Biofertilizers have become a viable substitute for chemical fertilizers. Biofertilizers contain the effective strains of potential organisms majorly included of bacterial and fungal strains providing desirable benefits to crop plants and soil. They are being prepared in different formulations suitable for diverse applications. Variations in production process, raw materials and storage conditions can lead to inconsistencies in microbial composition and nutrient levels, impacting their function in the fields. However, the shelf life and quality maintenance of biofertilizers are critical to their effectiveness and viability and present considerable hurdles throughout production, storage and application. Biofertilizers are easily affected by various factors resulting in eventual loss of viability. Variations in temperature, moisture content and exposure to UV radiation are a few examples of factors that might negatively impact microbial viability and activity. Furthermore, contamination by undesirable microorganism during production and storage can reduce the effectiveness of bio-fertilizers. To address these problems, innovative approaches such as different formulation techniques were developed. Addition of stabilizing agents to the formulation will add value to the products, since it gives protection to the cell, thus the efficacy and shelf life are maintained. Varied types of formulations have different issues with the maintenance of quality and shelf life. Widely used formulations and the problems and constrains with different formulations on application, in addition to shelf life and also the possible suggestions are discussed in this review.
Bio-fertilizers, Shelf life, Encapsulation, Aggregated Formulation, Nanoformulation, FBD
Over the past four decades, we have seen both the doubling of food productivity and the human population. Plant nutrition is the governing factor towards rising the food grain production and supply. The introduction of commercial man-made fertilizers has enabled rise in agricultural productivity with a substantial rise in the usage of chemical fertilizers. Fertilizer containing nitrogen (N) and phosphorous (P) are broadly used to meet out the plant nutrition. Additionally, the growing usage of fertilizers has led to environmental issues including degradation of groundwater, surface water, and soil quality; moreover, air pollution; decreased biodiversity; and inhibited ecosystem function.1 However a balanced and adequate quantity of nutrients should be available in the soil for better plant growth.2
Soil infertility is the primary factor restricting crop productivity in the underdeveloped countries overall the world, particularly for farmers with little resources. Farmers will not much benefit by using improved crop varieties and effective cultural practices unless the fertility is restored in these land. By implementing the Integrated Nutrient Management (INM) concept, which contains the nutrient management strategy based on the preservation of natural resources, biological nitrogen fixation (BNF), and enhanced input efficiency, soil fertility can be efficiently restored.3
Soil microorganisms are playing an important role in maintaining soil biodiversity and coordinated nutrient management. They are necessary for the development and growth of plants. At the cost of the ecology and health of all living creatures, the usage of chemical fertilizers in agriculture increases self-sufficiency in food production of the country. These fertilizers are expensive, and overuse in agriculture has a number of detrimental effects on soil fertility. Advantageous microorganisms are superior substitutes for traditional agricultural practices. Since they are more concentrated, not harmful to the surroundings and more effective when used in smaller amounts, bio-fertilizers are safer than chemical fertilizers.4
This review discussed the necessity of bio-fertilizer, types and challenges in maintaining the shelf life of bio-fertilizers.
In general, a bio-fertilizer, also known as “microbial inoculants”, are products containing living cells of various types of microorganisms that, when applied to seed, plant surface or soil, colonize the rhizosphere or the interior of the crop and promotes growth through the biological process by convertion of essential nutrients from unavailable forms to available forms.5 In addition to promoting and enhancing plant growth, beneficial organisms in bio-fertilizers shield the plants from pests and pathogens.6
Farmers have a long history of using microbial inoculum, which they pass down from century to century. The earliest step was the utilized for small-scale compost producing culture, which demonstrated the prospective of bio-fertilizer. This is evident when the cultures produce a healthy crop harvest while also speeding up the decomposition of wastes and agricultural by-products through a various processes.7
Many people have misconceptions about bio-fertilizer. Because of lack of knowledge and need of resources to develop bio-fertilizer products, it is frequently thought to be additional expense than conventional fertilizers. In addition to, out-turn on the crops is not as quick as of synthetic chemical fertilizers. For microbial inoculants we should provide care to maintain their effectiveness over an extended period of time in carriers and also in storage. Since bio-fertilizers are made up of live organisms, their effectiveness is influenced by the surrounding environment. Thus, the results are bound to uncertain.8 To achieve effective usage, the challenges such as short shelf life, devoid of appropriate carrier materials, sensitivity to high temperatures, issues with transportation and storage have to be resolved.
Between 2017 and 2021, the value of the Indian bio-fertilizer market grew by approximately 11.0%. This growth can be attributed to a number of factors, including rising farmer awareness, a growing number of registered organic farms in India, and a shift in consumer demand for sustainably or organically grown goods. As of 2021, India ranked first overall in producers and fifth globally in land used for organic agriculture.
While manufacturing bio-fertilizer, a number of factors must be taken into account, including the development profile of the microorganisms, the types and ideal conditions of the organisms, and the inoculum formulation. The efficacy of the biological product depends on the inoculum’s composition, application technique, and product storage.9
Types of bio-fertilizer
Organisms used as bio-fertilizer can execute certain functions in soil and on the host and they are of different kinds. Every plant requires specific nutrients for their growth and functions. By considering the plant requirement, bio-fertilizer organisms are grouped according to nature and function. Selection of appropriate type of biofertilizer will meet the demands of the specific crops and help the plants to grow and enhance the yield of the crops. Based on the functional attributes of the organisms they are classified as Nitrogen fixers, Phosphate solubilizers and P mobilizes, Potassium releasing organisms, Silica solubilizing and Zn solubilizing organisms.10 Bio-fertilizer include various kind of microbes such as fungi, bacteria and algae.11
Nitrogen fixing bio-fertilizers
The elemental nitrogen is converted into plant usable form by the process of biological nitrogen fixation.12 The inert N2 gas is transformed into organic molecules by the action of Nitrogen fixing bacteria (NFB).13 The nitrogen fixing bio-fertilizers are living fertilizers made up of efficient microbial strains or collections of microorganisms who can fix atmospheric nitrogen.14 Nitrogen fixing bacteria are classified as symbiotic, associative, free living and endophytic nitrogen fixers.
Bacterial nitrogen fixers
Symbiotic nitrogen fixers fix nitrogen only when they are in symbiotic association with host plant. Many soil microorganisms fix nitrogen symbiotically such as Rhizobium, Frankia, Glucanoacetobacter and BGA Anabaena. Among these Rhizobium for legumes and BGA in association with Azolla for lowland rice are widely used as bio-fertilizers. Beijerinck identified the first bacteria nodulating a legume in 1888. Previously this bacterium called Bacillus radicicola, was later renamed Rhizobium leguminosarum, where Frank (1889) first established Rhizobium.14 These Gram-negative bacteria belong to the family Rhizobiaceae, which fixes nitrogen 50-100 kg/ha only with legumes. They are members of the alpha subgroup of the phylum proteobacteria.15 While speaking with symbiotic nitrogen fixation, another organisms of interest is Frankia. It is a Gram-positive actinobacteria associate with wide actinorhizal plants which were not commercially exploited as that of Rhizobium due to its fastidious and slow growing nature.
Associative nitrogen fixers have non-symbiotic interaction with the host plant during biological nitrogen fixation. This group is dominated by Azospirillum, colonize the root surface and the intercellular spaces of the host roots and get attached to the roots by adhesion.
One of the most well-researched genera of rhizobacteria that promote plant growth, Azospirillum sp., can colonize hundreds of plant species and enhance their development, growth, and productivity. The effects of inoculation on plant growth promotion, particularly under stressful conditions, are explained by free nitrogen fixation and additive mechanisms linked to Azospirillum sp. capacity to create phytohormones and other related compounds.16 Atmospheric nitrogen fixation in the basic food crops worldwide, including rice, maize, sorghum, wheat, and millets is aided by Azospirillum. All soils contain significant numbers of Azospirillums species, and inoculating fodder and cereal crops has been shown to enhance productivity in variety of experiments.15 Many Azopsirillum species secrete the phytohormones like auxins, cytokininins and gibberellins as signals towards plant growth promotion,17 scientists are interested in using this genus for developing microbial inoculants in agriculture. When growth conditions are less than ideal, including low plant accessible nitrogen, these inoculants are said to have the capacity to enhance plant development ultimately leading to a higher yield when compared to non-inoculated treatments. But inoculation studies have not proven reproducible in numerous field trials conducted gobally, raising doubts about the use of these inoculants.16
Azotobacter, a free living diazotroph, use nitrogen gas from air to synthesize proteins in their cells. After cells die, their cell protein becomes decomposed in the soil, which makes a considerable quantity of nitrogen available to the crops from the soil source. Azotobacter spp. is sensitive to temperatures above 35 °C, high salt concentrations, and acidic pH. The use of Azotobacter as a bio-fertilizer to deliver N to soil. In addition to fixing atmospheric N2, it also helps in plant growth regulators such as auxins, cytokinins, gibberellins, amino acids, and vitamins, as well as solubilization of phosphate.18
Endophytic nitrogen fixers
Endophytic microbes are characterized as inhabiting the apoplasm or symplasm, or interior plant tissues, without infecting the host or producing symptoms. In general endophytes are more advantageous than many rhizobacteria and they hardly face a drastic change in the soil’s environment. Therefore, it is preferable to employ endophytic bio-fertilizers rather than rhizobacterial bio-fertilizers to increase yield and protection in rice.19 Among several model species, the diazotrophic endophyte Azoarcus sp. has been found to colonize the interior of rice plant’s root.20 Rice endophytic bacterial diversity is affected by soil types. In neutral pH soil, endophytes Rhizobium radiobacter and Pseudomonas oryzihabitans dominated, while in acid pH soil, Enterobacter-like organisms and Dyella ginsengisoli dominated. Ochrobactrum sp. and Stenotrophomonas maltophilia were segregated from rice seeds.21 Endophytic Burkholderia sp. SSG promotes plant development and plant growth found to be increased by 37%-76%. This endophytes was found to have four key characteristics that aids in promotion of plant growth such as nitrogen fixation, IAA production, siderophores and phosphorus solubilisation.22 Sugarcane roots are inhabited by the Gram-negative endophytic diazotrophs like Pantoeacypripedii and Kosakoniaarachidis, which could be helpful in the growth, development, and prevent pathogen growth in sugarcane. Numerous PGP traits were found in endophytic cultures, such as N2 fixation, enzyme and phytohormone production, and antifungal activity against plant diseases. More investigations are required to assess the commercial potential of these organisms as bio-fertilizers for increased sugarcane agricultural output23 and the potential bioinoculant for all crops.
