ISSN: 0973-7510
E-ISSN: 2581-690X
Exopolysaccharides (EPSs) are novel functional additives for low-fat yogurt. Pharmaceutical, medical, and food industries are using more LAB-based EPSs. In this study, Leuconostoc spp. was used to produce ninth bacterial EPSs in a modified molasses medium. Production of EPSs was concentration-dependent on all stains and the highest yield was obtained from the S3 strain (55.23 g/l), followed by S6 (49.95 g/l), S8 (45.68 g/l), and S7 (44.23), respectively. HPLC and FTIR analysis showed that all purified EPSs from Leuconostoc citreum (S3) and Leuconstoc holzaapfelii (S8) were related to exopolysaccharide glucan. Anticancer activity of all EPSs samples (EPSs1-9) against Caco-2 cells and normal MCR-5 cells were investigated using MTT assay. The results revealed that Caco-2 cells were more sensitive than the normal MCR-5 cells. The highest anticancer activity against Caco-2 cancer cells was recorded for EPS8 (IC50 = 22.94 µg/ml, SI=3.73), followed by EPS3 (IC50 = 36.15 µg/ml, SI=8.72), EPS1 (IC50 = 50.01 µg/ml, SI=3.73), and EPS4 (IC50 = 94.90 µg/ml, SI=3.26), respectively. The lowest cytotoxicity was recorded for EPS5 (IC50 = 130.5 µg/ml). The most active EPSs (EPS3 and EPS8) were used as fat replacements and stabilizers in low-fat set yogurt at non-toxic concentrations (0.4, 0.8, and 1.2%). EPS3 and EPS8 improved the low-fat yogurt’s organoleptic and rheological properties. EPS8 had the highest water holding capacity (77.26%), viscosity (3660 CP), and lowest syneresis (22.95%) and whey off (0.6 ml). Low-fat set yogurt enhanced with EPS3 and EPS8 recorded the highest sensory evaluation values with overall acceptability, especially EPS3b, EPS3c, EPS8c, and EPS8b; the total score point of 97.50, 97.43, 96.51, and 96.36, respectively in fresh age compared to control yogurt (92.64). In conclusion, Leuconostoc EPSs, especially EPS8, can be explored for anti-cancer effects on Caco-2 colorectal cancer cells. It could also improve the rheological and organoleptic qualities of low-fat set yogurt.
Anticancer, Caco-2 cells, Cytotoxicity, Molasses, Exopolysaccharide, Glucan, Leuconstoc Strains, Low-fat Set Yogurt, Organoleptic and Rheological Properties
Yogurt is fermented milk, and it is consumed worldwide due to its therapeutic and nutritive values and consider a key component of daily diet and ranks second among Children’s favorite snacks globally.1 Yogurt’s physical properties, including thickness and smoothness,2 and viscosity,3 are crucial for consumers’ acceptability. Yogurt also promoting health benefits4; as it contains live lactic acid bacteria, which cure some intestinal disorders, decrease the risk of cancer, lowering blood cholesterol, and improving digestion of lactose.5 Several researchers reported the association of LAB-produced EPS with anti-biofilm, immunomodulatory, antiulcer, cholesterol-binding, antioxidant, and anticancer effects.6,7
It is a rich source of proteins, carbohydrates, vitamins, fats, phosphorous, and calcium. Lactic acid fermentation of yogurt makes it easily digestible and increases the bioavailability of calcium in the intestine.8 Consumers prefer dairy items (non-fat or low-fat yogurt) as it is best for health; due to reduced heart diseases and obesity risk.9 Using the suitable type and adding the right amount of the stabilizer to fermented milk will improve the viscosity, and prevent whey separation and addition of skim milk powder will not be required. Since a low concentration of stabilizer may not satisfy the functional requirements, also too high concentration may result in unacceptable appearance, mouthfeel, surface sheen, and texture. So, to produce good quality yogurt; the concentration of stabilizer addition should be controlled.10 Fermented Yoghurt preparation through EPS-producing bacterial cultures exhibits varying gel firmness, ropiness, syneresis, mouth thickness, and rheological characteristics.11 Addition of EPSs or stabilizers enhancing the texture and sensory features of yogurt and to achieve a viscous final product. A few EPSs could affect the texture of yogurt, whereas other EPS could replace the fat and thicken the texture.12 Polysaccharides protect bacteria in harmful environments and facilitate microbial cell attachment to a solid surface to form an extracellular biofilm matrix.7,13 Food scientists are giving special attention to LAB-produced EPSs because of their GRAS (Generally Recognized as Safe) status and physicochemical, prebiotic, and health-promoting properties.14,15 The exopolysaccharides obtained from bacteria was used since a long time in therapeutic and other industrial applications with no adverse effects.7 The complex chemical structures of bacterial EPSs significantly affect their functional properties and health-promoting potential, including anticarcinogenic, cholesterol-lowering, immunomodulatory, glycemic control, anti-inflammatory, anti-biofilm, prebiotic, calcium and magnesium absorption, modulating the gut microbiome and antioxidant activities.7,16,17 EPSs have a bioactive function that enables drug delivery systems and as antitumor agents. EPSs production for drug delivery is comparatively simple than viable cells containing biological scaffold production. EPS drug carriers could be tailored for controlling drug release, enhancing the drug’s shelf life inside the body, and improving drug efficiency.17 EPSs from LAB are varied from species to species; they can be divided into two classes, homopolysaccharides such as glucans, fructans, or galactans, which either contain a single type of monosaccharide or multiple types of monosaccharides known as heteropolysaccharides.18 EPSs are important for the preparation of several dairy items, including cheese, drinking yogurt, yogurt, desserts, and cultured cream. LAB-produced EPSs have been used in situ for reducing yogurt syneresis and they could also potentially be used in other food preparations.14,17 However, low LAB-based EPSs production restricts their applications as a functional ingredient. Consumers’ acceptance is affected negatively by textural defects such as the low gel strength and syneresis of yogurt. Leakage of whey from yogurt is a common defect and must be controlled, whey network expulsion leading to visible surface whey which known as Whey-off, and this is negatively affecting consumer perception of yogurt. Hence, stabilizers (starch, gelatin, and pectin) are used during yogurt manufacturing for preventing whey-off, which may be indicative of faulty fermentation and off-flavors.19 EPSs remain an interesting tool for modulating the sensory properties of yogurt. LAB-produced EPSs as natural thickeners have received considerable importance in food manufacturing industries because of their better water-binding capacity, gelling, emulsification, texturization, sweetening, and bioactive properties.20
Cancer is a term refers to the uncontrolled proliferation of abnormal cells that have the ability to invade and damage surrounding tissue. The World Health Organization (WHO) lists cancer as a top 10 global killer. An increase in cancer incidence has been linked to a lack of education about the disease and access to effective chemotherapeutic medicines.21 Traditional cancer treatments include surgical removal, radiation, and drug therapy. Current cancer treatments include surgery, radiation, hormone therapy, anti-hormone therapy, and chemotherapy.22 Drug therapy can harm healthy organs and tissues, such as the bone marrow, kidneys, and oral mucosa, as well as disrupt a person’s regular metabolic process. Cytotoxic agents such as doxorubicin, taxol, 5-fluorouracil, and cyclophosphamide are commonly used in the treatment of different types of cancer.23 Thus, there is a growing demand for targeted medicines that can effectively treat cancer while having minimal side effects on healthy tissues or that can serve as adjuvants to lower clinical doses and boost the efficacy of standard chemotherapy drugs.24 Recent research has shown that LAB EPS could be used as potential antioxidants and anticancer. Polysaccharides from various sources are being explored for anticancer efficacy and acting as immunomodulators, increasing the body’s defense against cancer cells and reducing the immunosuppression caused by chemotherapy.7,25,26 In vitro and in vivo animal investigations have demonstrated that more than 100 polysaccharides are anticancer across a spectrum of cancer cell types. These drugs overcome several of chemotherapy’s disadvantages.27
This work aims to (i) evaluate the EPSs production from newly isolated Leuconostoc strains using sugar cane molasses as a cheaper fermentation medium, (ii) assess the cytotoxicity and anticancer activity of EPSs against human colorectal cancer (Caco-2) and normal fetal lung fibroblasts (MRC-5), (iii) using the EPS produced from some Leuconostoc strains as a fat replacer for making low-fat set yogurt and studying its effect on the rheological and organoleptic properties of the resulted yogurt.
