ISSN: 0973-7510
E-ISSN: 2581-690X
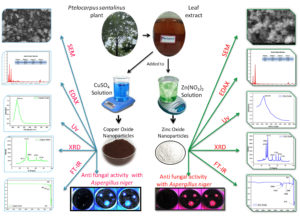
Copper oxide (CuO) and zinc oxide (ZnO) nanoparticles were synthesized by Pterocarpus santalinus leaf extract following the green method as a reducing and capping agent. Analytical methods like scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and UV-visible spectroscopy were followed for characterization of synthesized nanoparticles. Outcomes demonstrated that crystalline CuO and ZnO nanoparticles with distinct morphologies have successfully formed. The ability of metal oxide nanomaterials against the growth of Aspergillus niger fungus was tested by agar diffusion method on PDA plates. Pure culture of the isolated fungus from onions was used in this experiment. Fungal inoculum was prepared and spread on PDA plates and tested for growth inhibition with varying concentrations of CuO and ZnO nanoparticles using Disk Diffusion Method. A successful growth inhibition was identified in plates treated with CuO and ZnO.
Copper Oxide, Zinc Oxide, Pterocarpus santalinus, Aspergillus niger, Allium cepa
Metal oxide nanoparticles (MONPs) are gaining momentum in agriculture for its application in inhibiting the plant pathogenic microorganisms and for plant growth promotion. Green synthesis of MONPs is one of the efficient chemical synthesis in executing the functions of synthesized nanoparticles. It also reduces the usage of chemical reagents in synthesis process. The replacement of chemical pesticides with MONPs antimicrobial properties is potential in reducing the usage of chemical agents.1 The negative impact of synthetic chemicals such as fungicides on human health can be addressed with these latest technologies. It can target the fungicide resistant pathogen populations and ultimately may reduce the load of microbial toxins in vegetable crops.2 Onion (Allium cepa)is most widely consumed and cultivated vegetable. In India, onions are available in all seasons of the year3 (Rabi, Kharif and Late Kharif seasons). It is consumed raw in salads and a main ingredient in many Indian recipes and various dishes around the world. Onions are grown in all parts of India major cultivating states are Maharashtra, Madhya Pradesh, Gujarat and Karnataka and Andhra Pradesh. Onions are rich in nutrients having many health benefits. Onion crop is susceptible to various infections by bacterial, viral and fungal pathogens leading to crop loss.4 The present study focuses on inhibition of Aspergillus niger. The fungus A. niger infection in onion causes severe crop loss during various stages of plant growth. The fungus survives in harvested onions and infection increases with increase in storage time.5 Generally the onions are left to air for some days allowed for drying before marketing. The infected onions brought from field when kept for storage in storage units can spread the infections to healthy onions. All the infected onions turn black in color and they lose their market value. The spores of filamentous fungus A. niger on inhalation causes opportunistic infections that affects lower respiratory tract.6 The organism colonization leads to Aspergillus bronchitis and pneumonia. The tree Pterocarpus santalinus, red sandalwood belongs to the family Fabaceae. Its wood is dark red to purple in color. It’s a high commercial value wood used in medicines and cosmetics.7 It’s endemic to South India and found only in southern parts of the Eastern Ghats. It’s having high medicinal values with bioactive compounds is being used in Ayurveda from ancient times.8 In the present study P. santalinus leaf extract was utilized in green synthesis of Copper oxide and Zinc Oxide Nanoparticles. MONPs like zinc oxide and copper oxide nanoparticles may have antibacterial properties.9,10 These materials are prepared using copper sulphate, zinc nitrate precursors following standard methods.11 The inhibitory effect of the synthesized nonmaterial was tested on isolated fungus from onions.
Copper sulphate, zinc nitrate, sodium hydroxide, potato dextrose agar (PDA) were purchased from Hi-Media chemicals, sterilized glass petriplates, Lacto phenol cotton blue (LPCB), for disk diffusion method disks were made using sterile Whattman No. 1 filter papers and cotton swabs were used.