Alagal bio-fertilizers
Microalgae are photosynthetic organisms that include prokaryotic blue green algae and eukaryotic green algae. These fascinating organisms have the capacity to improve soil nutrients, which makes them useful in modern farming. They could have filamentous, saponaceous, multicellular, or unicellular characteristics. They are also the greatest primary producers in the planet as the species count is more than 2,00,000.24 Microalgae, such as Spirulina sp., Chlorella sp., and Cyanobacteria (blue-green algae), can produce plant growth hormones, polysaccharides, antibacterial compounds, and other metabolites in addition to improving soil fertility and quality.25 Cyanobacteria and green microalgae are important sources of organic matter in the agri-ecosystem since by photosynthesis they directly contribute to the absorption of atmospheric carbon dioxide into organic algal biomass. Half of all photosynthesis on earth is carried out by algae. Due to the direct absorption of carbon dioxide, they can significantly increase the amount of organic carbon in soil. Cyanobacteria’s heterocyst cells fix atmospheric nitrogen, supplying plants, flora, and soil micro- and macro-fauna with Nitrogen. Lot of studies have shown that crops applied with cyanobacteria greatly increased the quantity of nitrogen in the soil and can reduce the amount of chemical nitrogen fertilizer of crops by 25%-40% upon inoculation.26
Nitrogen supplying Fungal bio-fertilizer
Fungi are found in all environmental niches and habitats and they are associated in a number of biological processes, including the decomposition of organic materials which results in the nutrient cycling. In their natural habitat, fungi interact with plants, animals and bacteria through a variety of ways, metabolic processes, and nutritional adaptability.27 Yeast has the ability to provide nutrients and growth promoting substances for the enhancing crop growth and it can be utilized as biofertilizer.
Strain of Candida tropicalis isolated from soil shows great ability to fix nitrogen.28 On the other hand, studies of ammonia-producing yeasts have been reported with the genus Meyerozyma as the major NH3 producer in the same way that Pseudozyma rugulosa, Cryptococcus flavus and Pseudozyma antarctica.29 Generally, the enzyme 1-aminocyclopropane-1-carboxlyate (ACC), which is responsible for the cleavage of the ethylene precursor ACC into α-ketobutyrate and ammonia. By this way the above mentioned yeast species produce ammonia. Thus there is a double positive effect on the presence of this enzyme: one is in case of any adverse situation, the release of ethylene gets reduced since in large quantities Ethylene becomes harmful to plants and the second is the generation of nitrogen recycling mechanism by the release of ammonia with its symbiotic partner.
In a study, the yeast Saccharomyces cerevisiae from brewing industry waste product has been investigated for it’s potential as biofertilizer. The results revealed the increase in nutrient contents (Nitrogen and Phosphorus) of roots and shoots of tomato (Solanum lycopersicum) and young sugarcane plants was observed when live or dead yeast was added to the soil. In addition to this, yeast application also enhanced shoot biomass and tillering in sugarcane.30 This study demonstrated the utility of yeast for plant nutrition and growth as a biofertilizer.
Phosphatic bio-fertilizers
For the growth and development of plants the important element next to nitrogen is Phosphorus. The nutrient P is involved vital plant physiological process like reduction in nutrient stress and photosynthesis.31 Despite the huge quantity of P present as both inorganic and organic forms in soil, its availability is limited as it primarily exists in insoluble forms. The average soil contains approximately 0.05% (w/w) of P, but due to poor solubility and fixation, only 0.1% of the total P is available to plants. Nutrient immobilization occurs either indirectly or directly due to elevated pH and CaCO3 levels.32 Soil pH determines the availability of phosphorus.33 Increase in pH accelerates the transformation of P into insoluble forms, hence decreasing P availability and P use efficiency,34 which significantly affects the soil productivity. By binding with cations, P become unavailable as phosphates of Al3+, Ca2+ and Fe2+.35 The phosphorus solubilizers produce variety of organic acid. The hydroxyl or carboxyl group of the organic acid chelates the cations attached to the phosphorus and makes P available.36 Mainly the organic acids such as gluconic, succinic, fumaric, oxalic, formic, malic, citric, tartaric and lactic acid production were reported in several bacterial genera37 and different fungal genera.38
The organic P is converted into soluble P due to soil enzyme acid phosphatase and alkaline phosphatase.39 Some phytase producing microorganisms are Aspergillus parasiticus, Aspergillus niger, Aspergillus fumigatus, Aspergillus terreus, Penicillium zonatum and Penicilliumrubrumand Bacillus spp.40,41 Some soil microorganisms have the capability to transfer unavailable inorganic form of P like rock phosphate and tricalcium phosphate to available form of P like dibasic and monobasic by lowering the pH. This process increases the P absorption and enhances the yield of plants.42,43 Because of simple structure of Ca3(Po4)3, the amount of P dissolved from the Ca3 (Po4)3 was high compared to AlPO4 and FePO4.44 The bacterial strains of Pseudomonas spp. and Bacillus sp. are the most powerful phosphate solubilizers. Pseudomonas straita, Bacillus circulans, Bacillus subtilis and Bacillus megaterium are some active bio-fertilizers.45 Sclerotium, Aspergillus, Penicillium and Fusariumare are some genera of fungus who can solubilize the fixed phosphate.46 Among these, Penicillium bilaiae is commercially used as bio-fertilizer for phosphate solubilisation and it was formulated as wettable powder.47 Even-though many bacterial P bio-fertilizers are available in the market, fungal bio-fertilizers are preferred because they are very effective in acid soil.43
AMF for phosphorus mobilization
The Mycorrhiza is the symbiotic association between the plant root and fungi.48 More than 90% of terrestrial plants contain arbuscular mycorrhizae (AM), a common endotrophic symbiont that is taxonomically and functionally varied. They contain two distinct structures: balloon-like vesicles for nutrient storage inside the host’s plant root cortical cells, and minutely branching hyphal tip arbuscules for exchanges of nutrients.49 The phosphorus is the immobile nutrient that progressively being decreased in rhizosphere of the plant and the Phosphorus uptake is also reduced due to immobility.50 The phosphate solubilizer synthesis the enzymes like alkaline and acid phosphatases which are used to solubilize fixed phosphates and releases the mineral P.51 After solubilization of phosphorus, some microbes like AMF transfer the P nutrient from place of solubilization to the rhizosphere of the plant.52 Mainly in P deficient soil, the AMF mobilizes the P from rock phosphate and transfer the mobilized P through hyphae.53 The loading and unloading process determines the rate of translocation of P through arbuscles and extracellular hyphae.54 AMF also acts as a bio-control agents, it directly compete for nutrients in the rhizosphere and rhizoplane region with disease causing microbes.55,56 Application of AMF has the potency to improve the soil fertility, plant protection and plant nutrition.57
Since AMF increased the accumulation of Macro elements (Nitrogen and Phosphorus) and microelements (Zn, S, Cu, Fe, and Mn), it is essential to apply to crops for their production. AMF contributes either indirectly or directly to soil N-cycling activities. Alterations in soil aggregation and aeration have an effect on denitrification processes and reduce inorganic nitrogen leachate.58 The N availability of soil is impacted by the presence of AMF. AMF prefers to absorb nitrogen in ammonium from, which is then transported to the cytoplasm and translocated to intraradical hyphae via vacuole, where it is released in the apoplastic compartment. After release it is assimilated as arginine and used by plants.
Potassium releasing bio-fertilizer
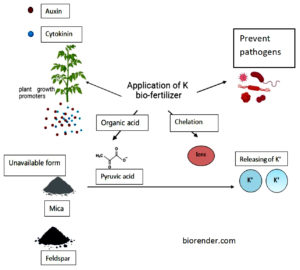
K is the third most important element for plant growth. It improves plant resistance against pathogen and also drought and extreme heat. Many crops, including banana, grapes, orange, mango, apple, sugarcane, pineapple, paddy, muskmelon, tomato, beans, wheat, watermelon, capsicum, pomegranate, gerbera, etc., can benefit from the use of potash bio-fertilizers. KRB plays lot of functions, including protection of plants from salinity by enhancing physiological processes associated to growth, such as lipid peroxidation, stomatal conductance, and electrolyte leakage.59 The weathering of K containing minerals leads to release of K which is utilized by plant roots.60 In soil system, the K nutrient is also released from the minerals with the help of various rhizobacteria.61 Some of the K releasing bacteria are Paenibacillus mucilaginosus, Acidothiobacillus ferrooxidans, Bacillus edaphicus, B. circulans, B. mucilaginosus, Pseudomonas, Burkholderia etc.62 B. mucilaginosus and B. edaphicus solubilize the feldspar by secreting the carboxylic acid and capsular polysaccharide and drastically improves the crop growth and yield63 (Figure 1). Through organic acid production some microbes have the capacity to release K and also from exchange reactions, and acidolysis64 (Figure 1).