Bacterial strain used for EPSs Production
Based on the previously published work,15 the most promising nine isolates submitted to the Gene bank database (Accession numbers: MN251629, MN251661, MN251606, MN396397, MN251658, MN251740, MN251663, MN251741, and MN251672) were assessed for production of EPS using Sugar cane molasses media.
Chemicals and Sugar Cane Molasses
Analytical grade chemicals were purchased from (Sigma and Merck). Sugar cane molasses was kindly provided from the Abo-Qurqas sugar factory, Al-Minia, Egypt.
Molasses Medium, Extraction and Purification of EPSs
The sugar cane molasses was clarified and diluted with distilled water containing NaH2PO4 (1.5 gl-1) as described by the methods of Mattos et al.28 for obtaining a serial dilution of sugarcane molasses media (W/V) with the following concentrations:130, 160, 200, 262 and 290 (g/l). The diluted molasses was autoclaved for 30 min at 120°C and kept for 24 h, followed by their separation from the solid material. Then, the addition of yeast extract (6g/L-1) was carried out at pH 7.0. The medium was inoculated with freshly prepared cultures (5%) of each strain and incubated under shaking at 37°C for 48h. The cell growth was measured at 600 nm using a Jenway, 6850 UV/Vis (USA) spectrophotometer.29 After incubation, molasses culture broth viscosity was reduced by adding deionized water. Then, centrifugation was carried out for 30 min at 4000g, and supernatant (cell-free) was slowly added to isopropanol (two volumes) along the conical flask wall and kept at room temperature for 2 hours for EPS precipitation. The cold ethanol was used to wash EPS (thrice) and dried at 60°C for two days and weighed.14,30
Chemical Analysis of EPSs
The two EPSs (EPS3 and EPS8) which produced from Leu. Mesenteroides S3 and Leu. Holzaapfelii S8 exhibited the highest anticancer activity against Human colorectal cancer (Caco-2), and normal fetal lung fibroblasts (MRC-5) cell line, were partially purified, dried, and kept for chemical characterization. A Gallen Kamp melting point apparatus determined the melting point. To determine their content of monosaccharides, the sample was hydrolyzed using 6N HCl, followed by dilution with water and titrated against Benedict’s solution.
Fourier Transform Infrared Spectrometer (FTIR) Analysis
To detect the function groups of EPSs, Fourier Transform Infrared Spectrometer (FTIR) was conducted. KBr (potassium bromide) pellets were used to ground partially purified and dried samples. A 50 mg disk of dried specimens (2%) and KBr was prepared to analyze EPSs spectra at a 400–4000 cm-1 wavelength31 using FT- IR Spectrometer (8201 PC Shimadzu) at Chemistry Department, Faculty of Science, Assiut University, Egypt.
LC-MS analysis
To determine the mass spectrum of the EPSs through UPLC (ultra-performance liquid chromatography), methanol was used to dissolve partially purified EPSs and assessed by following the procedure of Supaka et al.32 UPLC system and Quattro Premier triple quadrupole mass spectrometer (Micromass, Milford, MA, USA) were coupled through an electrospray ionization source (Z-spray) operating in negative ionization mode.
Preparation of Cancer Cell Lines and Anticancer Activity Assay of EPSs
Anticancer activity of EPSs samples produced from Leuconostoc strains was assessed against Human colorectal cancer (Caco-2) and normal fetal lung fibroblasts (MRC-5) using MTT assay as described by Mosmann.33 Both cell lines (Caco-2 and MRC-5) were employed for the cytotoxicity evaluation of exopolysaccharides (EPS1-9). Cell lines were cultured in DMEM (Dulbecco’s modified Eagle’s medium). Media was supplemented with penicillin/streptomycin (1%), fetal calf serum (10%), and L-glutamine. Experiments were conducted under optimum conditions (37°C, CO2 (5%), and humidity (95%)). Cells were detached using Trypsin/EDTA solution at the logarithmic growth phase. To determine the anticancer activity of EPSs samples, cell lines (2 × 104 cells/well) were cultured in 96-well plates and incubated for 24 h. Stock solutions (10 mg/ml) of EPSs produced from the selected strains (Table 1) were prepared in sterile distilled water and overnight sonicated. Serial fold dilutions of EPSs were prepared in the media and incubated for 24 h with cells. Doxorubicin (a chemotherapeutic agent) was used as a positive control. Then, the addition of MTT solution (0.5 mg/ml) was carried, out followed by incubation for 4 h. Formazan crystals were dissolved in DMSO (100 µl,) and a microplate reader was used to measure the light absorbance at 570 nm. Treatments were carried out in triplicate and 3 experimental repeats were performed.
The following equation determined cell viability:
Viability % = (OD sample treated cells – OD sample medium control)/(OD untreated cells – OD medium control) %
Selectivity Index (SI)
In the present study, the degree of selectivity of the isolated polysaccharides (EPS1-9) is expressed as reported previously with a minor modification.34
SI = (IC50 of PS in normal cell line)/(IC50 of PS in cancer cell line)
Preparation of EPSs for making Low-fat Set Yogurt
EPSs (EPS3 and EPS8) that exhibited the highest anticancer activity were prepared at different concentrations of 0.4, 0.8 and 1.2% for making the low-fat set yogurt treatments. Each of the EPS was prepared individually in beakers containing 50 ml of distilled water and then warmed to 45°C until completely dissolved full absorption occurred.
Preparation of Low-fat Set Yogurt Enhanced with EPSs Mediated Anticancer Activity
Yogurt was manufactured according to Tamime,35 the buffalo milk was skimmed to nearly 0.65% fat for making Low-fat set yogurt, then heated at 90°C for 10 min., to destroy unwanted microorganisms and to remove the dissolved oxygen because starter cultures are sensitive to oxygen so assisting starter growth, then cold to 45°C. After that, the EPS3 and EPS8 were added individually at a level of 0.4, 0.8 and 1.2% for making 6 treatments; coded as EPS3a, EPS3b, EPS3c, EPS8a, EPS8b, and EPS8c, respectively and compared with control (free of EPS). The different prepared treatments were well mixed with the remaining amounts of the milk and cooled down to 40°C, then all treatments and control were inoculated with a fresh yogurt starter culture (2% w/w). The treatments of inoculated milk were added into sterile plastic cups (150 ml) after stirrings and incubated at 40°C until curdling occurred. Subsequently, all treatments were cooled and stored at 5°C in cooling incubator.36 Physiochemical properties and sensory evaluation were assessed during storage periods for 15 days.
