DNA sequences result were compared with Gene Bank data base in National Center for Biotechnology Information (NCBI) and UNITE web site using Basic Local Alignment Search Tool Algorithm (BLAST) program. The sequence was matched with NCBI database to interprete species or genera identity.
Statistical Analysis
Data of seed germination and plant growth performance that include plants height, root length, and degree of root infection treated by endophytic fungi isolates were analysed using ANOVA.
Based on isolation of endophytic fungi of graminiae roots from Sumedang and Majalengka Regencies, there were a total of 124 isolates consisted of: 27 isolates from elephant grass (Pennisetum purpureum), 25 isolates from alang-alang (Imperata cylindrica), and 14 isolates from sugar cane (Saccharum officinarum L.), 9 isolates from maize (Zea mays), 23 isolates from rice (Oryza sativa) and 26 isolates from cyperus (Cyperus rotundus) (data not shown).
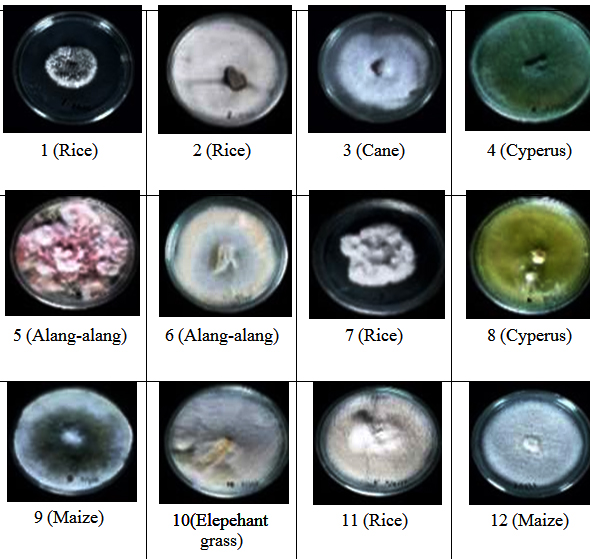
From all isolates obtained, only12 gave positive effect on germination and growth to seedlings during incubation time. These 12 isolates were considered to possess potential to go to further tests related to seedlings growth. The twelve isolates came from Oryza sativa (4 isolates), Saccharum oficinarum root (1 isolate), Cyperus rotundus (2 isolates), Imperata cylindrica (2 isolates), Pennisetum purpureum (1 isolate) and Zea mays (2 isolates) (Figure 1).
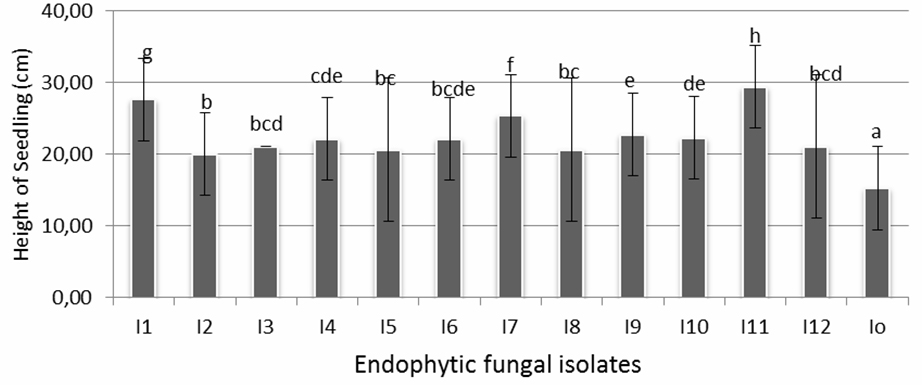
The influence of endophytic fungi isolates to seedlings’ height after 12 days of treatment is shown in Figure 2. It is shown that plants on all fungal treatments were significantly taller compared with controls. The best isolates which gave positive performance on seedling height (29,43 cm) were isolates I-11, followed by I-1 (27,67 cm), and I-7 (25,4cm) which statistically had significant result compared to other isolates. Isolates I-4, I-6, I-9, and I-10 (22,13-22,8 cm), were one cluster which showed significant influence to plant height, followed by next cluster consisting of isolates I-2, I-3, I-4, I-5, I-6, I-8, I-10 and I-12 (20,03-21,1 cm). The average height of seedlings in control was 15,27 cm, while the maximum height reached by isolate I-11 was 29,43 cm, which almost double in height.
