ISSN: 0973-7510
E-ISSN: 2581-690X
Psychobiotics are defined as the organisms that can provide the mental health benefit. The possible mechanism of psychobiotics is manipulation of neurotransmitter production and neurotransmitter production by the microbes. The lactobacillus group has been reported for the potential of neurotransmitter production, especially g-aminobutyric acid (GABA) which is an important inhibitory neurotransmitter. Therefore, GABA can be used for relaxation and applied in various psychiatric disorders. The aim of this study was determination of lactic acid bacterial isolates from Pak Sian Dong in Thailand for GABA producing ability. The results found that there were 3 isolates, SF66, SF80 and SF82, which revealed the ability to produce glutamic acid decarboxylase (GAD) enzyme. The GABA were detected by thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC) in the bacterial culture containing 3% monosodium glutamate. The survival in gastrointestinal synthetic condition found that only SF66 isolate showed the authentic percentage of survival then this isolate was selected. From the identification, the isolate was identified as Lactiplantibacillus pentosus and was designated as L. pentosus SF66 which exhibited with the potential for further investigation and development to be psychobiotics.
Psychobiotics, Probiotics, Gamma-Aminobutyric Acid, Pak Sian Dong
Spider plant or bastard mustard (Cleome gynandra L.) is a kind of plant which is known as Pak Sian in Thailand and these plants are world-wide distributed.1 Spider plant can be fermented to preserve this plant and produce the type of fermented food.2 During fermentation process, several microorganisms can grow and generate their products which lead to the signature taste of the fermented spider plant (Pak Sian Dong). For the community of microorganism in fermented spider plant, there are a group of lactic acid bacteria (LAB), such as Pediococcus pentosaceus, Lactiplantibacillus plantarum and Levilactobacillus brevis, and these bacteria were characterized for probiotic activities.3
Probiotics are commonly defined as the live organisms that can provide beneficial effects to the hosts.4 Various aspects of probiotics can be used such as prevention of infection, promotion of the health and treatment of the diseases. Interestingly, the approach for using the probiotics as psychobiotics, which is the probiotic that capable of giving profit to nervous system, is in the trend. From consuming of glutamic acid and using activity of glutamic acid decarboxylase (GAD) enzyme, several LAB have ability to produce g-aminobutyric acid (GABA) which is an important neurotransmitter for the nerves as described above. For instance, L. plantarum which is isolated from Thai fermented food exhibited GABA production.5 Moreover, the isolation of Lactobacillus buchneri from Kimchi had been determined for GABA production and studied the effect on neuronal cells.6
GABA is one of various neurotransmitters in cerebral cortex that is synthesized by GABAergic neuron and plays an important role in inhibitory neurotransmission. Abnormal GABA metabolism may induce abnormal cortical excitability.7 Biosynthesis of GABA requires the decarboxylation reaction of glutamate catalyzed by GAD.8 Not only central nervous system but also gastrointestinal tract require GABAergic neurotransmission via interneuron of the enteric nervous system. GABAergic signaling in the gut is involved in the activation of GABA receptor that modulate gastrointestinal motility and mucosal function.9
The bidirectional communication between the brain and the enteric nervous system, called gut-brain axis, is the network that regulates gastrointestinal homeostasis as well as higher cognition by integrating and linking brain function associated with emotion and cognition with gastrointestinal function by mechanisms such as neuroendocrine and immune response. Moreover, gut-brain axis can be influenced by the commensal gut microbiota.10 In accordance with impermeability of the blood-brain barrier to GABA; nevertheless, there is some evidence that small amount of GABA can pass through the blood-brain barrier.11
Previous study showed that mice chronically administered with L. rhamnosus (JB-1) had alterations in GABA receptor mRNA in the brain.12 Moreover, reduced stress hormone, corticosterone, and behavior related to anxiety and depression were also found. On the contrary, these results were not found in vagotomized mice.13 The results suggest that vagus nerve may play a key role in microbiome gut-brain axis.
Due to the discovery of GABA producing LAB which had been previously isolated from diverse fermented food and beneficial effect of probiotic on gut-brain axis and, therefore, the goals of this study are screening for GABA producing LAB which isolated from Pak Sian Dong and characterizing the isolates for using as probiotic bacteria.