Figure 1. The above image illustrates the mechanism of application of potassium (K) biofertilizer in plant growth and nutrient availability. Application of K bio-fertilizer enhances plant growth by producing plant growth promoters such as auxin and cytokinin, and by preventing pathogenic infections. Microbial activity leads to the secretion of organic acids (e.g., pyruvic acid) and chelating agents that solubilize unavailable forms of potassium present in minerals such as mica and feldspar. This process releases K⁺ ions into the soil, making potassium available for plant uptake and improving overall nutrient assimilation and health
The long history of employing fungi as bio-fertilizers has resulted in an increasing demand in the recent years for better understanding of their use and role as bio-control agents. The fungal species have natural ability to promote crop development and reduce dependency on artificial chemicals.65 Certain fungus includes Penicillium, Aspergillus and Fusarium also synthesis organic acids like gluconic, oxalic and citric acid. These acids degrade the mineral such as mica, feldspar and clay silicates and releases the nutrient K66 (Figure 1). When compost and Aspergillus niger are applied together, it may be possible to lessen the negative effects of calcareous soil and significantly lower the amount of potassium mineral fertilizer requirement without affecting the yield.67 Since Aspergillus niger produce organic acids, it has the ability to release the potassium from minerals.68 Organic acid production, ligands, protons and siderophore determine the weathering capacity of microbes. This activity was also found in some species such as Penicillium sp., Cladosporoides and Cladosporium.69
Bio-fertilizer for micronutrients
Micronutrients are crucial for the growth of plants and they are required in small quantities. They are boron (B), copper (Cu), iron (Fe), chloride (Cl), manganese (Mn), nickel (Ni), molybdenum (Mo), and zinc (Zn). For proper growth and development plants require these above micronutrient in balanced proportion. Several enzymatic functions require Zinc and also for functions like including protein synthesis, auxin synthesis, glucose metabolism, and cell membrane integrity. Growth of plants can be hindered by zinc deficiency through lower fruit and flower development, decreased amounts of carbohydrates, and lowering production of phyto-hormones. Zinc deficiency results in lower crop yields and lower nutritional quality.70 Based on the chemical reactions in soil, application of ZnSO4 in soil results in the formation of several forms of insoluble zinc, such as zinc oxide (ZnO), zinc hydroxide (Zn(OH)2), zinc carbonate (ZnCO3), zinc phosphate (Zn3(PO4)2), and zinc sulphide (ZnS). Crop plants are unable to absorb this insoluble zinc, leading to a zinc deficit. Additionally, zinc fertilizers spray on crops mitigates the zinc deficiency. But this method is expensive, and it can be hazardous for the environment and human health.71 The zinc solubilizers have the capacity to solubilize the insoluble Zn into soluble forms and it is the majorly used microorganisms as bio-fertilizer for micronutrient supply. Bacillus megaterium converts the insoluble ZnCO3 and ZnO into soluble zinc sulphate.72 Bacillus aryabhattai is less effective in solubilizing zinc oxide but achieves higher solubilization of zinc phosphate in comparison with zinc carbonate.73
Next to Zinc, silica is an essential micronutrient required for certain crop plants. Silica is abundantly present in soil (~27%) as insoluble silicate forms such as Aluminium silicate, magnesium silicate, sodium, and potassium silicate, calcium silicate and iron silicate.74 But Monosilicic acid is the only form in which plants can take Silicon, i.e. available form of Si. Silicate solubilizing bacteria (SSB) have the capacity to dissolute insoluble form of silicate to soluble form, boost plant bioavailability, and ultimately raise soil fertility to enable more effective agriculture.75 Silicate solubilizing bacteria (SSB) are recommended as a bio-fertilizers to solubilize silica. Nevertheless, there are few investigations on microorganisms that dissolve silicate. It was reported that Gram-negative Pseudomonas fluorescens and Gram-positive Bacillus flexus, B. megaterium76 and Bacillus amyloliquefaciens74 can solubilize the insoluble silicate. Dissolution of silicates by bacteria in soil releases the other plant nutrients like potassium, calcium, and magnesium from the silicates. With the increase in cost of potassic fertilizers, silicate minerals and ashes rich in silica and potassium are employed as fertilizers.
Microbial consortium
Utilizing a microbial consortium could be the perfect bioformulation to fully meet crops’ primary nutrient needs. Many researchers have created a mixed culture consortia and experimented in a variety of crops. The growth parameters shoot/root length and fresh/dry biomass of the barley crop were demonstrated to be improved by the microbial consortium containing N fixing (Erwinia sp.), P solubilizing (Chryseobacterium arthrosphaerae) and K solubilizing (Pseudomonas gessardii) strains.77 Application of microbial consortium has also reported to increase Phenols and Flavanoids. The beneficial response of the microbial consortium consists of Azospirillum brasilense and Pseudomonas fluorescens on enhancing the plant growth and grain yield in corn has been reported recently.78 They are used as bio fertilizers because of the beneficial response they offer on crop plants.
Formulations of biofertilizers
To use these advantageous microorganisms in crop production several formulations were evolved. Basically, a formulation is the combination of uniform mixture of beneficial strains with some appropriate carriers and made in an appropriate form so as to protect the cells. This process of bioinoculant formulation is mainly made in such a way to maintain the viability of the organisms during transportation, storage, and application either in a dormant or metabolically active condition and to make the successful delivery to the crop plant. An ideal bioformulation should have a high water retention capacity, be rapidly biodegradable, effective, and have an adequate shelf life. The microbial strains selected for formulation development should be efficient and having competence in the root zone and the microbial inoculant must successfully overcome the circumstances of temperature, salinity, humidity, water stress and UV radiation during its development upon application. Additionally, a carefully developed formulation provide the best environment for maximizing the persistence and activity of microbes in soil, enabling the greatest possible benefits upon inoculation on plants.79
The persistence and capacity of the bioinoculants to colonize plant roots is based on its physical form and method of application. The inoculant is classified into many such as liquid formulation, solid formulation or bio-encapsulated formulation based on its physical form.80 It is quite obvious that improper production, formulation, and/or application of a microbial inoculant cannot realize the advantages of biofertilizers.81 Similarly improper quality of inoculants in the market may cause inconsistent results of the applied product.
Solid formulations
Solid formations are the carrier based formulations prepared as dry powder/wettable powder/granules/capsulated/tablets. According to their particle sizes, the solid formulations are categorized and can be made in solid, granular, or powdery forms. They are based on either inorganic or organic carriers. The most significant solid formulations are based on carriers such peat, compost, agro-industrial wastes, vermiculite, rock phosphate, perlite, polysaccharides, and calcium sulphate.82 Increased attention in solid formulation technology has been received by Polysaccharide-immobilized inoculants in recent years83 and it can be a technological solution that can more effectively ensure the quality.
Carrier based powder formulation
Solid bio-formulations give the targeted bacteria a nourishing and protecting environment. It improves storage effectiveness and lowers contamination. Solid bio-formulation materials include soil-derived carriers like charcoal, fine clay, turf, and organic carriers like sawdust, wheat, soy, and oat bran, vermicompost, sewage sludge, animal manure, and compost; inert carriers include talc, peat, perlite, vermiculite, alginate, bentonite, kaolin, silicates, and charcoal.85 The carriers provide a protective and nutritive environment to those microbes that form micro-colonies. They should be adaptive, easily sterilizable, non-toxic with high water holding capacity.93 Based on the form of the product the carriers may vary.
Microbes are moved from a lab to the land via carriers, which are inert materials.94 Carrier based bio-fertilizers need to have a moisture content of 30%-40%. Over the time, bio-fertilizers’ moisture content progressively decline.95 The majorly used carrier materials in India are coal, charcoal, talc and lignite for mass production of carrier based bio-fertilizer. Among the above-mentioned carriers, selecting an appropriate material is very essential to keep the microbial cells alive and right quantity of carriers should be added with right amount of microbial cultures.96
The following qualities are ideal for a good carrier97: (1) Give the target microorganism(s) an appropriate microenvironment (2) Possess appropriate chemical and physical characteristics, such as easy pH adjustment, strong pH buffering ability, and good moisture absorption capacity (increased water holding capacity) (3) Maintain stability throughout the process: To assure the stability, the carrier needs to be as uniform both chemically and physically. It should be free of lump-forming ingredients, sterile or easily sterilized by autoclaving or other means, and suitable for fine grinding in order to mix with other compounds (adjuvants, nutrients), as well as conventional machinery application. Additionally, it should be simple to use and appropriate for the widest range of bacterial or fungal species and strains. (4) Enhance the storage and inoculation conditions: an effective carrier should ensure a longer shelf life, stick and survive on seeds, and permit a quick and control release.