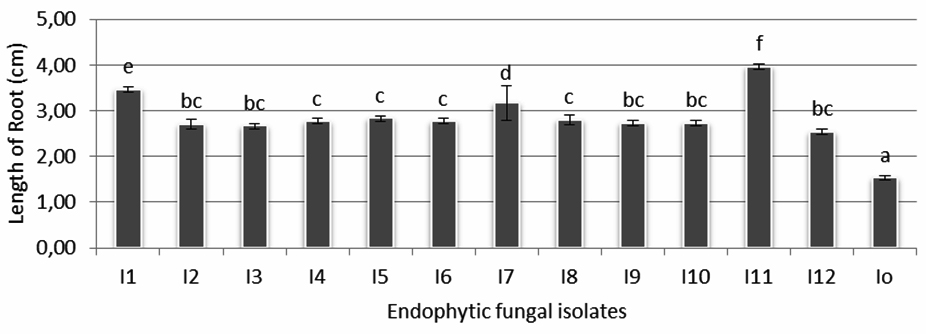
Figure 3 shows the comparison of endophytic fungi growth of isolate 11 (I-11) and control (I-0). The influence of endophytic fungi isolates on rice seedling growth (root length) is shown in figure 4. It is shown that the longest root (3,97 cm) found in seedlings treated by isolate I-11, followed by the ones treated by isolate I-1 (3,47 cm) and I-7 (3,17 cm) which were statistically significant compared with seedlings treated by the rest of isolates and control. Other isolates that gave positive influence to root length were I-2, I-3, I-4, I- 5, I-6, I-8, I-9, I-10 and I-12 with root length between 2,53-2,83 cm.
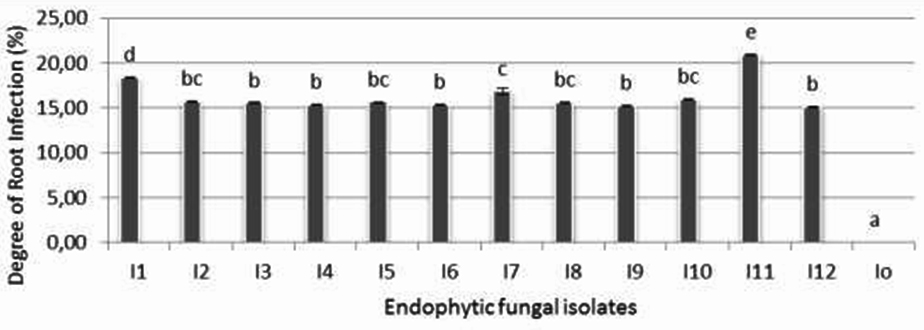
The ability of endophytic fungi isolates to colonize the root of rice seedlings is shown on figure 5. It is shown that isolate I-11 was statistically the best isolate and significantly showed the highest percentage of infection (22,3%) followed by isoate I-1 (18,4%). The rest of endophytic fungi isolates that give the same degree of root infection originated from one cluster.
Since the system was carried out axenically, negative control did not show any fungal infection.
All 12 of endophytic fungi characteristics were identified using ITS DNA marker approach. Based on their ITS DNA sequenses the 12 isolates identities are presented on table 1.
Table (1):
List of isolates species identity homology with NCBI databse.
No |
Isolates |
Host sources |
Data base species |
Homology (%) |
|---|---|---|---|---|
1 |
I-1 |
Rice |
Gaeumannomyces graminis |
97% |
2 |
I-2 |
Rice |
Meyerozyma guiliermondii |
97% |
3 |
I-3 |
Cane |
Bipolaris setariae |
99% |
4 |
I-4 |
Cyperus |
Trichoderma virens |
99% |
5 |
I-5 |
Alang-alang |
Fusarium oxysporum |
95% |
6 |
I-6 |
Alang-alang |
Fusarium keratoplasticum |
94% |
7 |
I-7 |
Rice |
Gaeumannomyces amomi |
98% |
8 |
I-8 |
Cyperus |
Trichoderma longibrachiatum |
81% |
9 |
I-9 |
Maize |
Dothiodeomycetes sp |
99% |
10 |
I-10 |
Elephant grass |
Pseudopestalotiopsis theae |
96% |
11 |
I-11 |
Rice |
Phialemonium dimorphosphorum |
97% |
12 |
I-12 |
Maize |
Starmerella bombicola |
99% |
As can be seen from table 1, eight isolates have homology of ITS DNA sequense minimum 97% compared to NCBI database. Four isolates had identity sequense less than 97% include isolates I-5, I-6, I-8, and I-10. The eight isolates matched with species database at NCBI database were: I-1 with Gaeumannomyces graminis, isolate I-2 with Meyerozyma guiliermondii, isolate I-3 with Bipolaris setariae, isolate I-4 with Trichoderma virens, isolate I-7 with Gaeumannomyces amomi, isolate I-9 with Phialemonium dimorphosphorum, islate I-11 with Phialemonium dimorphosphorum,and isolate I-12 with Starmerella bombicola.