Physiochemical Assays of the Low-fat Set Yogurt Treatments
To evaluate the quality of the prepared low-fat set yogurt enhanced with the EPSs, physiochemical properties including water-holding capacity (WHC), syneresis, wheying-off, viscosity, pH and acidity were determined.
According to Guzman-Gonzalez et al.,37 the water-holding capacity of low-fat set yoghurt samples of different treatments was estimated yogurt. The centrifugation yogurt samples (25g (Y)) was carried out at 4500 rpm (room temperature) for 30 min. Whey expelled (WE) was collected and weighed; WHC was measured as shown in the following formula:
WHC (%) = [1-WE/Y × 100]
Syneresis of low-fat set yogurt samples was estimated using the drainage method described by Isanga and Zhang.38 Separation of whey from all yogurt samples was measured and performed at 20°C. The samples were transferred into a filter paper (Whatman No. 4) placed on top of a funnel fixed on a graduated cylinder. Whey volume (ml/100g) was determined after 2h and taken as an index of syneresis.
Whey expulsion from the network leading to a visible surface whey, known as wheying-off negatively affects the perception of the consumer. Different stabilizers (starch, gelatin, and pectin) are used in large-scale yogurt preparations to prevent wheying-off. This parameter was determined by pulling and measuring the amount of expelled whey by a syringe after manufacturing for 24 h.19
A Brookfield viscometer (Brookfield viscometer DVII, USA) containing spindle no.4 and a rotation speed of 3 rpm at 25°C was used to determine the homogenized sample viscosity. The results (centipoise (CP) were recorded after 50 s of shearing.39
The yogurt sample’s pH values for the different treatments were observed through a digital pH meter (ORION, model 420, Germany). Samples were mixed with distilled water (10 ml) to assess the acidity through titration with 0.11 N NaOH and 0.5 % phenolphthalein, which presented a faint pink color at the endpoint.36 Fat content in yogurt samples was determined by the Gerber method, and the total solids were analyzed as described in AOAC.40
Sensory Evaluation of the Low-fat Set Yogurt Treatments
Consumer acceptability of the low-fat set yogurt treatments was evaluated during storage periods of 1, 5, 10 and 15 days at 4°C by staff members of the Dairy Science Department, Faculty of Agriculture, Fayoum University, Fayoum, Egypt. Samples of low-fat set yogurt treatments were presented at room temperature in coded cups using score card of 45, 35, 10 and 10 degrees for appearance, texture, body, flavor, and the acid taste, respectively.41
Statistical Analysis
SPSS general linear model was used for data analyses,42 and expressed as mean values + standard error, whereas means were compared through Duncan’s multiple range test at P ≤ 0.05.43
Exopolysaccharide Production using Sugar Cane Molasses Medium
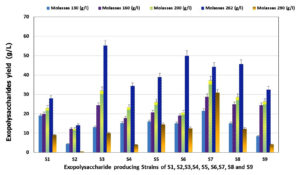
EPSs obtained from bacteria possess complex and diverse chemical structures, which significantly affect their functional properties and health-promoting potential, including anticarcinogenic, cholesterol-lowering, immunomodulatory, glycemic control, anti-inflammatory, anti-biofilm, prebiotic, calcium, and magnesium absorption, gut microbiome modulation, and antioxidant activities. EPSs are novel additives for dairy items, particularly low-fat yogurt. Our previous study selected and characterized the promising strains among various bacterial colonies isolated from dairy items, vegetables, and fermented fruits (genotypically and phenotypically).15 Isolates S1, S2 and S3, were identified as Leu. citreum, Leu. mesenteroides, and Leu. Fallax, respectively. Isolate S6 was identified as Leu. Lactis. While isolates S4, S5, S7, S8, and S9 were identified as Leu. Holzaapfelii, respectively. All strains were non-spore-forming, non-motile, Gram-positive, oxidase-negative, and catalase-positive bacteria. All selected isolates could be grown at different pH from 4 to 8, temperatures from 20 to 37°C, and tolerant to NaCl at concentrations from 0 to 6.5%. Sucrose is the most effective among the carbon sources tested.15 However, the increase in demand for polysaccharides due to their uses in different food, medicinal, cosmetic, agricultural, pharmaceutical, and chemical industries lead to searching for highly EPS-producing bacterial strains and optimization conditions. The high cost of EPS production is the major restriction in industrial production and the cost of sugar as a carbon source is considered one of the most important factors in determining the commercial success of EPS production.44 Therefore, an inexpensive substrate must be found to reduce the cost of the raw materials.45 Hence, the most economical by-products are produced from natural sources and industrial wastes. So, in this study, sugar cane molasses (which has a high concentration of sucrose) have been used not only as substrates instead of commercial sucrose as used by Razack et al.,46 but also as fermentation media for the production of EPS, to make the most economically effective as investigated by Sutherland et al.45 In this study, EPSs were produced from the selected isolates using different concentrations (13, 16, 20, 26.2, and 29%) of sugar cane molasses as a fermentation media (Figure 1). The results indicated that a gradual increase in EPS production was observed as molasses concentration increased in the medium from 13 to 20 % and reached the maximum with 26.2 % molasses. Then EPS yield was drastically decreased at the highest molasses concentration (29 %) for all tested bacterial strains. There were significant differences between the tested strains S1-S9 for EPSs production. The highest yield of EPSs was 55.23, 49.95, 45.68 and 44.23 g/l recorded for S3, S6, S8 and S7, respectively. In contrast, the lowest yield was 38.95, 34.27, 32.44, 27.99 and 27.99 g/l for S5, S4, S9, S1 and S2, respectively, when the molasses fermentation media was supplemented with 26.2% molasses. These results indicated that EPSs amounts inducted by the selected strains in the current study using the modified sugar cane molasses medium were higher than that recorded by Razack et al.,46 Moghannem et al.,47 and who reported the highest EPS yields obtained from Leuconostoc species in sugar cane molasses medium as 2.843, 5.5 and 7.6 gl-1, respectively.
Figure 1. EPS production (g/l) by the selected Leuconostoc strains (S1 to S9) using different concentration of sugar cane molasses as fermentation medium
Physiochemical Properties of EPSs Produced by the Selected Strains
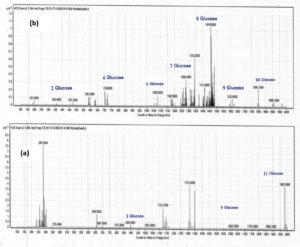
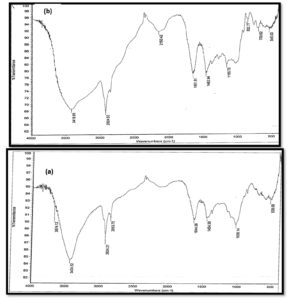
The most promising EPSs samples (EPS3 and EPS 8) that exhibited the highest anticancer activity and were used for making different low-fat set yogurt treatments were analyzed by FTIR and LC-mass. EPSs samples were insoluble in cold water but soluble in hot water and revealed a melting point of 300°C. The monosaccharide content of the purified EPSs was equal to 50% relative to the weight of starting sample, as detected by the Benedict reagent after hydrolysis. FTIR spectrum of the purified EPSs extracted from the selected strains was measured in the wavenumber range 4000–500 cm-1, and the FT-IR spectra concerning specific molecular groups were identified based on the published FT-IR spectra and reference standards.48 The purified EPSs extracted from EP S3, and EP S8 strains revealed prominent peaks at 3418, 2924, and 2150. 1651, 1452 and 1165 cm-1 as indicated in Figure (2 a, b). Absorption broadband ranging from 3600 – 3418 cm-1 indicated the stretching vibration of the O-H group, which may also help explain why EPSs soluble in ethanol and methanol. Also, the bands at 2924 cm-1 in both EPS3 and EPS8 were due to aliphatic CH group asymmetrical stretching vibration. Prominent bands at 1652 – 1632 cm-1 indicate the presence of CO group stretching vibration. However, IR data could not predict complete compound structure. Vibrational peaks mostly occur because of deformation or oxidation in the polyene chain.49 Furthermore, the high-performance liquid chromatography-mass spectra of the purified EPSs produced by Leuconstoc isolates were structurally related to glucan exopolysaccharide. The mass spectra of EPSs fragmentation (Figure 3 a, b) depicted a signal at an m/z of 1980, which corresponded to a fragment of 11 glucose units, and a signal at an m/z of 1830 corresponded to 10 glucose units. In addition, signals at m/z of 1620, 1440, 1260, 1080, 720, and 360 correspond to nine, eight, seven, six, four, and two glucose units, respectively Gangoiti et al.,50 reported that LAB species belonging to genera Weissella, Streptococcus, Lactobacillus, and Leuconostoc produce glucans (α-glucans or β-glucans) containing glucose residues, which form the backbone structure consisting of binding and branching sites. The obtained results in this study indicated that sugar cane molasses could be successfully utilized as an economical fermentation medium for large-scale industrial production of EPSs by Leuconostoc strains.