Plant Extract Preparation
P. santalinus leaves gathered from the Yogi Vemana University Botanical Garden in Kadapa, Andhra Pradesh, India. The fresh leaves washed followed by drying in the shade to reduce moisture and put away for later use. Separately, 2 grams of powdered substance was taken in a beaker with 100 ml of deionized water and boiled at 80°C for 25-30 minutes while in a stirring condition. The solution was cooled to room temperature then filtered using filter paper (Whattman No. 1) for removing heavy biomaterials, and centrifuged (5000 rpm/10 minutes). Clear solutions of plant extracts were obtained by keeping at 4°C until further use.
Copper Oxide nanoparticles synthesis
To synthesize copper oxide nanoparticles (CuO NPs) 30 ml leaf extract of P. santalinus plant was combined with 10 mM copper sulphate solution (70 ml) and continuously mixed at 60°C for 30 minutes. CuO NPs formation was verified by visual inspection that revealed a change in the solution’s color.12
Zinc Oxide nanoparticles synthesis
Zinc Oxide Nanoparticles (ZnO NPs) synthesis was done by rapidly rotating a zinc nitrate solution (0.1 M, 70 mL) at room temperature for 10 minutes while adding 30 mL of P. santalinus aqueous leaf extract. By using NaOH the solution was set to pH10. Then the solution was stirred for another 30 min at 60°C. The production of ZnO NPs were visibly noticed as a color change.13
Characterization of nanoparticles
CuO NPs and ZnO NPs were examined using an X-ray diffraction equipment (XRD) with Cuk radiation (=1.5412) at scanning 2θ range 100-800. By using UV-1800 Shimadzu UV Spectrophotometer in the wavelength range of 200-800 nm, UV-visible spectra was taken to confirm the absorbance of CuO and ZnO NPs. To detect the presence of distinctive functional groups on the surface of the CuO NPs and ZnO NPs, Fourier transform infrared (FTIR) spectra were taken using a JASCO INC 410, Japan, in the 400-4000 cm-1 range. Surface morphology of synthesized CuO and ZnO NPs were studied by using SEM and an elemental analysis was done by Energy dispersive x-ray analysis (EDAX).14
Collection of infected samples
The infected onions were collected from local vegetable market at Kadapa town. From the infected onions pure culture of A. niger was isolated. Isolated organisms were maintained on PDA (HiMedia) for further experiments.
Isolation of plant pathogens
Infected onions were collected and infection was identified by black colored powdery growth on onions. Infected onions were taken and surface layer was removed aseptically by wearing glove. Small piece of inner layer of onion was taken and kept on surface of PDA media plate. The plate with the infected onion piece was incubated at room temperature for three days and observed for fungal growth. After 3rd day of incubation fungus growing on the edges of the infected onion pieces was taken and subcultured into fresh PDA media. The fungus was identified based on fungal mycelia observation under microscope. Pure culture of identified fungus was maintained for further experiments.15
Simple microscopic observation of fungi
One drop of Lacto phenol cotton blue (LPCB) was taken on sterile clean slide. Using a sterile inoculating needle, a small portion of fungus growing from the pure culture plate was taken and added into a drop of Lacto phenol cotton blue on the slide. Tease with inoculating needle and cover slip and examined the wet preparation under low and high-power objectives. Observed and recorded the shape, types of spores, nature and color of the growth. The fungal growth was preserved by pure culture techniques at 4°C.16
Preparation of Inoculums
In 1 ml of sterile distilled water, from pure fungal culture a small amount of mycelium portion was taken and mixed well. With the help of vortex mixture the solution was mixed evenly. From this inoculum was taken for antifungal activity.
Antifungal activity
150 ml of PDA was prepared and poured into sterile petri plates each 20 mL under sterile conditions in a laminar air flow. After the solidification, a cotton swab was taken and dipped into the culture which is diluted in distilled water. To get rid of extra fluid, gently squeeze the swab against the tubes inside and used the swab to create a lawn of growth on a PDA plate. Three different concentrations (40, 80, 120 µg/mL) of CuO and ZnO NPs were taken in Eppendorf tubes separately and then sterile disks were taken and dipped in nanoparticles containing tubes. Using flame-sterilized forceps, discs were placed on the agar’s surface (all plates contains three concentrations of nanoparticle disks each Nanoparticles activity tested in duplicate). Then, the plates were placed in an incubator for overnight at 37°C for incubation. By assessing the zone of inhibition for the tested fungus, the antifungal activity was assessed.17 The (cm) size of the inhibitory zones was measured. Sterile distilled water dipped disc was maintained as a blank in single plate.