It has been known that symbiotic relationship between endophytic fungi and host, sspecially in grass family, is a constitutive mutual relationship. The association between endophytic fungi and their host is relatively strong because of mechanism that enables endophytes to colonize their host through hosts’ ovules and then spread along with seeds. In plant tissues, endophytic fungi live in suitable environment with appropriate pH, humidity, and nutrition source, which made it possible to live and propagate and was protected from competition with rhizosphere and phyllosphere microorganisms. The plants provide nutrition for endophytic fungi for their growth; in turn these endophytes contribute by inducing factors for plants health2.
The results showed that more endophytic fungi were found in elephant grass (Pennisetum purpureum), in cyperus (Cyperus rotundus) and alang-alang (Imperata cylindrica). Zhang et al. [2006]38 stated that almost all class of plants are known as host of endophytes microorganism. Endophytic fungi can be found in different species of plants all over the world. They can infect and colonize any or whole parts of host plants from root, stem, leaves, flower, fruits, and seeds35. Endophytic will stay in parts of plant tissues without causing any harmful effects to the hosts through their entire or partial life cycle.
Environment factors around plants affect the number and species of endophytic fungi that penetrate into plant tissues. Different host (graminiae) conditions predisposed the level of colonization of fungal endophytes in plants tissues. In this research, fungal endophytes found in graminiae roots also varies in number and types of species. Plants conditions, including genotype factor as well as their vigour play an important role in determining fungal success story in colonizing plant parts1. Internal factors of graminiae (age, parts of plants taken as samples) and environment factors (agricultural area, soil conditions, and climate) are believed to play important role to the varieties of fungal endophytes. Elephant grass, cyperus and alang-alang can be found easily around agricultural farms and they grow all year round. These grasses are also found in abundant. These condition is suitable for fungal species to infect, colonize and become endophytic partners to host plants. From those three species of grass, elephant grass (Pennisetum purpureum) is the most widely distributed.
In generally, endophytic fungi basically act as non-patogenic or epiphytic inside the host plants and do not cause any damage to host tissues. Based on their association with host plant,4 fungal endphytes are divided into two groups, constitutive and inductive mutualism. Constitutive mutualism is a close association between fungi and its host plant, specially the grasses. In this case, endophytic fungi are known to descend vertically to the progeny. Inductive mutualism is an association between fungi and its host plants which disseminate horizontally through air, soil or water. They infect vegetative parts of the host and remain metabolically inactive for a long period of time.
The process of endophytic fungi penetration and colonization into plants tissues and the involvement of all active substances produced have caused plants to develop different morphological and physiological changes to induce resistance3,12. Endophytic fungi are known to influence host plants physiology which in turn expanding root system, stimulating root hair elongation, increasing exudation of phenolic substances around rhizosphere in facing biological, Rodgriguez et al. [2009]30 or physical, Hubbard et al. [2012]15 environment stresses. From various researches, it was proven that plants containing endopohytes microbes show high defense genes expressions compared to control plants without endophytes, Gao et al. [2010]9. Endophytic fungi in plants has the ability to accelerate germination process, to survive in unfavourable condition, to speed up growth and increase sustainability under environment stresses, Yurnaliza et al. [2014]36. The beneficial impacts for plants by fungal endophyte against pathogenic infections was reported by Narisawa et al. [2000]22. Endophytic fungi were demonstrated to be useful in protecting host from pathogen infections by producing secondary metabolites which inhibit or induce plants protection system, Here et al. [2007]17. Furthermore, the positive contribution in accelerating of growth and enhancing the viability to drought and high temperature was reported by Lehtonen et al. [2005]18. In addition, the presence of fungal endophyte was determined as health bioindicator of plants10.