Figure 2. FTIR spectra analysis of Exopolysaccharides EPS3 (a), EPS8 (b) produced from Leuconostoc strains S3 and S8, respectively
Figure 3. LC-mass of Exopolysaccharides EPS3 (a), EPS8 (b) produced from Leuconostoc strains S3 and S8, respectively
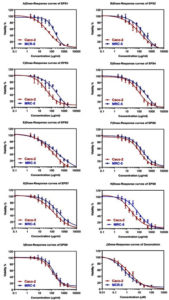
Cytotoxicity and Anticancer Activity of Microbial Exopolysaccharide
Cancer is an abnormal growth of the tissues when the body cannot control the cells because of irreversible DNA damage in response to tumor suppressor gene mutations, leading to a significant increase in the dividing cell population. The development of novel antitumor natural compounds with comparatively lower side effects and non-selective toxicity has become an important target in beneficial health effects and many immunotherapy studies.51 In this study, the cytotoxic activity of EPS and standard cytotoxic drug; doxorubicin, against colorectal cancer and normal fibroblast cell lines are represented in Figure 4. Different concentrations of EPS-1-9 were used to assess the inhibitory effects of these compounds on tested cells growth (Figure 4). It was revealed that the growth inhibition rate of tested cells increased significantly with increasing EPS concentrations, with a clear upward trend observed. The cytotoxic activity was categorized as highly active (IC50 ≤ 20 μg/ml), moderately active (IC50 21-200 μg/ml), weakly active (IC50 201-500 μg/ml), and inactive (IC50 > 501 μg/ml).52 The tested EPS showed variable moderate cytotoxicity on Caco-2 cells (Table 1), which were relatively more sensitive than normal MCR-5 cells (Figure 4, Table 1). The maximum cytotoxic effect of EPS on Caco-2 cells was observed in EPS8 (IC50 = 22.94 µg/ml) followed by EPS3 (IC50 = 36.15 µg/ml), EPS1 (IC50 = 50.01 µg/ml), and EPS4 (IC50 = 94.90 µg/ml). The lowest cytotoxicity was observed in EPS5 (IC50 = 130.5 µg/ml). The selective index (SI) values differentiated between the activity of EPS (Table 1). An SI value has been reported as less than 2 indicates general toxicity of the tested compound (14). Accordingly, the SI data in Table (1) indicate that EPS3, EPS8, and EPS1 exhibit a high degree of cytotoxic selectivity with SI values of 8.72, 3.73, and 3.61, respectively. However, unclear why EPS9 and EPS7 exhibited a low degree of selectivity in cytotoxicity with SI < 2 (Table 1), suggesting their general toxicity to cells, as per the earlier standard.53 The differences could be due to the application of different cell lines in our study. EPS targeting specificity and cellular sensitivity could also vary among cells. The difference in EPS chemical components with different targets could further elaborate on the dissimilar IC50 values of the tested samples. The difference in the collection, production, and environmental parameters and isolation sources might justify the variations in biological and chemical activities of the investigated EPS. In some studies, EPS biological activity corresponds to functional groups (amino, hydroxyl, carbonyl, and carboxyl groups).52,54 In the current study, FT-IR analysis of the sugar structure (Figure 2) of EPSs revealed the presence of hydroxyl and carbonyl groups, which may explain their significant anti-cancer properties. These components target different cytotoxic pathways in cancer cells. Anti-proliferative effects of EPSs on different cancer cells (breast, liver, and intestine) have been reported. However, the anticancer mechanisms of EPSs are unclear and complicated. However, the actual mechanism of cytotoxicity of EPSs produced from Leuconostoc strains in this study still needs further investigation and research. LAB-produced EPSs are known to inhibit tumor cell growth directly through enhanced apoptosis, induction of cell cycle arrest, anti-angiogenesis, anti-oxidative, and anti-mutagenic activities, or indirectly through immune stimulation.55,56 In addition, further clinical and in vivo investigations are needed to establish the anticancer potential of EPSs produced from Leuconostoc strains investigated in this study.
Table (1):
Anticancer activity of polysaccharides (EPS1-9) produced from different Leuconostoc strains on Caco-2 and MCR-5 cells compared to doxorubicin (positive control).
Compound |
Caco-2 |
MCR-5 |
SI |
|---|---|---|---|
EPS1 |
50.01 ± 4.86 |
180.7 ± 16.81 |
3.61 |
EPS2 |
112.00 ± 11.21 |
277.60 ± 25.53 |
2.48 |
EPS3 |
36.15 ± 3.29 |
315.3 ± 24.39 |
8.72 |
EPS4 |
94.91 ± 8.73 |
309.51 ± 26.17 |
3.26 |
EPS5 |
130.5 ± 12.89 |
276.50 ± 18.4 |
2.12 |
EPS6 |
129.3 ± 10.18 |
291.40 ± 19.45 |
2.25 |
EPS7 |
113.20 ± 8.79 |
189.20 ± 15.49 |
1.67 |
EPS8 |
22.94 ± 2.24 |
85.48 ± 6.73 |
3.73 |
EPS9 |
125.24 ± 9.83 |
144.50 ± 11.89 |
1.15 |
Doxorubicin |
1.57 ± 0.12 |
0.70 ± 0.05 |
0.45 |
Notes; Data expressed IC50 values (µg/ml) as mean ± SD. Caco-2: Human colorectal cancer, MCR-5: normal fetal lung fibroblasts, SI: Selectivity index.