Peat is majorly used carrier material for mass producing bio-fertilizer due to its suitable chemical and physical properties. Even though it is very suitable, the cost of sterilization of peat is high, there is a reduction in the production and use of carrier based bio-fertilizers.88 For many years, peat was the preferred and highly regarded transporter among the aforementioned materials. Even though they are effective transporters, peat and lignite are costly and difficult to find. The two main prerequisites for bio-formulation in underdeveloped nations are low cost and simple access to carrier material.98
Carrier materials of different kinds are accessible, choosing a right one is essential since the carrier keeps bio-agents alive. The selected carrier material should retain the moisture content and nutrients. The ideal carriers for extending the shelf life of the bio-formulation are those with a high moisture retention capacity, a low Carbon:Nitrogen ratio, and a pH close to 7.0. Different carrier materials were examined in a study to support microbial life which includes sand, bagasse, sawdust, wood ash, and coriander husk and reported that best carrier for this purpose is coriander husk, which retains 7.5 times its moisture content than the others. Nevertheless, an another study revealed that carriers with a low Carbon:Nitrogen ratio such as biogas slurry and compost are superior than carriers with a high Carbon:Nitrogen ratio in terms of extending the shelf life and improving the growth of plant and its development.99 To choose an efficient carrier, it is important to take into account the carrier’s water holding capacity and Carbon:Nitrogen ratio.
The mass production of carrier based bio-fertilizers is a traditional method having many challenges during storage and application like reduced quality, highly prone to contamination and short life span84 (Table). It also requires a lot of labour and energy, which increases the cost of production. While doing seed treatment, it is having the drawback of having a poor seed spread and uniform coating is not possible. The viable count has dropped every month, and the quality may be affected.95 Carrier based formulations develop heat when it is stored under room temperature. The organisms that are unable to withstand UV rays and temperature greater than 30 °C, less resilient to temperature changes83 and this makes reduction in populations leading to lesser shelf life. If the carrier material is improperly handled or sterilized, it may contaminate at every stage of manufacturing, including the mixing of organisms with carriers and packaging. Reduction in the quality of bio-fertilizers is also due to a result of inadequate storage conditions. It leads to varied response in field and also influencing the performance of it.100
Table:
Comparing the advantages and disadvantages of all the formulation mentioned
No. |
Formulation |
Advantages |
Disadvantages |
Reference |
|---|---|---|---|---|
1 |
Carrier based powder formulation |
• Gives the targeted bacteria a nourishing and protecting environment. |
• Reduction in quality of bioinoculants during storage and application. • Highly prone to contamination. • Short life span. |
84,85 |
2 |
Pellet formulation |
• Coating of bio-inoculants on the pellets is possible that increases the use efficiency of pellets. |
• Difficult to maintain microbial population in pellets. |
86 |
3 |
Liquid formulation |
• The best alternative for overcoming the drawback of solid based carriers. • Higher shelf life of 1-2 year. • No necessity for sticky materials Compatibility with modern day machinery. • Lack of contamination. Ability to tolerate temperatures as high as 45 °C. • Simple for handling and application. Addition of ingredients that promote the growth of microbial strains. • Easy during application on both soil and seeds. |
• Deprivation of nutrients for microbial inoculants. • Need special storage conditions for enhancing shelf life and this condition will not be provided by the farmers. |
87,88,89 |
4 |
Granular formulation |
• Simpler to handle, store, and apply, and dust free. • It is easy to adjust the positioning and application rate. |
• The effectiveness of granulated bio-fertilizer limited on wet surfaces. |
90 |
5 |
Encapsulated formulation |
• Beads have a very low volume and can be highly concentrated, handling and transportation are very easy. • Less space is needed for storage. • It produces a homogenous distribution of cells around the targeted region, increasing the application efficacy. |
• Encapsulated cells may undergo physiological, morphological, and metabolic changes. • There is a chance for cell death during the drying of encapsulated cells. |
90,82 |
6 |
Aggregated formulation |
• Encapsulation using aggregated cells always having high microbial load. • Formation of beads using aggregated cell shows superior enzymes activity. |
• The cost of the polymeric carrier is higher than the other components of the solid and liquid formulation. |
91,90,82 |
7 |
Nano formulation |
• Nanofertilizers that are used to increase the overall effect and reduce the negative effects of other type of formulations. • It helps in better and more gradual nutrient release characteristics. |
• Not cost effective formulation. |
92 |
Sometimes, the heat sterilized carriers used in formulation releases harmful components, which may affect survival and growth of the organisms.97 Furthermore, due to its complex organic nature, different batches of peat exhibit significant chemical variability, making it challenging to maintain consistent quality across all batches.101
Bio-char, a charcoal-based carrier, improves the strength of the bio-formulation and is safe for the environment because it doesn’t have any negative effects. Since charcoal contains less moisture content, it can be stored without sterilization, which is an additional benefit of using it. Bradyrhizobium japonicum based biochar had an improved bacterial survival efficiency and improved the nodulation in soyabean.
The dark brown or black mineral called lignite is generated when organic waste partially decomposes under high temperature and pressure conditions. The lowest grade of coal is lignite, which has a C concentration of 60%-70% and a mineral content of 6%-9%. Grinded to a particle size less than 40 µm, and it can be made available as a carrier material. Even though lignite contains less nutrients than peat, nitrogen-fixing bacteria were able to survive and develop when lignite was neutralized with 5% calcium carbonate. Also a significant alterations in microbial population was noted when lignite based inoculant was applied to soil.102 It is reported that adding 2% of an organic amendment (sawdust) can prolong the shelf life up to six months. Further, organic amendment addition maintains the moisture content of the microbial inoculant to certain level, the maximum moisture content was observed in the treatment with lignite + sawdust (36.23%) against using lignite alone (30.20%).103
Pellet formulation
One type of bio-fertilizer based on carriers is pellet formulation. Pellet is a solid, compacted, spherical shaped bio-fertilizer is produced by compressing microorganisms and carrier material. Applying force to the carrier based bio-fertilizer formulation until it turns into pellets is the basic idea behind the pellet formulation process. Starting with the selection of targeted microbial strains, the basic recipe for pellet bio-fertilizer is essentially the same as for other formulations. After mixing the carrier material and inoculant strains, the mixture is run through a pellet press machine to produce bio-fertilizer in the form of tiny pellets. With different sources of carrier materials such as compost and biochar the effectiveness of bio fertilizer can be tested. The results showed that compost based pellet formulation shows increased shoot weight, root weight, number of grains per panicle, grain weight and highest grain yield in rice104 than the others. Compared to the cells which are immobilized inside the pellets, it is most desired to go for coating the pellets. After pelleting instead of mixing with the carrier, spraying liquid bio-inoculant over the pellets is desirable86 (Table). For large scale production, one may think about the cell coating on the pellets, which is considered effective and practically feasible.
Liquid formulation
Products with liquid formulations are usually aqueous, oil-based, or polymer-based, containing the targeted microorganisms and their nutrients together with additional additives and a unique cell protector that enhances cell survival both during seed or soil application and storage.94 An alternative to carrier-based formulations is using liquid bio-fertilizers.89 Liquid bio-fertilizers are otherwise known as flowable and aqueous suspension. They are based on broth culture, organic oil and mineral, suspension based polymers and oil in water. Generally materials used in liquid biofertilizers are, 10%-40% microorganisms, 1%-3% suspender component, 1%-2% dispersant, 3%-8% surfactant, and 35%-65% carrier liquid (oil or water).85 Cell protectants which aids in the formation of dormant spores and cysts should be present in liquid bio-fertilizers,88 as it is an advantageous approach towards enhancing shelf life of liquid products.
In comparison with solid inoculants, liquid bio-fertilizers are more desirable because of the following features viz., higher shelf life of 1-2 years, no necessity for sticky materials, compatibility with modern day machinery, lack of contamination, ability to tolerate temperatures as high as 45 °C, simple for handling and application, addition of ingredients that promote the growth of microbial strains, and ease in application on both soil and seeds87 (Table). Because of higher microbial densities, better results can be achieved by applying lower dosages compared to solid inoculants.89,96 The additives used in the liquid bio-fertilizers should be affordable, readily available, harmless, and simple-to-use88 (Table). However liquid bio-fertilizers have a higher shelf life, the organisms may suffer to different environmental stressors like nutrient depletion, and hypoxia which can lead to decline in the microbial population. To reduce these risks, particular storage conditions such as cold temperatures are required.89 There should be a reduction in the chemical fertilizers application by 15% to 40% with liquid bio-fertilizers. Furthermore, their dosages are 10% lower than those of solid bio-fertilizer, meaning that less quantity is required and smaller storage areas are possible.42 Additionally, the by-products and wastes from different industries can be used to make liquid bio-fertilizers, which can be an affordable and suitable alternative to specifically prepared media for the growth of bacterial cells.
In broth culture, microbes do not live long and eventually lose their ability to colonize the seeds87 (Table). Therefore, some additives like polyvinylpyrolidone sucrose, arabic gum and glycerol are incorporated with liquid formulations to allow microbes to survive longer. These additives can inactivate toxic compounds, improve seed adhesion and also the survival of microbial strains under various environmental conditions is also increased. Some additives may provide cell protection through reducing the metabolic activity89 (Table).