The findings showed that there were three isolates which were identified as Phialemonium dimorphosphorum, Gaeumannomyces graminis and Gaeumannomyces amomi which gave significant stimulation on growth of rice seedlings. All these three isolates in fact originated from rice plant. This indicated that the mutualistic relationship between endophytic fungi and the host was specific. Coexistance or coevolution between endophytes and their host already well established since long time ago.The ability of endophytic fungi to promote rice plants growth depends on the ability to produce metabolites that enhance growth. Basically, endophytic fungi have the ability to help hosts in absorbing nutritions such as nitrogen (N) and phosphorous (P) 34. Better nutrition absorption will influence better growth and resistance against pathogens, Evan et al. [2013]7. Growth promoter substances such as gibberellin, auxin and cytokinin are also produced by endophytic fungi, Khan et al. [2012]17. Phialemonium dimorphosphorum was reported to produce phytohormone (Indole Acetic Acid) that can promote plants growth. IAA is a group of auxin which involved as promoter of plants growth. Plants also obtain growth hormones that are produced by endophytic microbes in addition to their own production. Endophytic fungi that produce IAA have the ability to distribute IAA into plants system more extensively. The role of endophyte microbes producing IAA in promoting host plants growth has been studied, particularly on certain strains of wheat plants, Etesani et al. [2009]8.
Endophytic fungi penetrate into plant tissues through different ways, actively or passively. Active mechanism is obtained by the growth of fungi through epidermis into plant tissues and then colonies are formed24. Endophytic fungi also penetrate plant tissues when there are wounds or carrier such as water or insects20. Haida et al. [2009]11, stated that there are differences of colonization levels of endophytic fungi in different part of plants of the same or different host plants. Sometimes, endophytic fungi are present in plant seeds as result of penetration of ovule during pollination, then grow along the development of embryo, Rodriguez et al. [2009]30. Endophytic fungi create the plants as their niche and food source, Selim et al. [2012]32. There was no significant evident showing differences in number and species of fungal endophytes found in similar or different types of plants. Physiologically, the plants determine types of species of fungal endophytes that can penetrate the tissues, especially root, Redman et al. [2001]28.
Type of interaction between plants and endophytic microbes is a dynaimic process. According to Redman et al. [2001]28, microorganisms occupy plants tissues successively and will select the next organisms, which in this case plant physiological responses depend on the first microorganism that form the first colony. Relationships created can be mutualism, commensalism, neutral or parasitism. Moreover, colonization of plants by symbiont can lead to resistance of plants to biotic and abiotic factors. Biotic factors include microorganism that increase host defense system by inducing intrinsic expression and supplying additional extrinsic defense mechanism such as antibiotic. Among the two factors, the dominant factor is determined by coevolutional process between hosts-endophytes-pathogens, Here et al. [2007]14.
The twelve isolates obtained from this research showed different characteristics in morphology and color, colony, hyphae and conidia. These showed that every isolate has its own specific characteristics. Colony or hyphal color differences was caused by intercelullar pigments produced by microbes including fungi, such as anthocyanin, melanin, carotenoid, tripirylmethane and phenozin31. Based on ITS DNA marker approach, only 8 isolates were able to be identified up to species level. This was particularly following the consensus on prokaryot system regarding homology status with gene bank data base, Madigan et al. [2012]19. By using this consensus, homology 97% or above data base can be considered as same species level, while homology minimum 95% can be considered as same genera with the data base. Isolate I-9 has a homology 99% with the database, however the reference DNA sequence on the data base is not up to species level. This reference is only up to genera level (Dothiodeomycetes sp), therefore identification can not go further to species level. In order to determine the isolate up to species level, it was suggested to use polyphasic approach.