Figure 4. Cytotoxicity (IC50) and anticancer activity of tested polysaccharides produced from Leuconostoc strains (EPS1-9) and doxorubicin (positive control) on Caco-2 and MCR-5 cells. Notes: the cytotoxic activity was categorized as highly active (IC50 ≤ 20 μg/ml), moderately active (IC50 21-200 μg/ml), weakly active (IC50 201-500 μg/ml), and inactive (IC50 > 501 μg/ml)
Physiochemical Properties of the Low-fat Yogurt Enhanced with EPSs-mediated Anticancer Activity
Effect of EPSs on pH and Acidity of Low-fat Set Yogurt
LAB-produced Exopolysaccharides are gaining popularity in the food industry because of their potential applications as natural functional food compounds and safe additives, leading to reduced external hydrocolloid usage.14,17 These EPS are natural bio-thickeners, which could enhance the rheological characteristics of fermented foods. In this study, three different concentrations of EPS3 and EPS8, which exhibited the highest anticancer activity were chosen for making different low-fat yogurt treatments, and the physiochemical properties and sensory evaluation were assessed. Results in Table 2 showed the pH values of EPSs enriched Low-fat set yogurt samples during storage (4°C) compared to controls. The pH values of EPS enriched low-fat yogurt samples were significantly different (P≤0.05) compared to the control samples. In general, pH values of all EPSs enriched low-fat yogurt samples and control were decreased during the storage periods. Also, it was noticed that as EPSs concentration in low-fat yogurt treatments increased, the pH values decreased compared to control. Table 2 reveals that the lowest pH values were recorded for the EPS8c sample (4.28), followed by EPS8b and EPS3c (4.35), EPS8a (4.37), EPS3a (4.39) at 15 days of storage and EPS8c (4.39) at 10 days of storage, respectively. In contrast, TA% (Table 2) values of the low-fat yogurt control samples were significantly increased compared to the low-fat yogurt treatments supported by EPSs during the storage periods. At the same time, the highest TA% values were recorded for the EPS8c sample (0.90), followed by EPS8b (0.86) and EPS3c (0.84%) at 15 days of storage compared with control and the other EPS low-fat yogurt samples (0.83%). The increase in acidity was mainly due to lactose hydrolysis during the fermentation processes occurred by lactic acid bacteria.57 and this was more in samples of low-fat yogurt enriched with EPS, which may activate lactic acid bacteria. These findings were similar to other previous studies.39,58 However, decreased pH during yogurt fermentation could cause syneresis because of casein micelles destabilizations.59,60 Therefore, low-fat yogurt’s rheological properties could be enhanced by adding EPS. In this context, nineteen EPS-producing strains were isolated from Chinese fermented dairy items and applied for yogurt preparation. A high EPS-producing starter was selected, which produced better texture, lower syneresis, and better sensory evaluation as compared to sample fermentation using a commercial starter culture.61
Table (2):
Changing in pH values and titratable acidity (TA %) of low-fat set yogurt enriched with EPSs mediated anticancer activity during storage periods at 5°C.
| Treatments* | Age (day) | pH | TA (%) | |
|---|---|---|---|---|
| Plain yogurt (free of EPS) as control | 1 | 4.65a..d | 0.72g | |
| 5 | 4.55a..e | 0.775d…g | ||
| 10 | 4.45e…h | 0.81b…f | ||
| 15 | 4.41e…h | 0.83bcd | ||
| EPS3** | EPS3a | 1 | 4.64abcd | 0.76e…g |
| 5 | 4.43e…h | 0.793b…f | ||
| 10 | 4.40e…h | 0.80b…e | ||
| 15 | 4.39e…h | 0.83bcd | ||
| EPS3b | 1 | 4.66abc | 0.773c…g | |
| 5 | 4.50b…g | 0.775c…g | ||
| 10 | 4.48d…g | 0.815b..f | ||
| 15 | 4.43e…h | 0.83bcd | ||
| EPS3c | 1 | 4.67abc | 0.765d…g | |
| 5 | 4.40e…h | 0.77d…g | ||
| 10 | 4.39e…h | 0.815b…f | ||
| 15 | 4.35fgh | 0.84abc | ||
| EPS8*** | EPS8a | 1 | 4.695a | 0.768d…g |
| 5 | 4.53a…f | 0.773c…g | ||
| 10 | 4.44e…h | 0.798b…f | ||
| 15 | 4.37e…h | 0.828b..e | ||
| EPS8b | 1 | 4.68ab | 0.748fg | |
| 5 | 4.49c…g | 0.77d…g | ||
| 10 | 4.45e…h | 0.825b..e | ||
| 15 | 4.35fgh | 0.86ab | ||
| EPS8c | 1 | 4.68ab | 0.72g | |
| 5 | 4.52a…g | 0.763d…g | ||
| 10 | 4.49c…g | 0.793b…f | ||
| 15 | 4.28h | 0.9a | ||
| SE+ | 0.054 | 0.020 | ||
Notes: a, b,…and h: Means having different superscripts within each column are significantly different (P≤0.05). SE: Standard Error. *: Control: fermented low-fat Plain set yogurt (free exopolysaccharide), treatments of EPS3a, EPS3b and EPS3c, EPS8a, EPS8b and EPS8c are the fermented low-fat yogurt enhanced with of 0.4, 0.8 and 1.2% EPSs, respectively. **EPS3: Exopolysaccharide produced from isolate S3. ***EPS8: Exopolysaccharide produced from isolate S8.
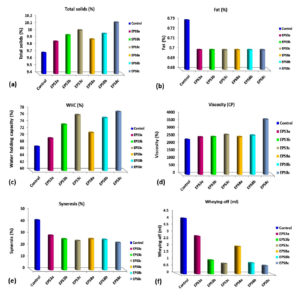
Effect of EPSs on Fat Content and Total Solids of Low-fat Set Yogurt
Results presented in Figure (5a) reveal non–significantly different fat content among low-fat yogurt treatments supplemented with EPS and control. On the other hand, the total solids (TS %) (Figure 5b) of low-fat yogurt supplemented with EPS were significantly increased (P≤0.001) as EPSs concentration increased in comparison to controls. Regarding the total solids content, the highest TS% value (10.13%) was recorded for the low-fat yogurt enriched with 1.2% of EPS3. In comparison, the lowest one was recorded for control yogurt (9.71%), which indicated that EPS increase the TS% and that reported by Omer62 as he mentions that polysaccharide is one of the factors which can improve consistency and hence will increase the total solids. The results of TS (%) were close to that mentioned by Abd El-Galeel et al.63
Effect of EPSs on Water Holding Capacity (WHC%) of Low-fat Set Yogurt
WHC depicts the sample’s gel stability and syneresis.64 Results illustrated in Figure (5c) showed the effect of EPSs on the WHC of low-fat yogurt enriched with different concentrations of EPS of Leuconstoc strains compared to control. It was noticed that as EPS increased, the water holding capacity increased. Whereas the highest value of WHC was recorded for EPS8c at a level of 1.2% (77.2%), followed by EPS3c (76.34%), EPS8b (75.48%) and EPS3b (73.52%), respectively, whereas control treatment demonstrated the lowest value (67.05%). Thus, EPS improves the water holding capacity of the yogurt by interacting with casein micelles and holding water in between.65 Similar researchers reported that EPS from Leuconostoc pseudomesenteroides improved the WHC, yogurt viscosity, and texture profile in cold storage compared to controls.66
Effect of EPSs on the Viscosity of Low-fat Set Yogurt