Bacterial isolation and culture condition
Pak Sian Dong were collected from local area in Pathum Thani province and used as the source for LAB isolation. The LAB were isolated by spreading the sample on MRS agar containing CaCO3 and nystatin. After incubation at 37°C for 48 h, the colonies with clear zone were picked from the plate and then characterized for lactobacilli properties which included gram positive, rod shape and catalase negative. The selected isolates were grown in MRS broth and stored at -80°C in glycerol until used.
Glutamic acid decarboxylase (GAD) activity and thin layer chromatography (TLC)
GAD activity was measured with color changing of indicator in reagent. The reagent for GAD activity was prepared. To determine GAD activity, the pellet of overnight culture was collected by centrifugation at 10,000 rpm for 2 min. The pellet was washed twice with normal saline solution to remove culture medium. A 500 µl of The GAD activity reagent (a liter of water which consisted of 1 g of L-glutamic acid, 300 µl of TritonX-100, 90 g of NaCl and 0.05 g of bromocresol green) was added to the pellet and mixed well then incubated at 37°C for 4 h. The color changing of the reagent was observed and blue color was indicated as strong GAD activity which remarked as positive result.14
For TLC, the isolate was inoculated in MRS containing 3% (w/v) of monosodium glutamate and the supernatant was collected from 48 h of the culture. The culture supernatants were spotted on TLC plate (Loba Chemie, India) and GABA was used as standard positive control. The solvent which containing butanol: acetic acid: distilled water (5:3:2) was prepared and used as mobile phase. After TLC running, the plate was sprayed with ninhydrin solution and placed at 70°C for 15 min.15 Finally, the bands were observed and compared with GABA standard control then picked the positive isolates for further study.
GABA quantification by HPLC
Following the method which described by Kanklai et al.15 and Li et al.16 with slightly modification. In brief, the LAB isolates were inoculated and culture in MRS containing 3% (w/v) of monosodium glutamate and the supernatant were collected after incubation for 48 h. The supernatant cultures were dried with lyophilization and resuspended with 1000 µl of the mixture which consisted of ethanol: water: triethylamine (2: 2:1). The derivatization was done by adding 80 µl of the solution which consisted of ethanol: water: triethylamine: phenyl isothiocyanate (7: 1: 1: 1) and incubated in the dark environment at room temperature for 20 min. After derivatization, the GABA content was analyzed by HPLC (Shimadzu, Japan) with ODS-3 column (4.6 x 250 mm, 5 µm). The GABA standards were prepared for 0.1, 0.25 0.5 0.75 and 1.0 mg/ml.
Acid and bile tolerance
The acid tolerance of the isolate was determined with synthetic gastric fluid incubation. Overnight culture of the isolate was washed twice with normal saline solution and prepared bacterial suspension by adjusting with McFarland 0.5. After preparation of bacterial suspension, a 100 µl of suspension was added to 900 µl of synthetic gastric fluid and then incubated at 37°C for 3 h. For control tube, the suspension was added to normal saline solution instead of synthetic gastric fluid. The survival of the isolate was calculated by plate count technique on MRS agar compared with control.17
To examine resistant to bile, MRS with 0.3% (w/v) of bile was prepared and inoculated with bacterial isolate. After incubation at 37°C for 24 h, viable plate count was performed by using MRS agar and then calculated for percentage of survival.
In vitro hydrophobicity
Adhesion to hydrocarbon was determine to assess ability of cell adherence. The overnight culture was washed twice with normal saline solution and the concentration of cell suspension was prepared the optical density (OD) to 0.5 at 600 nm (A0). The suspension was pipetted 1200 µl and mixed with 200 µl of xylene for 1 min. After mixing, the tube containing bacterial suspension and xylene was statically placed at room temperature for 1 min. Subsequently, the aqueous phase was collected and measured the OD (A1) and calculate for percent of hydrophobicity by following formula (A0-A1 x 100) /A0.17
Blood hemolysis and gelatinase determination
Sheep blood agar (SBA) were used to determine blood hemolysis. The isolates were streaked on the SBA and incubated at 37°C for 48 h. The hemolysis activity positive was presented as clear zone surrounding colony of LAB isolates while negative result was showed as the growth without clear zone.