Collection of infected samples
Infected onions were collected from vegetable market in Kadapa. The infection was identified by black colored powdery growth on onions (Figure 1). Infected onions were taken and surface layer was removed aseptically by wearing glove. Small piece of inner layer of onion was taken and kept on surface of PDA media plate. The plate with the infected onion piece was incubated at room temperature for three days and observed for fungal growth. After 3rd day of incubation fungi growing on the edges of the infected onion pieces were taken and subcultured into fresh PDA media. The fungi were identified based on fungal mycelia observation under microscope. The identified fungi were used for further experiments.
Isolation of plant pathogens
Isolated and sub-cultured fungus was tested for pure culture of Aspergillus niger by microscopic observation. The organism was identified by septet hyphae black conidia.18 Pure culture of A. niger was observed under microscope (Figure 2).
Characterization of nanoparticles
UV-Spectrophotometer
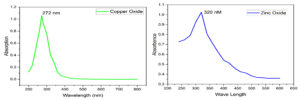
The absorbance peak at 272 nm (Figure 3) by UV-visible absorption spectra verified the production of CuO NPs. After that, the CuO NPs were purified and the metal ions were bio-reduced based on a qualitative analysis of the supernatant. Cuprous CuO NPs formation was confirmed when the reaction liquid turns brown and exhibits UV-visible absorbance peak at 272 nm.19 Secondary metabolites in plants can change zinc ions in solution into ZnO NPs, Plant extract is used for its reducing activity and as stabilizing agent. By looking at the UV-visible spectra in the 190-800 nm range, this was confirmed. ZnO nanoparticles had a noticeable peak in the spectra at 320 nm (Figure 3). The absorbance peak of ZnO nanoparticles is said to occur between 310 and 360 nm in wavelength.
Figure 3. UV absorption spectra of CuO and ZnO Nanoparticles. (λmax at 272 nM and 320 nM, respectively)
SEM with EDAX
Surface morphology of green synthesized CuO and ZnO nanoparticles was observed under scanning electron microscope (SEM).20 Shape and structure of green synthesized CuO and ZnO NPs are shown in Figure 4. The EDAX findings, which displayed a strong signal for CuO and ZnO NPs, verified the presence of copper and zinc in the oxide form. Significant peaks of 53.13% copper and 28.41% oxygen in CuO NPs and 74.1% zinc and 19.95% oxygen in ZnO NPs are provided by the EDAX (Figure 5). This report makes it possible to ascertain the relative amounts of every component in the analyte. Two different peaks in CuO and ZnO, respectively, indicated the elemental composition of the synthesized molecules. Apart from CuO and ZnO, no further residues were detected.
Figure 4. SEM images of green synthesized CuO NPs at different magnification; A) 2 µm, B) 1 µm, C) 1 µm, D) 500 nm and ZnO NPs; E) 1 µm, F) 500 nm, G) 200 nm, H) 100 nm
Figure 5. EDAX image showing Copper and Oxygen, Zinc and Oxygen peaks along with the percentage of the elements
XRD
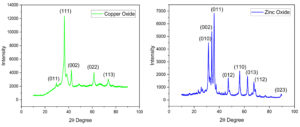
The X-Ray Diffraction (XRD) patterns of CuO and ZnO NPs as shown in Figure 6. The [011], [111], [002], [022] and [113] planes of CuO can be indexed to the reflections recorded at 29.66, 36.43, 42.43, 61.39 and 73.59 (2θ Degree). The peaks are indexed to be typical monoclinic structures of CuO NPs21 and ZnO NPs are shown results with hexagonal shape (wurtzite structure) and crystalline nature of ZnO.22 The [010], [002], [011], [012], [110], [013], [112], [023] planes of ZnO can be indexed to the reflections recorded at 31.41, 34.10, 35.86, 47.26, 56.36, 62.56, 67.57 and 89.26 (2θ Degree). It is discovered that green synthesized CuO and ZnO NPs having average crystallite size of 10.62 nm and 6.12 nm, respectively. The absence of any diffracted peaks indicating the presence of impurities demonstrates the high purity of the samples synthesized using green synthesis method.23
FTIR
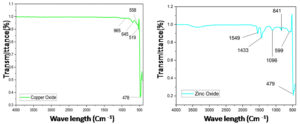