Results illustrated in Figure (5d) indicated that EPSs significantly increased the viscosity of low-fat yogurt and increased the viscosity, indicating more water binding increasing, the internal structure denser and uniform.65 The highest viscosity value was noticed for low-fat yogurt treatment of EPS8c, followed by EPS3c, EPS8b and EPS3b which were 3660, 2640, 2600 and 2500 CP, respectively, compared to the yogurt control treatment (without EPS) that recorded the lowest viscosity (2313 CP). Similar studies indicated that the viscosity and texture properties of yogurt were improved by adding EPSs to low-fat yogurt, because polysaccharides in the gel-like structure interact with free water.67
Effect of EPSs on the Syneresis and Whey-off of Low-fat Set Yogurt
Syneresis (Whey separation) occurs as a textural defect in yogurt. Due to the shrinkage of gel, water will expel from the network, which leads to syneresis, causing an unstable gel network with the poor capability of entrapping serum phase.68,60 Wheying-off or separation of the whey on the surface affects consumers’ perception because it indicates something wrong microbiologically with the product.69 Results in Figure (5e) summarize the potential effect of ESPs to reduce and decrease the syneresis (%) and Wheying-off (ml) in low-fat yogurt containing EPSs samples compared to the control. In general, it was noticed that additions of EPSs decrease the syneresis and hence improve the stability of yogurt. In addition, in this study, as EPS concentration increased, the syneresis and Wheying-off were decreased,. Ge et al.64 also reported that adding the polysaccharide effectively reduces syneresis. Whereas the lowest syneresis value was recorded for EPS8c (22.95%), followed by EPS3c (24.57%) and the highest amount was recorded for control treatment; 42.03%. Likewise, Wheying-off results take the same trends as syneresis. The results illustrated in Figure (5f) show the whey-off expressed as ml/100g for low-fat yogurt enriched with different EPS produced from Leuconstoc strains. The highest separated amount of whey (4.05 ml) was recorded for the control treatment (low-fat yogurt without EPS). In the contrast, all low-fat yogurt supplemented with different EPSs exhibited less separated amounts of whey. Whereas the lowest whey-off values were recorded for EPS8c (0.6 ml), EPS8b (0.8 ml) and EPS3c (0.75 ml), respectively, while the highest value was recorded for the control treatment (4.005). As reported by Korcz et al.,60 syneresis formation during the fermentation process of yogurt is the main yogurt defect that occurs in response to gel network rearrangements resulting from the destabilization of casein micelles due to decreasing the pH and increasing the acidity. The results obtained in this study indicated that additions of EPSs decrease the syneresis whey-off and hence improve the stability of yogurt. However, rheological issues, including high syneresis, gel fracture, and low viscosity, frequently occur during the preparation of fermented milk, which could be avoided through EPS-producing LAB strains and also by fat replacements.11,70
Figure 5. Some physiochemical properties of low-fat set yogurt enhanced with different levels of Exopolysaccharides (EPS3 and EPS8) produced from Leuconostoc strains S3 and S8. Notes: Control: fermented low-fat Plain set yogurt (free exopolysaccharide), treatments of EPS3a, EPS3b and EPS3c, EPS8a, EPS8b and EPS8c are the fermented low-fat set yogurt enhanced with of 0.4, 0.8 and 1.2% EPSs, respectively. Total solids (a), Fat (b), Water holding capacity (c), Viscosity (d), Syneresis (e) and Wheying off (f)
Organoleptic Characteristics of Low-fat Set Yogurt with EPSs
Recently, Low-fat yogurt has gained interest due to consumer awareness about health concerns. Complete or partial yogurt fat removal negatively affects its quality. Therefore, fat replacer, in particular EPSs as an ingredient to improve the function of the product as well as low-calorie yogurts has recently emerged on the market.11,70 Results in Table (3) revealed that, addition of EPSs of Leuconostoc strains significantly (P≤0.001) improved the mouth feel, gel firmness, syneresis, and rheological parameters of low-fat yogurt in comparison to control. The highest total score points were recorded for low-fat yogurt supplemented with 0.8% EPS (EPS3b) and 1.2% (EPS3c), followed by EPS8b, EPS8c and EPS8a compared to control yogurt, respectively. Similar studies have incorporated different exopolysaccharides (EPSs) and stabilizers for improving sensory features of yogurt (clean cut, mouthful, creaminess, shininess, and ropiness).4,14,61
Table (3):
Organoleptic properties of low-fat set yogurt enriched with EPSs mediated anticancer activity during storage periods at 5°C.
| Treatments | Age (day) | Flavor (45) |
Body&Texture (35) | Acidity (10) | Appearance (10) | Total (100) | |
|---|---|---|---|---|---|---|---|
| Plain yogurt as control (C)* | 1 | 42.50ABC | 32.286A..D | 9.14A..D | 8.71C..G | 92.64A..G | |
| 5 | 42.80AB | 30.20DE | 9.00A..F | 7.50I | 89.50FG | ||
| 10 | 38.20D | 29.60E | 7.70G | 7.40I | 82.90H | ||
| 15 | 38.20D | 29.00E | 7.70G | 7.80HI | 82.70H | ||
| EPS3** | EPS3a | 1 | 43.14AB | 33.86ABC | 9.50AB | 9.36A..D | 95.86ABC |
| 5 | 43.60A | 32.60ABC | 9.80A | 9.30A..F | 95.30A..D | ||
| 10 | 42.40ABC | 32.80ABC | 8.60B..G | 9.00A..G | 92.80A..G | ||
| 15 | 41.80ABC | 31.70CD | 8.10FG | 8.40F..H | 90.00EFG | ||
| EPS3b | 1 | 44.00A | 34.50A | 9.50AB | 9.50A..D | 97.50A | |
| 5 | 42.90AB | 34.20AB | 8.20EFG | 8.90A..G | 94.20A..F | ||
| 10 | 42.80AB | 32.80ABC | 9.00A..F | 8.70C..G | 93.30A..g | ||
| 15 | 42.80AB | 32.40ABC | 9.10A..D | 8.90A..G | 93.20A..g | ||
| EPS3c | 1 | 43.79A | 34.36A | 9.64A | 9.64AB | 97.43A | |
| 5 | 42.60ABC | 33.70ABC | 9.50AB | 9.60ABC | 95.40A..D | ||
| 10 | 42.40ABC | 33.30ABC | 9.30ABC | 8.20GHI | 93.20A..G | ||
| 15 | 41.10BC | 32.40ABC | 8.30EFG | 8.80B..G | 90.60D..G | ||
| EPS8*** | EPS8a | 0 | 43.43AB | 33.79ABC | 9.36A..D | 9.29A..F | 95.86ABC |
| 5 | 43.40AB | 32.00A..D | 9.80A | 8.90A..G | 94.10A..F | ||
| 10 | 41.70ABC | 33.20ABC | 8.50C..G | 8.50E..H | 91.90B..G | ||
| 15 | 40.90C | 31.70CD | 8.65B..G | 7.30I | 88.55F | ||
| EPS8b | 1 | 43.64A | 33.86ABC | 9.50AB | 9.36A..E | 96.36ABC | |
| 5 | 42.90AB | 32.30A..D | 9.40ABC | 9.00A..G | 93.60A..F | ||
| 10 | 41.90ABC | 32.60ABC | 8.60B..G | 8.60D..H | 91.70B..G | ||
| 15 | 41.80AB | 33.00ABC | 8.10FG | 8.50E..H | 91.40C..G | ||
| EPS8c | 1 | 43.29AB | 34.29A | 9.43ABC | 9.50A..D | 96.51AB | |
| 5 | 42.50ABC | 33.30ABC | 9.30A..D | 9.80A | 94.9A..D | ||
| 10 | 42.80AB | 33.10ABC | 8.40D..G | 8.80B..G | 93.10A..F | ||
| 15 | 40.30C | 31.90BCD | 8.10FG | 8.20GHI | 88.5F | ||
| SE+ | 0.729 | 0.70 | 0.301 | 0.282 | 1.534 | ||
Note: A, B,…and I: Means having different superscripts within each column are significantly different (P ≤ 0.001), SE: Standard Error, *: Control: low-fat Plain set yogurt (free exopolysaccharide), treatments of EPS3a, EPS3b and EPS3c, EPS8a, EPS8b and EPS8c are the low-fat set yogurt enhanced with of 0.4, 0.8 and 1.2% EPSs, respectively. **EPS3: Exopolysaccharide produced from isolate S3. ***EPS8: Exopolysaccharide produced from isolate S8.