While comparing with the solid based carriers, liquid based inoculants are sticky during application. The liquid based inoculants are the best alternative for overcoming the drawback of solid based carriers. The cell protectants are used for the mass production of liquid bio-fertilizers because it involved in the formation of resting structures such as cysts and spores.88 The ability of additives to shield bacterial cells during storage and on seeds in harsh circumstances, such as high temperatures, desiccation, and hazardous seed conditions and chemical conditions, is the basis for their selection. Good additives are high molecular weight polymers that are non-toxic, have good water solubility, can minimize heat transfer, have strong rheological qualities, and have high water activities.105 Some additives which are incorporated with liquid inoculants are Poly vinyl pyrrolidone (PVP), methyl cellulose, polyvinyl alcohol, polyethylene glycol, gum arabica, trehalose, glycerol and Fe-EDTA.106 Among many cell protectants, trehalose (15 mM) proved to be the best supplement for prolonging the shelf life of Azospirillum sp.107 Also at 1% and 2% levels of PVP and PEG, populations of Azospirillum spp. were found greater. Higher population density of Azotobacter sp. was supported by the incorporation of 2% glycerol.108 Addition of PVP/ gum arabic in the liquid inoculants of Rhizobium had an improved shelf life up to 12 months.109 In PVP K-15 addition at 2% concentration, Pseudomonas sp. and Bacillus sp. exhibit the best performance compared to other microbial inoculants without PVP.100 Hence the main aim of adding protectants into biofertilizer is for enhancing the quality of inoculant, product stabilization, detoxification of toxin compounds and enhancing the strain survival when exposed to extreme temperature, drying and storage. It is observed to have a significant correlation between the strains and the incorporation of additives. Addition of preservatives, like glycerol, help microorganisms survive by retaining large quantity of water, which prevents cells from desiccating by slowing down the drying process. To improve efficacy and stability of bio-inoculants, a liquid inoculant of organisms with a CFU count of 109 per millilitre was developed with the addition of preservatives such as glycerol and PVP, which enhances the shelf life of the microbial inoculant up to 2 years.101 These additives may act by detoxifying the accumulated toxic metabolites and reducing the metabolic activity of the cells. Polyethylene glycol, by it’s sticky consistency and adhesive qualities, it will improve cell adherence to the seed, and its viscous nature will delay the inoculant’s drying process. Gum arabic is a biopolymer with a significant molecular weight that has high water activity and sticky, emulsifying, and stabilizing qualities which inhibit heat transfer. Sodium alginate is also, a large molecular weight, non-toxic substance with adhesive qualities, inhibit heat transfer, and having high water activity and this has been utilized as a cell protectant in liquid formulations. These characteristics help to promote the inoculant’s long-term survival.110 Easily available, harmless and cheaper cell protectants must be used for the production of liquid bio-fertilizer. Selected cell protectants should not have any adverse effect on the organisms and should have desired physical and chemical properties.98 The microbial density is always higher in liquid bio-fertilizer when compared to carrier based bio-fertilizers. Due to higher microbial densities the amount of liquid inoculum needed for application is less to obtain same effect.89 One kind of liquid bio-fertilizer is the suspension concentrate, which is produced by mixing solid active substances that have a low solubility in water and adequate hydrolysis stability. Suspension concentrations need to be diluted in water before use. Their solubility and storage can be improved by adding surfactants and other substances. The ready-to-use composition known as ultra low volume suspension can also be prepared. This can be sprayed as an extremely fine spray using ultralow volume aerial or ground spray equipment. Mainly the requirement of low volume will be the advantage with this formulation compared to common liquid formulation.
There are several challenges in the liquid bio-fertilizers for maintaining the microbial densities. Storing the liquid bio-fertilizers for more than twelve months leads to deprivation of nutrients for microbial inoculants. Liquid bio-fertilizers need special storage conditions for enhancing shelf life and this condition will not be provided by the farmers.89 For extending the durations of storage, the viability of bio-fertilizer is preserved by combining bacterial cells with polymeric ingredients. The cell protectants improve the bio-fertilizer’s adhesion to seeds and shelf life. Additionally, it was discovered that the production of a liquid bio-fertilizer with a 180 days storage shelf life and outstanding efficiency was achieved by combining glycerol with polyvinyl pyrrolidone.111
Granular formulations
Because of various limitations in carrier based powder formulation and liquid formulation and increasing interest in alternative formulations the granular formulation is highly utilized now-a-days. The granulated bio-inoculants addressed the problems of the traditional formulations.
Granule formulation is a kind of bio-fertilizer because of carriers that is manufactured into tiny particles that resemble grains. In 1990, the granular form was invented. This formulation’s primary goal is to produce dust-free bio-fertilizer without worrying about powder segregation.112 This formulation is crucial in guaranteeing that every granule particle contains the same amount of bio-fertilizer. Granule formulation is usually prepared using carrier materials mixed with the additives and the powder form of concentrated cells of the selected cultures79 and then after thorough mixing it is granulated mechanically. In granular formulations, normally cell count seems to be high. Further based on the form of inoculum addition shelf life can also be extended with this during storage.
Encapsulation of granular inoculant formulations with different polymers and subsequent drying has received more attention for the past ten years. Adjusting the application rates and placements to prevent damage to delicate seed coats, mitigate the harmful effects of pesticides and fungicides on seeds, and lower the possibility of losing viable bacteria through seed drilling equipment or when the seed coat is lifted out of the ground during germination are some benefits of granular inoculants. Granular inoculant formulations made using various grain flours as carriers seem to be a preferable option in this situation than liquid or powder formulations.113
Granulated bio-fertilizers are simpler to handle, store, and apply, and dust free. The restrictions on seed applications are removed, and it is easy to adjust the positioning and application rate: To encourage lateral-root interactions, the inoculant is positioned in a furrow near the seed, but it is kept away from any chemicals or pesticides which are harmful to the microorganisms90 (Table).
Cell coating of granules or spore coating technology
In this technology, the selected carrier materials are first formulated into pellets or granules and then bacterial cultures are coated on the surface of the pellets or granules. Beneficial microbes in agricultural applications are more viable and effective when bacterial cell coating is applied to granular bio-fertilizers. With an importance on enhancing soil health and growth of plants, numerous research have investigated various techniques and materials for producing these coated fertilizers. To improve stability and nutrient release, the majority of techniques involve fermenting organic materials with beneficial microbes, then granulating and coating the substance. These particles improved crop health and nutrient availability by using a variety of organic ingredients to reach a living bacterium population of 62 million CFU/g. A study recorded Serratia entomophila coated granules had higher survival rates, which makes it useful for managing pests such as the New Zealand grass grub.114 Due to quorum sensing (QS) mechanisms, which are essential for the stability of these granules, aerobic granules exhibit enhanced bacterial adhesion and biofilm formation. Granules containing the necessary microorganisms were used in a series of studies to optimize and obtain appropriate coating conditions. For a good formulation, granulating conditions should be optimized. In a study on the production of probiotics, the coating conditions such as inlet air temperature, fluidized air flow rate, atomizer pressure, and spray rate were considered crucial during optimization, since they could impact the viability of probiotics during coating.115 The above method can also be used for bio-fertilizer. Apart from coating of microbial cultures the formulated carriers materials are coated by spores. Some spore producing bacteria such as Bacillus and AMF spores are produced in lab which are used for coating of selected carrier materials.
Encapsulated formulation
These days, immobilized bio-fertilizer is acknowledged as the most advanced bio-fertilizer formulation. Microbial cells are encapsulated and adhered to an insoluble and inert substance in this formulation. The development of bio-fertilizer encapsulation increased the cell’s ability to withstand environmental stress and adverse soil conditions. It is therefore more stable with respect to pH and temperature and more tolerant to changes in its surroundings. Apart from that, immobilization helps release the microbe or enzyme into the soil gradually and steadily. Based on the intended use, encapsulation might contain both macro and microform.111 Natural and synthetic polymers, alginate, carrageenan, agar-agar, and agarose, polyacrylamides, polystyrene, and polyurethane, gums and proteins, carbohydrates, starches and products, humic acid, skim milk, clay and sodium alginate are the most commonly used products for the bio-encapsulation of microorganisms116 (Figure 2). A naturally occurring polymer is alginate which is frequently used for encapsulating microorganisms. It is made up of L-guluronic acid and D-mannuronic acid connected by β-1,4 bonds. Some of the amendments are added to improve the formulation based on alginate’s. Compared to alginate beads alone, the addition of clay and skim milk to the beads dramatically enhanced bacterial survival.83 During formulation, Pseudomonas fluorescens, A. brasilense, and Aspergillus (filamentous fungal) strains were encapsulated. It has been noted that adding beneficial nutrients, like skim milk, can increase the strain’s viability when glycerol is present. They have the greater survivability at adverse conditions. In a study, glycerol-alginate beads exposed under the UV radiation has significantly higher percentage of survival.100
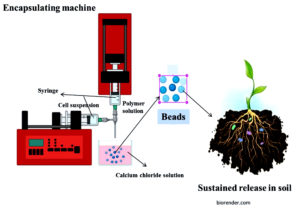
Figure 2. The above picture gives the schematic illustration of encapsulation process of biofertilizer and sustained release in soil. To create stable polymeric beads that encapsulate the cells, the encapsulating machine combines a polymer solution with a microbial cell suspension and extrudes the mixture dropwise into a calcium chloride solution. In order to boost plant growth, encapsulated bio-fertilizers can be applied directly to the soil and release bacteria gradually into the soil, reducing the frequency of applications and increasing the stability of the living cells
During encapsulation the polymer solution and cell suspension homogenized then it is sprayed into hot chamber containing calcium chloride that leads to beads formation. In order to boost plant growth, encapsulated bio-fertilizers can be applied directly to the soil and release bacteria gradually into the soil, reducing the frequency of applications and increasing the stability of the living cells (Figure 2).