Possible biological capability or functional groups of plant extracts involved in stabilizing nanoparticles (NPs) were categorized using Fourier transform infrared spectroscopy. Formation of NPs by green synthesis is supported by the FTIR spectrum in the 400-5000 cm-1 range. The results of the study demonstrated the existence of vibrational bands in the samples within the 400-2000 cm-1 range (Figure 7). The primary infrared absorption peaks in the CuO NPs data indicate that the CuO nanostructures vibrate in the 500-700 cm-1 range. Major peaks observed were at 519 cm-1, 558 cm-1 and 645 cm-1. Peak observed at 519 cm-1 noted as stretching of Cu-O.23 Peaks in the 450-1500 cm-1 range were absorbed in the ZnO NPs data. Major peaks observed were at 479 cm-1, 599 cm-1, 841 cm-1, 1096 cm-1and 1433 cm-1 respectively. The peak at 479 cm-1 noted as stretching of Zn-O.24
Disk diffusion method for antifungal activity
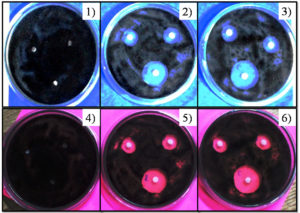
A 20 ml of Potato dextrose agar (PDA) was prepared and poured in sterile Petri plates. Swab was used to prepare lawn of fungal growth. Three different concentrations (40, 80, 120 µg/mL) of CuO and ZnO NPs were prepared on disks and each nanoparticles activity was tested in triplicate. Then the plates placed in incubator for overnight at 37°C. Zone of inhibition against the fungus was assessed. Size of the inhibitory zones (mm) was measured. The growth inhibitory effect of synthesized CuO and ZnO NPs was tested on Aspergillus niger by Disk diffusion method. After the incubation for 48 hours clear zones of growth inhibition were observed. For experiment 6 plates of Potato Dextrose Agar Medium was prepared, two plates used as a Blank (control) and remaining four plates were used as test plates (Duplicate) (Figure 8). Here, we have used three different concentrations of nanoparticles sample for that we got all three concentrations positively showing zone of inhibitions, with diameter of zone of inhibitions were as shown in the Table. These results demonstrate the considerable potential for inhibiting A. niger by Copper Oxide and Zinc Oxide Nanoparticles (Figure 9).
Table:
Showing diameter of zone of inhibitions with three different concentrations of Green synthesized Copper Oxide and Zinc Oxide Nanoparticles
No. |
Concen. of Nanoparticles |
Zones of growth inhibition with Copper Oxide Nanoparticles |
Zones of growth inhibition with Zinc Oxide Nanoparticles |
|---|---|---|---|
1. |
40 µL |
1.2 ± 0.1 cm |
1.3 ± 0.1 cm |
2. |
80 µL |
1.4 ± 0.1 cm |
1.5 ± 0.1 cm |
3. |
120 µL |
2.3 ± 0.1 cm |
2.9 ± 0.1 cm |
Figure 8. Zone of growth inhibitions of Aspergillus niger
Plate 1: Blank for Copper Oxide Nanoparticles; Plate 2 and 3: Inhibition with Copper Oxide Nanoparticles with three different concentrations (Duplicate Plates); Plate 4: Blank for Zinc Oxide Nanoparticles; Plate 5 and 6 Inhibition with Zinc Oxide Nanoparticles with three different concentrations (Duplicate Plates)
Nanoparticles of several metals and their oxides, such as Ag, ZnO, Fe2O3, Fe3O4, Al2O3, TiO2 and CuO, exhibit antimicrobial effect against fungi as well as Gram-negative, Gram-positive bacteria.25-27 CuO and ZnO have broad range of applications and its antimicrobial activity is one of the significant application in agriculture.28 In this study the metal oxides such as CuO and ZnO were selected for testing their antimicrobial properties. Copper oxides have high surface area and crystal morphology. CuO known for its potential antimicrobial property.29 It inhibits the growth of microorganisms by various mechanisms. Mechanisms involved in antimicrobial property of CuO nanoparticles includes its interaction with fungi. As CuO is positively charged it adhere to the fungal cell walls. Highly ionic CuO nanoparticles in high doses are known to inhibit many fungal species. Evidenced studies with fungi such as S. cerevisiae. Similarly, in the present study, CuO also exhibited good inhibition of Aspergillus niger growth. ZnO nanoparticles having varying structures exhibits significant antimicrobial activity.30 Similarly, in our study, the ZnO exhibited good antimicrobial property and able to inhibit the growth of the fungus. The potential antimicrobial activity depends on specific surface area to volume. The toxic effects of ZnO on fungal surface and core results in death of the microorganism. The effect of nano material on environment and plant growth was analyzed in various studies.31, 32 It is needed to further study the effects of these materials on environment for extensive utilization in agricultural fields. The problems like emerging and reemerging infectious diseases, food contaminants can be addressed with effective antimicrobial metal oxide nanomaterials.