The major problems and limitations in the large-scale application of exopolysaccharides include their high cost and lower yield by LAB strains. So, EPS production should be optimized through new techniques for industrial usage. In this study, Leuconostoc strains inducted considerable EPSs yield reached up to 55 g/L using molasses media. Furthermore, the EPSs produced from Leuconostoc species particularly EPS8 can be explored as anti-cancer activity against colorectal cancer cells Caco-2. In addition, beside the health benefits of the EPSs, it improves rheological properties, texture, flavor, stability, and shelf life of the fermented low-fat yogurt with desirable characteristics for consumers comparing with control. Whereas, using EPSs in particular EPS8; increased water holding capacity, viscosity and decreases both syneresis and the whey off, indicating its high ability of binding water especially at concentration of 1.2%. However, further in vivo and clinical investigations are needed to establish the anticancer potential of EPSs produced from Leuconostoc strains investigated in this study.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Sharma S, Padhi S, Kumari M, Rai, AK, Sahoo, D. Bioactive Compounds in Fermented Foods. Health Aspects; CRC Press: Boca Raton, FL, USA. 2021:48.
Crossref - Jaworska D, Waszkiewicz-Robak B, Kolanowski W, Swiderski F. Relative importance of texture properties in the sensory quality and acceptance of natural yoghurts. Int J Dairy Technol. 2005;58(1):39-46.
Crossref - Marshall VM, Rawson HL. Effects of exopolysaccharide producing strains of thermophilic lactic acid bacteria on the texture of stirred yoghurt. Int J Food Sci Technol. 1999;34(2):137-143.
Crossref - Behare PV, Singh R, Tomar SK, Nagpal R, Kumar M, Mohania D. Effect of exopolysaccharide-producing strains of Streptococcus thermophilus on technological attributes of fat free lassi. J Dairy Sci. 2010;93(7):2874-2879.
Crossref - de Leblanc AdM, Pedrigon G. Yogurt feeding inhibits promotion and progression of cancer. Med Sc Monit. 2004;10(4):96-104. PMID: 15039638
- Singh P, Saini P. Food and health potentials of exopolysaccharides derived from Lactobacilli. Microbiol Res J Int. 2017;22:1-14.
Crossref - Khalil MA, Sonbol FI, Al-Madboly LA, Aboshady TA, Alqurashi AS, Ali SS. Exploring the Therapeutic Potentials of Exopolysaccharides Derived From Lactic Acid Bacteria and Bifidobacteria: Antioxidant, Antitumor, and Periodontal Regeneration. Front Microbiol. 2022;13:803688.
Crossref - Singh G, Muthukum K. Influence of calcium fortification on sensory, physical and rheological characteristics of fruit yogurt. LWT-Food Science Technology. 2008;41(7):1145-1152.
Crossref - Temesgen M. Effect of Application of Stabilizers on Gelation and Synersis in Yoghurt. Food Science and Quality Management. 2015;37:90-102.
- Staff MC. Cultured milk and fresh cheese. In R. Early (Ed.). The technology of dairy products. New York: Chapman and Hall. 1998:123-157.
- Zhang S, Zhang LW. Effect of Exopolysaccharide Producing Lactic Acid Bacterial on the Gelation and Texture Properties of Yogurt. Advanced Materials Research. 2012;430-432:890-893.
Crossref - Gawai KM, Mudgalm SP, Prajapati JB. Chapter 3- Stabilizers, Colorants, and Exopolysaccharides in Yogurt In: Yogurt in Health and Disease Prevention. 2017:49-68.
Crossref - Oleksy-Sobczak M, Klewicka E, Piekarska-Radzik L. Exopolysaccharides production by Lactobacillus rhamnosus strains-Optimization of synthesis and extraction conditions. LWT. 2020;122:109055.
Crossref - Elbanna K, Metry W, Elgarhy H. Exopolysaccharide from lactobacillus pentosus strain h2 and its impact on rheological properties and the sensory evaluation of low fat yoghurt and UF-soft cheese. Int J Nutr Food Sci. 2015;4(5):555-564.
Crossref - Abdalrahim S, Zohri ANA, Khider M, et al. Phenotypic and Genotypic Characterization of Exopolysaccharide Producing Bacteria Isolated from Fermented Fruits, Vegetables and Dairy Products. J Pure Appl Microbiol. 2019;13(3):1349-1362.
Crossref - Amiri S, Mokarram RR, Khiabani MS, Bari MR, Khaledabad MA. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int J Biol Macromol. 2019;123:752-765.
Crossref - Juraskova D, Ribeiro SC, Silva CCG. Exopolysaccharides produced by lactic acid bacteria: from biosynthesis to health-promoting properties. Foods. 2022;11:156.
Crossref - Hussain A, Zia KM, Tabasum S, et al. Blends and composites of exopolysaccharides; properties and applications: A review. Int J Biol Macromol. 2017;94(Part A):10-27.
Crossref - Lee WE, Lucey JA. Formation and Physical Properties of Yogurt. Asian-Aust J Anim Sci. 2010;23(9):1127-1136.
Crossref - Jaiswal P, Sharma R, Sanodiya BS, Bisen PS. Microbial Exopolysaccharides: Natural Modulators of Dairy Products. J Appl Pharm Sci. 2014;4(10):105-109.
Crossref - Sritharan S, Sivalingam N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021;278:119527.
Crossref - Qin JY. Current situation and prospect of human cancer prevention and control. Sci Technol Rev. 2015;33:125.
Crossref - Peter S, Alven S, Maseko RB, Aderibigbe BA. Doxorubicin-Based Hybrid Compounds as Potential Anticancer Agents: A Review. Molecules 2022;27(14):4478.
Crossref - Liu, CT, Chu, FJ, Chou, CC, Yu, RC. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat Res. 2011;721(2):157-162.
Crossref - Algotiml R, Gab-Alla A, Seoudi R, Abulreesh HH, El-Readi MZ, Elbanna K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci Rep. 2022;12(1):2421.
Crossref - Wang J, Yuan P, Zhang W, et al. Separation, Purification, Structural Characterization, and Anticancer Activity of a Novel Exopolysaccharide from Mucor sp. Molecules. 2022;27:2071.
Crossref - Khan T, Date A, Chawda H, Patel K. Polysaccharides as potential anticancer agents-A review of their progress. Carbohydr Polym. 2019;210:412-428.
Crossref - Mattos KA, Volpon AGT, Previato LM, Previato JO. Aplicacao de microrganismos produtores de biopolímeros na recuperacao de petroleo, avaliacao do melaco como fonte alternativa para a producao de biopolimeros. CENPES/SEBIO, Rio de Janeiro, Brazil. Technical report. 1997.
- Prasanna PHP, Grandison AS, Charalampopoulos D. Bifidobacteria in milk products: An overview of physiological and biochemical properties, exopolysaccharide production, selection criteria of milk products and health benefits. Food Res Int. 2014;55:247-262.