For gelatinase activity, MRS containing 12% gelatin was prepared in test tube and inoculated with LAB isolates by stabbing. After incubation at 37°C for 24 h, inoculated tubes were placed at 4°C to assess the hydrolyzing by gelatinase. The positive was presented as liquefaction of medium containing gelatin while negative was presented as solidified medium.
Bacterial isolate identification
The isolate, which had ability to produce GABA and showed high percentage of survival in synthetic gastric fluid and bile containing condition, was identified by API 50 CHL kit (bioMérieux, France) for phenotypic identification and determined the 16 s rRNA sequence for molecular identification. To differentiate between L. plantarum and L. pentosus, the primers and PCR condition for recA gene were used with slightly modification.18
Antibiotic susceptibility profile
Ten antibiotics, which included ampicillin, chloramphenicol, ciprofloxacin, erythromycin, kanamycin, nalidixic acid, novobiocin, penicillin, tetracycline and vancomycin, were selected to examine the susceptibility profile of the isolate. The procedure was done following Yasiri et al.19
Screening for GABA producing lactic acid bacteria
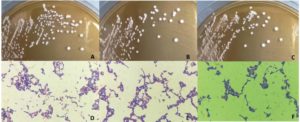
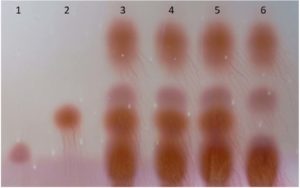
The 96 isolates were isolated and revealed lactic acid bacterial characteristics from Pak Sian Dong. The LAB isolates were screened for GABA producing ability by determination of GAD activity. There were 3 isolates, SF66, SF80 and SF82 (Figure 1), which changed indicator color from yellow to blue in GAD reagent representing an ability to produce GAD enzyme. All 3 isolates were cultured and collected the supernatants to run with TLC. The GABA expected bands, which could be assumed from comparison with the GABA standard, showed on the TLC plate after sprayed with ninhydrin and developed by heating (Figure 2). This result brought these 3 isolates for probiotic determination.
Figure 1. The colony of LAB isolates on MRS agar were showed on the upper row. The isolates SF66, SF80 and SF82 were represented with A, B and C, respectively. The Gram’s staining results of LAB isolates were showed on the lower row. The isolates SF66, SF80 and SF82 were represented with D, E and F, respectively.
Figure 2. The supernatant of MRS with 3% MSG cultures was determined for GABA production by TLC. Lane 1; MSG, Lane 2; GABA standard, Lane 3; isolate SF66, Lane 4; isolate SF80, Lane 5; isolates SF82, Lane 6; L. plantarum SF21 which is non-GABA producing strain.
Probiotic characteristics and antibiotic susceptibility
To assess colonizing characteristics, survival in simulated gastrointestinal tract conditions and hydrophobicity were determined. The isolate SF66 had ability to survive in the condition which contained acid and bile salt while SF80 and SF82 did not survive in these conditions (Table). This result indicated that SF66 isolate has potential to use as orally administered probiotics. According to ability of survival rate in gastrointestinal tract condition, only SF66 isolate was selected to investigate in further experiments.
Table:
Probiotic characteristic determination for the selected isolates and safety examination.
| Isolates | Probiotic characteristics | Safety examinations | |||
|---|---|---|---|---|---|
| Gastric fluid survival rate (%) | Bile salt survival rate (%) | Hydrophobicity (%) | Blood hemolysis test | Gelatinase test | |
| SF66 | 36.71 | 91.88 | 85.05 | No hemolysis | Negative |
| SF80 | 0 | 75.06 | 85.09 | No hemolysis | Negative |
| SF82 | 0 | 83.31 | 89.46 | No hemolysis | Negative |
For in vitro hydrophobicity, the percentage of hydrophobicity was 63.29 for the SF66 isolate. According to criteria from Mota et al, the percentage more than 50 was defined as hydrophobic characteristic.17 Hydrophobicity is a force that is related with ability to attach the epithelial cells and microorganisms can use this cellular mechanism to adhere the surface of host cells.20 This suggested that SF66 isolate was able to colonize in the intestine and provide beneficial effect to their host.
According to safety of LAB isolate, blood hemolysis and gelatinase test were determined. The results showed that there was no beta hemolysis from the isolate which inoculated on sheep blood agar and liquified gelatin was also not observed. Therefore, safety of probiotics, the strains should not express virulence activity which including blood hemolysis and gelatinase activity. Thus, the isolate in this study could be assigned as a safe probiotic.