The 12 out of 124 isolates identified as Gaeumannomyces graminis, isolate Meyerozyma guiliermondii, Bipolaris setariae, Trichoderma virens, Gaeumannomyces amomi, Phialemonium dimorphosphorum, Phialemonium dimorphosphorum, Starmerella bombicola, Fusarium sp., Trichoderma sp., Dothiodeomycetes sp., and Pseudopestalotiopsis sp. were positive to support rice seedlings growth. In addition, there were 3 isolates identified as Phialemonium dimorphosphorum, Gaeumannomyces graminis, and Gaeumannomyces amomi were significantly increase plant height, root length and root colonization.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expertise of staff of the Department of Microbiology, School of Life Science and Technology – Bandung Institute of Technology.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Arnold, A.E. and Here, A.E. (2003). “Canopy cover and leaf age affect colonization by tropical fungal endophytes” : Ecological pattern and process in Theobroma cacao (malvaceae). Mycologia, 95(3): 388-98.
- Backman, P.A. and Sikora, R.A. (2008). “Endophytes: An emerging tool for biological control”. Biological Control. 46:1-3.
- Bailey, B.A., Bae, H., Strem, M.D. Roberts, D.P., Thomas, S.E., Crozier, J., Samuels, G.J., Choi, I-Y., and Holmes, K.A. (2006). “Fungal and Plant Gene Expression during the Colonization of Cacao Sedlings by Endophytic Isolates of Four Trichoderma Species”. Planta, 224 (6): 1449-64.
- Carrol, G. (1998). “Fungal Endophytes in Stem and Leaves: From Laten Pathogen to Mutualistic Symbiont. Ecology”, 62: 2-9.
- Central Statistic Agency. (2016). “Production of Fixed Food Crops, 2015”.
- Durham, N.C. 2004., “Armies of Fighting Fungi Protect Chocolate Trees”. www.rpi.edu/ajayan/locker/publications/natureajayanjan202004.pdf
- Evan P. Ramdan, Widodo, Ef T. Tondok, Suryo Wiyono, Sri Hendrastuti Hidayat. “Fungal Endophytic Nonpatogen Origin of Chili Plants and Its Potential as a Growing Agent for Growth”. (2013). Journal of Indonesian Phytopathology. Bogor Agricultural Institute. Bogor. Vol 9. No. 5. Pg. 139-144. ISSN: 2339-2479.
- Etesami H, H.A Alikhani, and A.A Akbari, (2009).” Evaluation of plant hormones production (IAA) ability by iranian soils rhizobial strains and effect of superior strains application on wheat growth indexes”. World Apllied Sciences Journal 6(11): 1576-1584.
- Gao, F-K., Dai C-C., and Liu, X-Z., “Mechanisms of fungal endophytes in plant protection against pathogens”. (2010). African Journal of microbiology Research. 4(13), 1346- 1351.
- Genaro, M., Gonthier, D.& Nicolotti, G. (2003). “Fungal Endophytic Communities in healthy and declining Quercus robur L. anf Q. Cerris in Northern Italy”. Journal of Phytopatholy 151:529-534.
- Haida, S.N., and Idris, A.S. (2009). “In invitro colonization and nursery evaluation of endophytic fungi as biological control of Ganoderma Basal Stem Rot in oil palm”. Proceedings of Agriculture, Biotechnology and suistanability Conference. PIPOC 2009 Int. P.O. Cong-Agric., Biotech. And Sustain. Conf-Vol. 3:397-407.
- Harman, G.E. Howell, C.R. Viterbo, A., and Chat, I. (2004). “Trichoderma spp. Opportunistic avirulent plant symbionts”. Nat. Rev. Microbial. 2:43-56.
- Hawksworth, D. C., and A. Y. Rossman, (1987). “Where are the undescribed fungi?” Phytopathology 87:888-891.
- Here, E.A., Mejia L.C., Kyllo, D.A., Rojas E., Maynard Z., Butler A., and Bael, S.A.V. (2007). “Ecological implications of Anti-pathogen Effects of Tropical Fungal Endophytes and Mycorrhizae”. Ecology, 88 (3): 550-558.
- Hubbard, M.J., Germida, and V. Vujanovic. (2012). “Fungal endophytes improve wheat seed germination under heat and drought stress”. Botany 90: 137-149.
- Istikorini Y.(2008). “Potential Endophytic Fungus for Controlling Anthracnose Disease in Chili”. (Dissertation). Bogor: Graduate School, Bogor Agricultural Institute.
- Khan, S.A., Hamayun M, Khan AL, Lee IJ, Swinwari ZX, Kim J. (2012). “Isolation of Plant Growth Promotic Fungi from Dicots Inhabiting Coastal Sand Dunes of Korea” J. Bot. 44 (4): 1453-7031.