Crossref - Kumar MA, Anandapandian KTK, Parthiban K. Production and characterization of exopolysaccharides (EPS) from biofilm forming marine bacterium. Braz Arch Biol Technol. 2011;54(2):259-265.
Crossref - Singh M, Kalaivani R, Manikandan S, Sangeetha N, Kumaraguru AK. Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga. Appl Nanosci. 2013;3(2):145-151
Crossref - Supaka N, Juntongjin K, Damronglerd S, Delia ML, Strehaiano P. Microbial decolorization of reactive azo dyes in a sequential anaerobic-aerobic system. Chem Eng J. 2004;99(2):169-176.
Crossref - Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63.
Crossref - Kaminsky RC, Schmid R. Brun, An In Vitro selectivity index for evaluation of cytotoxicity of antitrypanosomal compounds. 1996:9:315-323.
- Tamime A. Fermented Milks (First Edit). 2006.
Crossref - Dave RI, Shah NP. Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J Dairy Sci. 1998;81:2804-2816.
Crossref - Guzman-Gonzalez M, Morais F, Ramos M, Amigo L. Influence of skimmed milk concentrate replacement by dry dairy products in a low fat set-type yoghurt model system. I: Use of whey protein concentrates, milk protein concentrates and skimmmed milk powder. J Sci Food Agric. 1999;79(8):1117-1122.
Crossref - Isanga J, Zhang G. Production and evaluation of some physicochemical parameters of peanut milk yoghurt. LWT – Food Sci Technol. 2009;42(6):1132-1138.
Crossref - Gassem MA, Frank JF. Physical properties of yogurt made from milk treated with proteolytic enzymes. J Dairy Sci. 1991;74(5):1503-1511.
Crossref - AOAC. Association of Official Analytical Chemists-official Method of Analysis (18th Ed.). Benjamin Franklin Station Washington, DC, USA. 2007.
- Hamdy AM, El-Kousey LA, AbdelLatif R. A study in the fermented milk (zabady). Agric Res Rev. 1972;50:159-168.
- SPSS. Statistical Package for Social Sciences. 2008; Version 17.0.0, SPSS Corporation.
- Duncan D. Multiple range and multiple F test. Biometrics. 1955;11(1):1-45.
Crossref - Gayathiri E, Bharathi B, Velu S, et al. Isolation, identification and optimization of exopolysaccharide producing lactic acid bacteria from raw dairy samples. Inter J Pharma Chem Res. 2017;3(2):202-211.
- Sutherland IW. Bacterial exopolysaccharides. In: Kamerling J.P. (Ed.), Comprehensive glycoscience. Oxford: Elsevier, Amsterdam. 2007:521-557.
Crossref - Razack SA, Velayutham V, Thangavelu V. Influence of various parameters on exopolysaccharide production from Bacillus subtilis. Int J Chem Tech Res. 2013;5(5):2221-2228.
- Moghannem SA, Farag MM, Shehab AM, Azab MS. Media optimization for exopolysaccharide producing Klebsiella oxytoca KY498625 under varying cultural conditions. Int J Adv Res Biol Sci. 2017;4(3):16-30.
Crossref - Krishnan R, Maru GB. Isolation and analyses of polymeric polyphenol fractions from black tea. Food Chem. 2006;94(3):331-340.
Crossref - Yuan L, Koehler M, Baudelet M, Richardson M. Fusion of infrared and Raman spectroscopy for carotenoid analysis. Pittcon Orlando FL USA. 2012;3:1-13.
- Gangoiti J, Pijning T, Dijkhuizen L. Biotechnological potential of novel glycoside hydrolase family 70 enzymes synthesizing a-glucans from starch and sucrose. Biotechnol Adv. 2018;36(1):196-207.
Crossref - Saadat YR, Khosroushahi AY, Gargari BP. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr Polym. 2019;217:79-89.
Crossref - Srisawat T, Chumkaew P, Heed-Chim W, Sukpondma Y, Kanokwiroon K. Phytochemical screening and cytotoxicity of crude extracts of Vatica diospyroides Symington type LS. Trop J Pharm Res. 2013;12:71-76.
Crossref - Koch A, Tamez P, Pezzuto J, Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol. 2005;101(1-3):95-99.
Crossref - Sathiyanarayanan G, Dineshkumar K, Yang YH. Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Crit Rev Microbiol. 2017:43(6):731-752.
Crossref - Jiayi T Wu, Yuheng Zhang, Ling Ye, Chenglin Wang. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohydr Polym. 2021;253:117308.
Crossref - Dammak MI, Salem YB, Belaid A, et al., Partial characterization and antitumor activity of a polysaccharide isolated from watermelon rinds. Int J Biol Macromol. 2019;136:632-641.
Crossref - Hussein MM, Hassan FAM, Abdel-Daym HH, Salama A, Enab AK, Abd El-Galil AA. Utilization of some plant polysaccharides for improving yoghurt consistency. Ann Agric Sci. 2011;56(2):97-103.
Crossref - El Batawy O. and Khalil OSF. Manufacture and Properties of Low-Fat Bio Yoghurt Containing Probiotic Strains and Maltodextrin as Prebiotic. Journal of Probiotics and Health. 2018;6(1):1-9.
Crossref - Berthold-Pluta AM, Pluta AS, Garbowska M, Stasiak-Roza L. Exopolysaccharide-producing lactic acid bacteria-Health-promoting properties and application in the dairy industry. Adv Microbiol. 2019;58(2):191-204.
Crossref - Korcz E, Varga L. Exopolysaccharides from lactic acid bacteria: Techno-Functional application in the food industry. Trends Food Sci Technol. 2021;110:375-384.
Crossref - Han X, Yang Z, Jing X, et al. Improvement of the texture of yogurt by use of exopolysaccharide producing lactic acid bacteria. BioMed Res Int. 2016;2016:e7945675.
Crossref - Omer SHM. Chemical and Physical Properties of Yoghurt from Khartoum Dairy Product Company (KDPC). M.Sc. Thesis, Faculty of Animal Production, University Khartoum, Sudan 2003:8-9.
- Abd El-Galeel AA, Atwaa EH, Abdelwahed EM. Improving Properties of Non-Fat Yoghurt Using Fat Replacers. Zagazig J Agric. Res. 2017;44 (2):583-590.
Crossref - Bruckner-Gühmann M, Benthin A, Drusch S. Enrichment of yoghurt with oat protein fractions: Structure formation, textural properties and sensory evaluation. Food Hydrocolloids. 2019;86:146-153.
Crossref - Ge Z, Yin D, Li Z, Chen X, Dong M. Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts. Foods. 2022;11:1764.
Crossref - Pan L, Wang Q, Qu L, et al. Pilot-scale production of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its application in set yogurt. J Dairy Sci. 2021;105(2):1072-1083.
Crossref - Guzel-Seydim ZB, Sezgin E, Seydin AC. Influences of exopolysaccharide producing cultures on the quality of plain set type yogurt. Food Control. 2005;16(3):205-209.
Crossref - Lucey JA, Singh H. Formation and physical properties of acid milk gels: A review. Food Res Int. 1997;30(7):529-542.
Crossref - Lucey JA, Munro PA, Singh, H. Whey separation in acid skim milk gels made with glucono-δ-lactone: Effects of heat treatment and gelation temperature. J Texture Stud. 1998;29(4):413-426.
Crossref - Sanli T. Effects of using transglutaminase and fat replacer on functional properties of non-fat yoghurt. Kafkas Univ Vet Fak Derg. 2015;21(6):907- 913.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.