The antibiotic susceptibility profile of the SF66 isolate was determined and the results found that the SF66 isolate was susceptible to chloramphenicol, erythromycin, novobiocin, penicillin and tetracycline.
GABA quantification and identification of GABA producing LAB
The GABA content from LAB SF66 was quantified by HPLC. After determination, the GABA content of LAB SF66 supernatant was 0.9 mg/ml.
The isolate was identified by using API 50 CHL commercial identification kit combined with and the result showed that the SF66 was shown possibility to be Lactiplantibacillus pentosus. The 16s rRNA sequencing was needed for confirmation. However, L. plantarum and L. pentosus showed highly identity from 16s rRNA sequencing then the amplification of recA gene by multiplex PCR was performed and the result found that the product of PCR was approximately 218 bp, which could be interpreted as the specific size for L. pentosus. From the above results, this novel isolate was L. pentosus firmly and could be designated as L. pentosus SF66.
The colonizing properties are the essential characteristics of commensal bacteria. It is crucial for novel LAB isolation for using probiotics as an oral administration.21 This study found that the isolate SF66 could resist harsh condition of gastrointestinal tract, which were gastric fluid and bile salt containing environment, and had high percentage of hydrophobicity that related with ability to attach the surface of epithelial cells. Besides probiotic and colonizing properties, the safety of LAB was commonly concerned for screening the novel strain to use as probiotics. From the results, the isolate SF66 revealed the susceptible to 5 from 10 antibiotics and presented with no hemolysis and gelatinase activity. According the above results, this noticed that novel LAB SF66 which isolated from Pak Sian Dong has ability to produce GABA with safe and good colonizing characteristics.
The probiotic characteristics for the LAB isolates from Pak Sian Dong were presented in numerous reports. As for example, the study of Yasiri and Seubsasana22 revealed that bile salt hydrolase and uricase activity of L. brevis can be applied for hypercholesterolemia and hyperuricemia. In addition to antimicrobial activity, LAB which is/are isolated from Pak Sian Dong showed ability to inhibit gram-positive and gram-negative bacteria. Moreover, L. pentosus which is isolated from Pak Sian Dong was reported for GABA producing activity and could be developed as a novel probiotic drink.23
From GABA quantification by HPLC, the LAB SF66 could produce 0.9 mg/ml of GABA which was determined from 48 h supernatant of the MRS containing 3% MSG culture. The previous study reported the concentration of GABA which was produced by LAB with various amount.24,15,3 The concentration of GABA related with several variable parameters such as starter inoculum, MSG concentration and glucose supplementation.25 According the amount of GABA from this study, this suggested that the optimization for high yield production was needed to be investigated in the future.
From bacterial identification, the sequencing of 16s rRNA showed the high identity of the isolate with L. plantarum and L. pentosus. Unambiguous identification of these species has been reported and solved by using recA gene amplification.18,26 Regarding to the result of identification kit and recA gene amplification, the novel isolate was finally characterized and identified as L. pentosus. This result suggested that the identification of LAB isolates probably need more than one methods to characterize the species for achieving the rigid identification results.
The isolation of L. pentosus from Pak Sian Dong has been reported previously.3 In general, L. pentosus is a gram positive, rod shape which can be detected in various environments including plants, animals and fermented foods. Several studies reveald that L. pentosus can be used as probiotics in various aspects such as antimicrobial activity,27 cholesterol reduction28 and immunomodulation.29 Using as probiotics with antimicrobial activity, L. pentosus SLC13 which is/are isolated from mustard pickles exhibit broad spectrum inhibitory activity for gram-positive and gram-negative bacteria.30 For ability to reduce cholesterol, feeding with L. pentosus KF923750 in animal model found significant lowering of cholesterol and triglyceride in blood plasma.28 In addition, L. pentosus S-PT84 which is/are isolated from pickles accomplished in modulation of cytokines production.31
The capability of GABA production could be observed in various several L. pentossus strains which are isolated from various sources, e.g., fermented mulberry fruits,24,15 natural black Conservolea olives32 and Thai pickle weed.23 The advantages of GABA producing bacteria were shown in various aspects. In accordance with ability to convert MSG to GABA, reduction of MSG absorption was determined in mice which was administered with L. brevis G-101 and could be assumed to diminishing of MSG side effects.33 For immunomodulation activity, the GABA producing L. brevis showed ability to regulate cytokine production in mesenteric lymph node cells and also cause autophagy in some populations of these cells.34 Moreover, improvement of glucose homeostasis and lipid metabolism were observed in the mice which provided with GABA producing L. brevis strains.35 From several reports above, the lactobacilli with ability to produce GABA have the potential for using in health improvement and promotion aspects.