By using immobilized-cell technologies, two or more microorganisms can be immobilized. Co-immobilization has been shown to generally lower the production costs and resolve issues with process parameters, nutrient use, oxygen consumption, etc., between co-cultures. The co-immobilization of several microbes in a single porous matrix appears to be a commonly employed technique in certain fermentation processes; nevertheless, it has not been extensively employed in the production of microbial inoculants that are helpful to plants. It is feasible to co-immobilize plant-beneficial microorganisms, and once the immobilized microorganisms are introduced into the plant-soil systems, the required microbial bioactivities are retained.81
The cells undergo chemical solidification after being combined with polymer following mass multiplication. It creates the homogeneous beads that contain living cells (Figure 2). For additional development in the polymer matrix, these beads are fermented and then dried. When these beads are applied, soil microbes break them down and release the confined cells to the soils.83 Encapsulated formulation having higher microbial population compared to liquid and carrier based formulations.117 When the capsules are deposited in the soil, soil microorganisms break them down gradually, releasing the target cells into the soil in enormous quantities over time. This process often occurs during seed germination or seedling emergence (Figure 2).
Cells are not stressed during encapsulation procedures, contamination is minimized by aseptic conditions and the carriers are harmless and biodegradable. Because the beads have a very low volume and can be highly concentrated, handling and transportation are made easier and less space is needed for storage. They are easy to use, having a longer shelf life and are consistently of high quality. They can even be dried and kept at room temperature for longer periods of time. The microencapsulation produces a homogenous distribution of cells around the targeted region, increasing the application efficacy. Thus, there is less chance of off-site drift during application and less cell movement in the soil80 (Table). Immobilization of microbes has several benefits over free-cell systems, including higher inoculant production from increased metabolic activity and stability, relative ease of product separation, enhanced process control, and decreased susceptibility to contaminations. The formulations based on encapsulation (entrapment) within polysaccharide beads, in particular, offer additionally a superior protection of cells against biotic and abiotic stress factors for agricultural and environmental applications81 (Table).
Aggregated formulation
The process of clustering of cells that produce contiguous, fairly stable and multicellular association under liquid culture which is named as bacterial aggregation. Clumping, biofilm coagulation, flocculation are other terms of aggregation. For cell to cell aggregation phenomenon the production of exopolysaccharides and capsular polysaccharides act as a molecular glue. Aggregation of cells are considered as excellent practice for inoculant production, survival during storage and after application in the field. Aggregated cells of Azospirillum grown in 12 different carrier materials that shows the superior survivability in all the carriers and enhance the crop growth and yield in sunflower. The cultures of Azospirillum brasilense, Pseudomonas psychrotolerans and Methylobacterium thiocyanatum shows cell aggregation in low C:N ratio medium. The above cultures are used for the production of the beads through immobilization techniques, in which chitosan was used as carrier material. Encapsulation using aggregated cells always having high microbial load or population in immobilized beads compared to using non aggregated cells. The formation of beads using aggregated cell shows superior enzymes activity and crop growth in rice under pot condition.91
The drawbacks of the encapsulated formulation are the higher cost of polymers than peat-based inoculants, needs greater attention from the sector, require more workers for mass production, the inoculum’s survival is limited by the minimal oxygen transfer. Although there are clear advantages to immobilized-cell formulations of plant-beneficial microbes with regulated cell-release, there are still hurdles to their widespread manufacture and field use. Since the cost of the polymeric carrier is higher than the other components of the solid and liquid formulation, which is one of the primary causes for the comparatively high production cost. Moreover, the structure of a polymer carrier (like alginate) is typified by a low mechanical strength, which dictates an unstable, uncontrollable release of its substance. Another important aspect of the bio-encapsulation process that has been identified is cell death during the drying of encapsulated cells.82 Encapsulated cells may undergo physiological, morphological, and metabolic changes. Since the cells may not establish outside of the beads, successive applications of beads may be necessary79 (Table).
Fluid bed dried inoculants
Fluid bed dryer (FBD) is a dryer in which material is maintained in suspended state against gravity in an upward flowing air stream creating a fluidized condition. Heat is produced by electrical heaters in order to dry the material. As a result of the hot air expanding the material bed at its terminal velocity, turbulence is produced in the final product. This process is termed “fluidization”. The benefit of FBD for bio-inoculant drying is low temperature drying. The product can be dried at 37-38 °C or at ambient temperatures.118 The temperature of the drying chamber is adjustable, and even less temperature can be used for more sensitive organisms. After drying, the moisture content of inoculants reduces to a level that does not allow the contaminants to grow and outcompete the target microorganisms.83
The some of the advantages of the FBD are the relatively little decrease in the number of cells and reported for zero contamination.94 It is possible for mixing many ingredients and drying can be achieved. It is having the advantage of adjusting the drying temperature as necessary.119 After drying different type of products can be made. In case of tablet form fluid bed dried product, it is having the advantage of lesser disintegration time. The hardness of the pellets/granules prepared from the product of fluid bed dryer was less compared to the freeze dryer and hence the disintegration time for the product from the fluid bed dryer was less compared to freeze dryer.
There are some constrains in this type of bio-formulation. The mass production of bio-fertilizer by using fluid bed dryer is very expensive. It needs technically skilled person. The dried cell powder from fluid bed dryer contains lesser microbial cell load compared to the cell powder from the freeze dryer.120 During drying process some species can withstand high temperatures, but it might be challenging for heat sensitive microbes. Compared to sticky carriers, friable carriers can be easier to dry. The inoculant and carrier can be mixed uniformly to speed up drying process. In FBD, blockage is likely to occur. Therefore, it needs to be thoroughly cleaned to prevent blockage.
Research on the field survival of FBD inoculants is necessary. It is necessary to test the response of various crops to FBD inoculants in order to determine their compatibility with crops. To create more appropriate protocols, formulating can be done in various time and temperature cycles. Research on protein profiling and gene expression evaluates how well FBD inoculants stimulate stress-related signalling and how that affects the inoculants’ rhizo competence. Experiments with various additives and carriers can improve the organisms’ efficacy. Since it avoids contamination and minimizes viability loss during storage, this novel approach to biofertilizers may have encouraging results in addressing the issue of inconsistent performance.
Scaling up is the primary issue with industrial fluidized bed dryers because there aren’t many theoretical models that can take the place of costly laboratory experiment. Centrifugal or spinning fluidized bed dryers are still unavailable for industrial usage, despite the successful use of both vibrated and agitated fluidized bed dryers.120
Freeze dried incoulants
Freeze dried products can be prepared using the instrument such as freeze dryers or lyophilizers. Freeze drying can be defined as the drying of the substance by freeing and removing the proportion of any associate solvent by direct sublimation from solid phase to the gaseous phase, without passing through the intermediate liquid phase. Bacterial cells can be freeze-dried using a lyophilizer will be used for storage of the cultures. For freeze drying on high volume, the cell concentrates can be used. Recent days cell concentrates have been prepared using Tangential flow filtration method. The cell concentrates can be mixed with buffers and carriers at certain proportion and the materials are arranged in the trays kept in the instrument. The process was started after the trays were arranged. The three stages of the freeze-drying process are freezing, primary drying, and secondary drying. In a study, the cell dried powder obtained from freeze dryer contains higher microbial cell load compared to the cell powder derived from the fluid bed dryer (Figure 3). The cell count of the final product of freeze dryer was 11.0 per cent higher than the final product from the FBD. Though this preparation contains higher cell load, when it is made into tablets or granules, the hardness was found to be higher and hence it takes more time for disintegration.121
Figure 3. The above image depicts the freeze dried formulation of microbes. This is referred to as freeze dried cell powder biofertilizer formulation which is created by cultivating beneficial microorganisms, harvesting their biomass, and subsequently freeze-drying (lyophilizing) the material. This procedure maintains cell viability, stability, and shelf life by removing water under low temperature and vacuum
It is necessary to know the freeze-dryer’s limitations such as the maximum sublimation and the lowest chamber pressure, time consuming process and highly requiring a technically competent persons to operate the freeze dryer. Another crucial freeze-drying process component is the container closure system. The appropriate selection and characterization of heat and mass transfer in these container closure systems, which may differ greatly from the conventionally used glass vial are crucial for the development and scaling up of the freeze-drying process, in addition to the characterization of the interaction between the product and the system. If a lab-scale dryer’s set cycle time is applied to a pilot or production-scale dryer, the final product might not satisfy the required standards122 and this demonstrate the need for process optimization to pilot scale as well as to the large scale.