The present work effectively demonstrated the green synthesis of CuO and ZnO NPs using Pterocarpus santalinus leaf extract, demonstrating an environmentally benign and cost-effective method. Strong antifungal activity against Aspergillus niger was demonstrated by the biosynthesized ZnO and CuO NPs. These nanoparticles inhibitory action highlights their potential as useful biocontrol agents in agricultural settings, offering an alternative to traditional chemical treatments that frequently carry dangers to human health and the environment. Subsequent research endeavors may centre on refining the synthesis procedure, investigating the mechanism of antifungal activity, and assessing the effectiveness of the nanoparticles in practical agricultural situations.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Yugandhar P, Vasavi T, Uma Maheswari Devi P, Savithramma N. Bioinspired green synthesis of copper oxide nanoparticles from Syzygiumalterni folium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl Nanosci. 2017;7:417-427.
Crossref - Gelaye Y, Nakachew K, Ali S. A Review of the Prospective Effects of Spacing and Varieties on Onion Yield and Yield Components (Allium cepa L.) in Ethiopia. Sci World J. 2024;2024:2795747.
Crossref - Kim SH, Yoon JB, Han J, et al. Green Onion (Allium fistulosum): An Aromatic Vegetable Crop Esteemed for Food, Nutritional and Therapeutic Significance. Foods. 2023;12(24):4503.
Crossref - Shin JH, Lee HK, Back CG, et al. Identification of Fusarium basal rot pathogens of onion and evaluation of fungicides against the pathogens. Mycobiology. 2023;51(4):264-272.
Crossref - Silva JJ, Bertoldo R, Fungaro MHP, et al. Black aspergilli in Brazilian onions: From field to market. Int J Food Microbiol. 2021;337:108958.
Crossref - Atchade E, Jean-Baptiste S, Houze S, et al. Fatal invasive aspergillosis caused by Aspergillus niger after bilateral lung transplantation. Med Mycol Case Rep. 2017;17:4-7.
Crossref - El-Badawy RE, Ibrahim KA, Hassan NS, El-Sayed WM. Pterocarpus santalinus ameliorates streptozotocin-induced diabetes mellitus via anti-inflammatory pathways and enhancement of insulin function. Iran J Basic Med Sci. 2019;22(8):932-939.
- Bulle S, Reddyvari H, Nallanchakravarthula V, Vaddi DR. Therapeutic potential of Pterocarpus santalinus L.: an update. Pharmacogn Rev. 2016;10(19):43-49.
Crossref - Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015;7(3):219-242.
Crossref - Yusof HM, Rahman NAA, Mohamad R, Hasanah Zaidan U, Samsudin AA. Antibacterial potential of biosynthesized zinc oxide nanoparticles against poultry-associated foodborne pathogens: an in vitro study. Animals. 2021;11(7):2093.
Crossref - Phiwdang K, Suphankij S, Mekprasart W, Pecharapa W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia. 2013;34:740-745.
Crossref - Wu S, Rajeshkumar S, Madasamy M, Mahendran V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif Cells Nanomed Biotechnol. 2020;48(1):1153-1158.