In conclusion, the GABA producing LAB strains could be isolated from Pak Sian Dong, and this indicates that Pak Sian Dong is a healthy diet with a possibility to be a source of beneficial LAB. The potential isolate in this work was identified and designated as L. pentosus SF66. According to ability to survive in simultaneous gastrointestinal tract condition and present with high hydrophobicity, this isolate can be applied to use as a probiotic for oral administration as well. The GABA producing isolate from this study can potentially be used in the functional food aspect. However, more studies are further needed to investigate for GABA highest yield condition and approval in animal models.
ACKNOWLEDGMENTS
The authors would like to thank Staffs of Chulabhorn International College of Medicine Laboratory for technical assistance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This work was supported by Research Fund of Chulabhorn International College of Medicine Contract No: G 7/2561, Thammasat University, Pathum Thani, Thailand.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Wu TH, Solberg SO, Yndgaard F, Chou YY. Morphological patterns in a world collection of Cleome gynandra. Genet Resour Crop Evol. 2018;65(1):271-283.
Crossref - Cleome gynandra L. (Capparaceae). https://www.jircas.affrc.go.jp/project/value_addition/Vegetables/030.html. Accessed 8 August, 2022.
- Pumriw S, Luang-In V, Samappito W. Screening of probiotic lactic acid bacteria isolated from fermented Pak-Sian for use as a starter culture. Curr Microbiol. 2021;78(7):2695-2707.
Crossref - Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66(5):365-378.
Crossref - Sahab NRM, Subroto E, Balia RL, Utama GL. g-aminobutyric acid found in fermented foods and beverages: current trends. Heliyon. 2020;6(11):e05526.
Crossref - Cho YR, Chang JY, Chang HC. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol. 2007;17(1):104-109.
- Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.
Crossref - Buddhala C, Hsu CC, Wu JY. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem Int. 2009;55(1-3):9-12.
Crossref - Krantis A. GABA in the mammalian enteric nervous system. News Physiol Sci. 2000;15(6):284-290.
Crossref - Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203-209.
- Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, Nieuwenhuis S. Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front Psychol. 2015;6:1520.
Crossref - Kochalska K, Oakden W, Slowik T, et al. Dietary supplementation with Lactobacillus rhamnosus JB-1 restores brain neurochemical balance and mitigates the progression of mood disorder in a rat model of chronic unpredictable mild stress. Nutr Res. 2020;82:44-57.
Crossref - Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050-16055.
Crossref - Pennacchietti E, D’Alonzo C, Freddi L, Occhialini A, De Biase D. The glutaminase-dependent acid resistance system: qualitative and quantitative assays and analysis of its distribution in enteric bacteria. Front Microbiol. 2018;9:2869.
Crossref - Kanklai J, Somwong TC, Rungsirivanich P, Thongwai N. Screening of GABA-producing lactic acid bacteria from Thai fermented foods and probiotic potential of Levilactobacillus brevis F064A for GABA-fermented mulberry juice production. Microorganisms. 2020;9(1):33.
Crossref - Li Y, Chen X, Shu G, Ma W. Screening of gamma-aminobutyric acid-producing lactic acid bacteria and its application in Monascus-fermented rice production. Acta Sci Pol Technol Aliment. 2020;19(4):387-394.
Crossref - Mota RM, Moreira JL, Souza MR, et al. Genetic transformation of novel isolates of chicken Lactobacillus bearing probiotic features for expression of heterologous proteins: a tool to develop live oral vaccines. BMC Biotechnol. 2006;6:2.
Crossref - Torriani S, Felis GE, Dellaglio F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl Environ Microbiol. 2001;67(8):3450-3454.