Nanoformulation
Nanobiofertilizer means the fertilizer or any supplement needed for plant growth is reduced in size to the nanoscale by reformulating the available powdered solid or liquid biofertilizer. Both chemical and mechanical techniques can be used to obtain the nanoformulation of biofertilizer. These fertilizers provide additional benefits over conventional fertilizers, including a longer shelf life, a lower amount requirement, and the capacity to act as both insecticides and heavy metal scavengers.123 Nano-biofertilizers, whether applied on the leaves, seeds, or soil, have a unique ability to enter plants.124 Nano-bio-fertilizers are a mix of bio-fertilizers and nanofertilizers that are used to increase the overall effect and reduce the negative effects of other type of formulations. Depending on the kind of nanoparticles that contain bio-fertilizers or the bio-fertilizers that stick to nanoparticles, there are a variety of approaches and strategies used to accomplish this. In addition to lowering fertilizer manufacturing costs and possibly lowering the amount of fertilizer that plants need to receive, this invention results in better and more gradual nutrient release characteristics. The gradual release of nutrients also increases the efficacy of the product. Encapsulation incorporates bio-fertilizer into the nanomaterial cover. This method involves the use of starch with a non-toxic material like calcium alginate, which stimulates the growth of bacterial strains93 (Table). Preparing a microbial culture, encapsulating it with nanoparticles, and verifying its effectiveness, quality, and shelf life are the three essential phases in manufacturing a nano-biofertilizer. PGPR suspension is combined with sodium alginate, starch, and bentonite, and then cross-linked with calcium chloride to produce it. Salicylic acid and nanoparticles have also been used to make nano-biofertilizers. This method entails mixing the biofertilizer with salicylic acid, ZnONPs, and sodium alginate, then adding calcium chloride.125 The nanomaterial may improve the dissolution and diffusion of insoluble nutrients in the soil, increase the nutritional bio-availability of soil and plants, and enable the controlled and gradual release of nutrients that are directly absorbed and internalized by plants when applied as a coating or immobilization substrate for bio-fertilizers. Throughout a plant’s life cycle, the amount of nutrients accessible is progressively raised by controlled release.126 Biofertilizers are loaded in nanomaterials. By altering the loading system’s structure, active substances can be released in a variety of ways (particle, emulsion, porous, based, fiber, capsule). Nanoparticles have high specific surface area and small size effect allow them to stick to the target as much as possible, increasing the active components’ bioavailability127 (Figure 4).
Figure 4. The above graphical image schematically shows nanobiofertilizers in different loading techniques. Emulsion-based, capsule-based, porous-based, fiber-based, and particle-based systems are the various loading system of nanobiofertilizers. These delivery methods enhance plant growth and soil health by enabling the regulated release, protection, and improved bioavailability of nutrients or microbial inoculants. The figure also illustrates the dimensional difference between conventional liquid biofertilizer, which measures 1 μm, and nanobiofertilizer, which has a size of 500 nm
Bio-fertilizers are proved as an essential component in organic farming as well as in integrated nutrient management programmes facilitating the agriculture sustainable. These microbial preparations have many advantages for the environment, but they can also lose their effectiveness and degrade over time for a variety of reasons. Low population, short shelf life, contamination, and environmental sensitivity are some of the issues that carrier-based formulations frequently incurred. In order to overcome these problems carriers should have a low carbon-to-nitrogen (C:N) ratio, which preserves microbial viability and improves nutrient availability, and a high water-holding capacity. To increase shelf life, proper storage conditions are crucial, including controlling humidity and temperature. Contamination hazards can be decreased by using hygienic procedures during manufacture and by keeping everything clean at all times. Furthermore, the stability and efficacy of the formulation can be enhanced by avoiding the contaminants as well as improving the moisture content through the addition of some additives. These steps guarantee bio-fertilizers operate better and have a longer shelf life.
Issues with liquid bio-fertilizer formulations include the requirement for appropriate storage conditions to avoid nutrient loss and hypoxia, as well as nutrient depletion, which results in cell death. Preventing contamination and using less expensive ingredients to save production costs are crucial to overcome in liquid inoculant production. Reusing industrial waste can also be an economical and environmentally friendly alternative. The stability of the formulation is improved by adding protectants, which increase the generation of cysts and spores. While preservatives assist retain huge amounts of water, extending shelf life, standardizing the level of additives guarantees uniform performance. The shelf life of liquid bio-fertilizers can be increased by up to two years with concentrated cell suspensions, and long-term stability and efficacy can be greatly increased by mixing different ingredients.
For producing combined granular formulations, each species is separately formulated into granules and then mixed. This ensures the presence of all member of the consortium. Cell coating can also be extended to this granular formulation. Further advantages come from the potential for a biofilm formation by the coated microorganisms, which can improve microbial interactions, encourage nutrient cycling, and boost the bio-fertilizer’s overall effectiveness.
Cell viability loss during the immobilization process is a common problem for immobilized formulations, which might lower the efficacy of the bioformulation. In order to help the organisms stay viable, skim milk can be utilized as a source of nutrients. To further protect the cells during immobilization, cell protectants such as glycerol might be added as amendments. The benefits of co-immobilization include a longer shelf life and delayed release, which enable a more regulated and consistent distribution of living microorganisms. All things considered, immobilization of cells offers a number of advantages over free cell systems since it shields the cells from environmental stressors and enhances their stability and functionality under various circumstances. Aggregated formulation is the novel formulation in bio-fertilizer technology, in which cells are grouped together and become aggregated to improve their contact with one another. This aggregation encourages synergistic activity, which raises enzyme activity and may increase the formulation’s overall efficacy. These aggregated cells are immobilized latter.
Freeze dried and fluid bed dried cells can also be used to prepare various formulation as the way to get increased cell count. However, the high production costs of freeze-dried and fluidized bed inoculants can limit their widespread use. When freeze-dried and fluidized bed dried inoculants are made into granules, they become more stable, easier to distribute, and possibly more cost-effective while still retaining the desired microbial viability and activity. This can make freeze-dried and fluidized bed dried inoculants a more accessible option for large-scale use.
By reformulating the available powdered solid or liquid biofertilizer, the fertilizer or any supplement required for plant growth is decreased in size to the nanoscale, a process known as nanobiofertilizer. The nanoformulation of bio-fertilizer can be produced by mechanical or chemical methods. The nanobiofertilizer may enhance the dissolution and diffusion of insoluble nutrients in the soil, raise the nutritional bio-availability of soil and plants, and allow for the controlled and gradual release of nutrients that are directly absorbed and internalized by plants this may be the future formulations.
Future of the bioformulation depends on the effectiveness as well as the shelf life. Also care should be taken to reduce the bulkiness of the inoculum. Mostly the preferred ones are the concentrated cell powder, which can be easily dispersable at the time of application. At the same the new formulations should also provide the cell load higher than the traditional formulation. These can be taken into account where prepare newer formulations. More research is needed on the development of consortia based formulations having various functions towards plant and soil health.
In conclusion, the continuous advancements in bio-fertilizer technologies are revolutionizing sustainable agriculture by overcoming key limitations, enhancing efficiency, and ensuring long-term viability, thereby contributing significantly to environmental conservation and organic farming practices.
ACKNOWLEDGMENTS
The authors acknowledge the support of Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India, for organizing the technical writing workshops to facilitate the students’ writing this review article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
Not applicable.
ETHICS STATEMENT
Not applicable.
- Vance CP, Graham PH, Allan DL. Biological Nitrogen Fixation: Phosphorus – A Critical Future Need?. In: Pedrosa FO, Hungria M, Yates G, Newton WE. (eds) Nitrogen Fixation: From Molecules to Crop Productivity. Curr Plant Sci Biotechnol Agric, vol 38. Springer, Dordrecht. 2000:509-514.
Crossref - Chen JH. The combined use of chemical and organic fertilizers and/or bio-fertilizer for crop growth and soil fertility. Presented at: International Workshop on Sustained Management of the Soil-Rhizosphere System for Efficient Crop Production and Fertilizer Use. 2006;16(20):1-11.
- Vlek PLG, Vielhauer K. Nutrient management strategies in stressed environments. In: Stressed Ecosystems and Sustainable Agriculture. New Delhi: Oxford and IBH Publishing Co. 1994:203-229.
- Suyal DC, Soni R, Sai S, Goel R. Microbial Inoculants as Biofertilizer. In: Singh D, Singh H, Prabha R. (eds) Microbial Inoculants in Sustainable Agricultural Productivity. Springer, New Delhi. 2016;1:311-318.
- Rokhzadi A, Asgharzadeh A, Darvish F, Nour-Mohammadi G, Majidi E. Influence of plant growth-promoting rhizobacteria on dry matter accumulation and yield of chickpea (Cicer arietinum L.) under field conditions. 2008. https://www.semanticscholar.org/paper/Influence-of-plant-growth-promoting-rhizobacteria-Rokhzadi-Asgharzadeh/c55dcdc78dfd8c5365d8e6b37eeba7ac6f21484d
- Halim NA. Effects of using enhanced bio-fertilizer containing N-fixer bacteria on patchouli growth [doctoral dissertation]. Universiti Malaysia Pahang; 2009.
- El-Yazeid AA, Abou-Aly HE, Mady MA, Moussa SAM. En-hancing Growth, Productivity and Quality of Squash Plants Using Phosphate Dissolving Microorganisms (Bio Phosphor) Combined with Boron Foliar Spray. Res J Agric Biol Sci, 2007;3,274-286.
- Rahim KA. Biofertilizers in Malaysian agriculture: perception, demand and promotion. Presented at: 2002 FNCA Joint Workshop on Mutation and Bio-fertilizer. 2002. https://www.fnca.mext.go.jp/english/bf/country_img/malaysia.pdf
- Mohammadi K, Sohrabi Y. Bacterial biofertilizers for sustainable crop production: a review. ARPN J Agric Biol Sci. 2012;7(5):307-316.
- Nayak T, Patangray AJ. Biofertilizer-beneficial for sustainable agriculture and improving soil fertility. Asian J Multidiscip Stud. 2015;3(2):189- 94.