Crossref - Sonia S, Kumari HLJ, Ruckmani K, Sivakumar M. Antimicrobial and antioxidant potentials of biosynthesized colloidal zinc oxide nanoparticles for a fortified cold cream formulation: a potent nanocosmeceutical application. Mater Sci Eng C Mater Biol Appl. 2017;79:581-589.
Crossref - Mohammadi-Aloucheh R, Habibi-Yangjeh A, Bayrami A, Latifi-Navid S, Asadi A. Green synthesis of ZnO and ZnO/CuO nanocomposites in Mentha longifolia leaf extract: characterization and their application as anti-bacterial agents. J Mater Sci Mater Electron. 2018;29(16):13596-13605.
Crossref - Solairaj D, Legrand NN, Yang Q, Zhang H. Isolation of pathogenic fungi causing postharvest decay in table grapes and in vivo biocontrol activity of selected yeasts against them. Physiol Mol Plant Pathol. 2020;110(22):101478.
Crossref - Amin N, Shenoy MM, Pai V. Clinical and Mycological Characterization of Chronic and Recurrent Dermatophytes using Various Staining and Microscopic Methods. J Pure Appl Microbiol. 2023;17(4):2598-2608.
Crossref - Parvathalu K, Chinmayee S, Preethi B, et al. Green synthesis of silver nanoparticles using Argyreia nervosa leaf extract and their antimicrobial activity. Plasmonics. 2023;18(3):1075-1081.
Crossref - Simonovicova A, Vojtkova H, Nosalj S, et al. Aspergillus niger Environmental Isolates and Their Specific Diversity Through Metabolite Profiling. Front Microbiol. 2021;12:658010.
Crossref - Binjawhar DN, Alsharari SS, Albalawi A, Abdulhasan MJ, Khat M, Ameen F. Facile green synthesis inorganic cuprous oxide nanoparticles and their antibacterial properties. Micro Nano Lett. 2023;18(1):e12154.
Crossref - Prasad RD, Prasad RS, Prasad RB, et al. A review on modern characterization techniques for analysis of nanomaterials and biomaterials. ES Energy & Environment. 2024;23:1087.
Crossref - Nzilu DM, Madivoli ES, Makhanu DS, Wanakai SI, Kiprono GK, Kareru PG. Green synthesis of copper oxide nanoparticles and its efficiency in degradation of rifampicin antibiotic. Sci Rep. 2023;13(1):14030.
Crossref - Atchudan R, Edison TNJI, Perumal S, Karthikeyan D, Lee YR. Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J Photochem Photobiol B Biol. 2016;162:500-510.
Crossref - Padil VV, Cernik M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomed. 2013;8(1):889-898.
Crossref - Rajendran SP, Sengodan K. Synthesis and characterization of zinc oxide and iron oxide nanoparticles using Sesbania grandiflora leaf extract as reducing agent. J Nanosci. 2017;2017:8348507.
Crossref - Wanag A, Rokicka P, Kusiak-Nejman E, et al. Antibacterial properties of TiO2 modified with reduced graphene oxide. Ecotoxicol Environ Saf. 2018;147:788-793.
Crossref - Liu J, Vipulanandan C, Cooper TF, Vipulanandan G. Effects of Fe nanoparticles on bacterial growth and biosurfactant production. J Nanoparticle Res. 2013;15(1):1-3.
Crossref - Arakha M, Pal S, Samantarrai D, et al. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci Rep. 2015;5(1):14813.
Crossref - Consolo VF, Torres-Nicolini A, Alvarez VA. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci Rep. 2020;10(1):20499.
Crossref - Hans M, Erbe A, Mathews S, Chen Y, Solioz M, Mücklich F. Role of copper oxides in contact killing of bacteria. Langmuir. 2013;29(52):16160-16166.
Crossref - Tiwari V, Mishra N, Gadani K, Solanki PS, Shah NA, Tiwari M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front Microbiol. 2018;9:1218.
Crossref - Kasana RC, Panwar NR, Kaul RK, Kumar P. Biosynthesis and effects of copper nanoparticles on plants. Environ Chem Lett. 2017;15:233-240.
Crossref - Rambabu K, Bharath G, Banat F, Show PL. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater. 2021;402:123560.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.