- Lehtonen, P., Helander, M., Saikkonen, K. (2005). “Are endophyte-mediated effects on herbivores conditional on soil nutriens?” Oecologia 142: 38-45.
- Madigan M.T., Martinko J.M. Stahl D.A., Clark D.P. (2012). “Biology of Microorganism”.13th ed. San Francisco. P.140-141.
- Maheswari, R. (2006) : What is an endophytic fungus?” Current Science, 90. (10) 1.
- Munif, A. Wiyono. S, Suwarno. (2012). “Isolation of Endophytic Bacteria from Gogo Rice and Its Potential as Biocontrol Agent and Growth Booster”. Bogor Agricultural Institute. Bogor. Journal of Indonesian Phytopathology. Volume 8, Number 3, Page. 57-64. ISSN 0215-7950.
- Narisawa, K., Ohki, T and Hashiba, T. (2000). “Suppresion of Clubroot and Verticilum Yellows in Chinese Cabage in the Field by the endophytic fungus, Heteroconium chaetospira”. Plant Pathology 49, 141-146.
- Niere B. (2002). “Banana Endophyte : Potential for Pest Biocontrol”. IITA-ESARC. Kampala. Uganda.
- Petrini, O., T.N Sieber, L Toti dan O. Viret. (1992). “Ecology Metabolite Production and Substrate Utilization In Endophytic Fungi”. Natural Toxins (1) : 185 – 196.
- Peters, S., S. Draeger, Aust, and B. Schultz. (1998). “Interactions in Dual Cultures of Endophytic Fungi with Host and Non Host Plant Calli”. Mycologia 90: 360-367.
- Rayner ADM. (1991). “The Challenge of The Individualistic Mycelium”. Mycologia 83 : 48- 7.
- Redline, S.C. L.M. Carris. (1996). “Endophytic Fungi in Grasses and Woody Plants Systematic, Ecology and Evolution”. Minnesotta : American Phytopathological Society (APS) Press.
- Redman, R.S., Dunigan, D., Rodriguez, R.J., and Rodriguez, R. (2001). “Fungal Symbiosis from mutualism to parasitism”. Who controls the outcome, host or invader? Controls the outcome, host or invader? New Phytologist, 151, 705-716.
- Reininger, V., Grunig, C.R. and Sieber, T.N. (2012). “Host species and Strain combination determine growth reduction of spruce and birch seedlings colonized by root-associated dark septate endophytes”. Environmental Microbiology 14: 1064-1076.
- Rodriguez, R.J., White, J.F., Arnold, A.E., and Redman, R.S. (2009). “Fungal endopyhtes : Diversity and Functional Roles”. The New Phytologist. 182 (2): 314-30.
- Safrida, Yuni,. (2012).”Isolation and Characteristics of Potential Probiotic Bacteria on Bloated Fish (Rastrelliger sp.)” Journal. University of Syiah Kuala, Aceh.
- Selim, K.A., El-beih, A.A., Abd El-Ranman, T.M., El-Diwany, A.I. (2012). “Biology of endophytic fungy. Curr”. Res. Environ. Appl. Mycol. 02.31-82.
- Tellenbach, C., Grunig, C.R. Sieber, T.N. (2011). “Negative effects on survival and performance of Norway spruce seedlings colonized by dark septate root endophytes are primarily isolated-dependent”. Enviromental Microbiology 13: 2508-2517.
- Tan, R.X., and W.X., Zou. (2001). “Endophytes: as a rich source of functional metabolites”. Nat. Prod. Rep. 18: 448-459.
- Worang, R.L. (2003). “Endophytic Fungi as An Antibiotic Producer”. Bogor Agricultural Institute.
- Yurnaliza , Aryantha I.N.P, Esyanti, R.R and Susanto A. (2014). “Antagonistic Activity Assessment of Fungal Endophytes from Oil Palm Tissues Against Ganoderma boninense Pat”. Plant Pathology Journal, 13 (4): 257-267.
- Zakaria,L, Yaakop, A.S, Salleh, B. and Zakaria M. (2010). “Endophytic Fungi from Paddy”, Trop Life Sci Res. 21(1): 101–107.
- Zhang, H.W., Y.C. Song and R.X. Tan. (2006). “Biology and Chemistry of endophytes”. Nat. Pro. Rep., 23:753-771.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.