Crossref - Yasiri A, Vanaxay E, Kiatmontri J, Seubsasana S. Isolation and determination of bile salt hydrolase-producing lactic acid bacteria from fermented spider plant. J Pure Appl Microbiol. 2018;12(3):1055-1060.
Crossref - Farrag HA, A-Karm El-Din A, Mohamed El-Sayed ZG, Abdel-Latifissa S, Kamal MM. Microbial colonization of irradiated pathogenic yeast to catheter surfaces: Relationship between adherence, cell surface hydrophobicity, biofilm formation and antifungal susceptibility. A scanning electron microscope analysis. Int J Radiat Biol. 2015;91(6):519-527.
Crossref - Santini C, Baffoni L, Gaggia F, et al. Characterization of probiotic strains: an application as feed additives in poultry against Campylobacter jejuni. Int J Food Microbiol. 2010;141(Suppl 1):S98-S108.
Crossref - Yasiri A, Seubsasana S. Isolation of bile salt hydrolase and uricase producing Lactobacillus brevis SF121 from Pak Sian Dong (fermented spider plant) for using as probiotics. J Pure Appl Microbiol. 2020;14(3):1715-1722.
Crossref - Kittibunchakul S. Yuthaworawit N, Whanmek K, Suttisansanee U, Santivarangkna C. Health beneficial properties of a novel plant-based probiotic drink produced by fermentation of brown rice milk with GABA-producing Lactobacillus pentosus isolated from Thai pickled weed. J Funct Foods. 2021;86:104710.
Crossref - Zhong Y, Wu S, Chen F, He M, Lin J. Isolation of high gamma-aminobutyric acid-producing lactic acid bacteria and fermentation in mulberry leaf powders. Exp Ther Med. 2019;18(1):147-153.
Crossref - Li H, Qiu T, Gao D, Cao Y. Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids. 2010;38(5):1439-1445.
Crossref - Spano G, Beneduce L, Tarantino D, Zapparoli G, Massa S. Characterization of Lactobacillus plantarum from wine must by PCR species-specific and RAPD-PCR. Lett Appl Microbiol. 2002;35(5):370-374.
Crossref - Dai M, Li Y, Xu L, et al. A novel bacteriocin from Lactobacillus pentosus ZFM94 and its antibacterial mode of action. Front Nutr. 2021;8:710862.
Crossref - Bendali F, Kerdouche K, Hamma-Faradji S, Drider D. In vitro and in vivo cholesterol lowering ability of Lactobacillus pentosus KF923750. Benef Microbes. 2017;8(2):271-280.
Crossref - Birhanu BT, Lee JS, Lee SJ, et al. Immunomodulation of Lactobacillus pentosus PL11 against Edwardsiella tarda infection in the head kidney cells of the Japanese eel (Anguilla japonica). Fish Shellfish Immunol. 2016;54:466-472.
Crossref - Huang JY, Kao CY, Liu WS, Fang TJ. Characterization of high exopolysaccharide-producing Lactobacillus strains isolated from mustard pickles for potential probiotic applications. Int Microbiol. 2017;20(2):75-84.
Crossref - Izumo T, Izumi F, Nakagawa I, Kitagawa Y, Shibata H, Kiso Y. Influence of Lactobacillus pentosus S-PT84 ingestion on the mucosal immunity of healthy and Salmonella Typhimurium-infected mice. Biosci Microflora. 2011;30(2):27-35.
Crossref - Pavli F, Gkana E, Adebambo O, Karatzas KA, Panagou E, Nychas GE. In vitro screening of gamma-aminobutyric acid and autoinducer-2 signalling in lactic acid bacteria exhibiting probiotic potential isolated from natural black conservolea olives. Foods. 2019;8(12):640.
Crossref - Jang SE, Han MJ, Kim SY, Kim DH. Lactobacillus brevis G101 inhibits the absorption of monosodium glutamate in mice. J Microbiol Biotechnol. 2014;24(11):1592-1596.
Crossref - Bajic SS, Dokic J, Dinic M, et al. GABA potentiate the immunoregulatory effects of Lactobacillus brevis BGZLS10-17 via ATG5-dependent autophagy in vitro. Sci Rep. 2020;10(1):1347.
Crossref - Patterson E, Ryan PM, Wiley N, et al. Gamma-aminobutyric acid-producing Lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci Rep. 2019;9(1):16323.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.