- Mishra S, Mishra DR, Lee Z, Tucker CS. Quantifying cyanobacterial phycocyanin concentration in turbid productive waters: a quasi-analytical approach. Remote Sens Environ. 2013;133:141-151.
Crossref - Gothwal RK, Nigam VK, Mohan MK, Sasmal D, Ghosh P. Screening of nitrogen fixers from rhizospheric bacterial isolates associated with important desert plants. Appl Ecol Environ Res. 2008;6(2):101-109.
Crossref - Bakulin MK, Grudtsyna AS, Pletneva AY. Biological fixation of nitrogen and growth of bacteria of the genus Azotobacter in liquid media in the presence of perfluorocarbons. Appl Biochem Microbiol. 2007;43(4):399-402.
Crossref - Bhat TA, Ahmad L, Ganai MA, Khan OA. Nitrogen fixing bio-fertilizers; mechanism and growth promotion: a review. J Pure Appl Microbiol. 2015;9(2):1675-1690.
- Mazid M, Khan TA. Future of bio-fertilizers in Indian agriculture: an overview. Int J Agric Food Res. 2014;3(3).
Crossref - Cassan F, Diaz-Zorita M. Azospirillum sp. in current agriculture: from the laboratory to the field. Soil Biol Biochem. 2016;103:117-130.
Crossref - Cecagno R, Fritsch TE, Schrank IS. The plant growth-promoting bacteria Azospirillum amazonense: genomic versatility and phytohormone pathway. Biomed Res Int. 2015;2015(1):898592.
Crossref - Martin del Campo JS, Rigsbee J, Bueno Batista M, et al. Overview of physiological, biochemical, and regulatory aspects of nitrogen fixation in Azotobacter vinelandii. Crit Rev Biochem Mol Biol. 2022;57(5-6):492-538.
Crossref - Schultz B, Boyle C. The endophytic continuum. Mycol Res. 2005;109(6):661-686.
Crossref - Hurek T, Reinhold-Hurek B. Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J Biotechnol. 2003;106(2-3):169-178.
Crossref - Hardoim PR, Hardoim CCP, van Overbeek LS, van Elsas JD. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS One. 2012;7(2):e30438.
Crossref - Kong P, Hong C. Endophytic Burkholderia sp. SSG as a potential biofertilizer promoting boxwood growth. Peer J. 2020;8:e9547.
Crossref - Singh RK, Singh P, Guo DJ, et al. Root-derived endophytic diazotrophic bacteria Pantoea cypripedii AF1 and Kosakonia arachidis EF1 promote nitrogen assimilation and growth in sugarcane. Front Microbiol. 2021;12:774707.
Crossref - Balasubramaniam V, Gunasegavan RDN, Mustar S, Lee JC, Mohd Noh MF. Isolation of industrial important bioactive compounds from microalgae. Molecules. 2021;26(4):943.
Crossref - Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019;9(4):192.
Crossref - Guo S, Wang P, Wang X, Zou M, LIu C, Hao J. Microalgae as Biofertilizer in Modern Agriculture. In: Alam, M., Xu, JL., Wang, Z. (eds) Microalgae Biotechnology for Food, Health and High Value Products. Springer, Singapore. 2020:397-411.
Crossref - Zeilinger-Migsich S, Mukherjee PK. Fungus-fungus interactions. Open Mycol J. 2014;8(Suppl-1, M1):27.
Crossref - Mukherjee S, Sen SK. Exploration of novel rhizospheric yeast isolate as fertilizing soil inoculant for improvement of maize cultivation. J Sci Food Agric. 2015;95(7):1491-1499.
Crossref - Fernandez-San Millan A, Farran I, Larraya L, Arregui LM, Veramendi J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: benefits for seedling development. Microbiol Res. 2020;237:126480.
Crossref - Lonhienne T, Mason MG, Ragan MA, et al. Yeast as a biofertilizer alters plant growth and morphology. Crop Sci. 2014;54(2):785-790.
Crossref - Xiong Y, Liao B, Proffitt E, et al. Soil carbon storage in mangroves is primarily controlled by soil properties: A study at Dongzhai Bay, China. Sci Total Environ. 2018;619-620:1226-1235.
Crossref - Amanullah, Zakirullah M, Khalil SK. Timing and rate of phosphorus application influence maize phenology, yield and profitability in northwest Pakistan. Int J Plant Prod. 2010;4(4):281-292.
Crossref - Chavez-Ortiz P, Tapia-Torres Y, Larsen J, Garcia-Oliva F. Glyphosate-based herbicides alter soil carbon and phosphorus dynamics and microbial activity. Appl Soil Ecol. 2022;169:104256.
Crossref - Naeem A, Akhtar M, Ahmad W. Optimizing available phosphorus in calcareous soils fertilized with diammonium phosphate and phosphoric acid using Freundlich adsorption isotherm. Sci World J. 2013;2013(1):680257.
Crossref - Siedliska A, Baranowski P, Pastuszka-Wozniak J, Zubik M, Krzyszczak J. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021;21(1):1-17.
Crossref - Iftikhar A, Aijaz N, Farooq R, et al. Beneficial role of phosphate solubilizing bacteria (PSB) in enhancing soil fertility through a variety of actions on plants growth and ecological perspective: An updated review. Journal of Xi’an Shiyou University, Natural Science Edition, 2023;19(9):520-547.
- Ortiz A, Sansinenea E. Succinic acid production as secondary metabolite from Bacillus megaterium ELI24. Nat Prod J. 2020;10(2):153-157.
Crossref - Yin Z, Shi F, Jiang H, Roberts DP, Chen S, Fan B. Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil. Can J Microbiol. 2015;61(12):913-923.
Crossref - Dasila H, Sah VK, Jaggi V, et al. Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status. Front Microbiol. 2023;14:1135693.
Crossref - Aseri GK, Jain N, Tarafdar JC. Hydrolysis of organic phosphate forms by phosphatases and phytase producing fungi of arid and semi-arid soils of India. Am Eurasian J Agric Environ Sci. 2009;5(4):564-570.
- Dechavez RB, Serrano AE Jr, Nunal S, Caipang CMA. Production and characterization of phytase from Bacillus spp. as feed additive in aquaculture. Aquac Aquarium Conserv Legis. 2011;4(3):394-403
- Riaz U, Mehdi SM, Iqbal S, et al Bio-fertilizers: Eco-Friendly Approach for Plant and Soil Environment. In: Hakeem, K., Bhat, R., Qadri, H. (eds) Bioremediation and Biotechnology. Springer,. 2020:189-213.
Crossref - Elfiati D, Delvian D, Hanum H, Susilowati A, Rachmat HH. Potential of phosphate solubilizing fungi isolated from peat soils as inoculant biofertilizer. Biodiversitas. 2021;22(6):3042-3048.
Crossref - Zhang Y, Chen FS, Wu XQ, et al. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS One. 2018;13(7):e0199625.
Crossref - Xu X, Mao X, Van Zwieten L, et al. Wetting-drying cycles during a rice-wheat crop rotation rapidly (im) mobilize recalcitrant soil phosphorus. J Soils Sediments. 2020;20(9):3921-3930.
Crossref - Pradhan N, Sukla LB. Solubilization of inorganic phosphates by fungi isolated from agricultural soil. Afr J Biotechnol. 2006;5(10):850-854.
- Burton EM, Knight JD. Survival of Penicillium bilaiae inoculated on canola seed treated with Vitavax RS and Extender. Biol Fertil Soils. 2005;42(1):54-59.
Crossref - Lee EH, Eo JK, Ka KH, Eom AH. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology. 2013;41(3):121-125.
Crossref - Pepe A, Giovannetti M, Sbrana C. Different levels of hyphal self-incompatibility modulate interconnectedness of mycorrhizal networks in three arbuscular mycorrhizal fungi within the Glomeraceae. Mycorrhiza. 2016;26(4):325-332.
Crossref - Kruse J, Abraham M, Amelung W, et al. Innovative methods in soil phosphorus research: A review. J Plant Nutr Soil Sci. 2015;178(1):43-88.
Crossref - Sato T, Hachiya S, Inamura N, Ezawa T, Cheng W, Tawaraya K. Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza. 2019;29(6):599-605.
Crossref - Ibrahim M, Iqbal M, Tang YT, Khan S, Dx G, Li G. Phosphorus immobilization in plant-soil environments and inspired strategies for managing phosphorus: A review. Agronomy. 2022;12(10):2539.
Crossref - Etesami H, Jeong BR, Glick BR. Contribution of arbuscular mycorrhizal fungi, phosphate-solubilizing bacteria, and silicon to P uptake by plant. Front Plant Sci. 2021;12:699618.
Crossref - Cao Y, Wu X, Zhukova A, et al. Arbuscular Mycorrhizal fungi (AMF) species and abundance exhibit different effects on saline-alkaline tolerance in Leymus chinensis. J Plant Interact. 2020;15(1):266-279.
Crossref - Poveda J, Abril-Urias P, Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front Microbiol. 2020;11:992.
Crossref - Santoyo G, Guzman-Guzman P, Parra-Cota FI, et al. Plant growth stimulation by microbial consortia. Agronomy. 2021;11(2):219.
Crossref - Thirkell TJ, Charters MD, Elliott AJ, Sait MS, Field KJ. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J Ecol. 2017;105(4):921-929.
Crossref - Valkov VT, Sol S, Rogato A, Chiurazzi M. The functional characterization of LjNRT2.4 indicates a novel, positive role of nitrate for an efficient nodule N2 fixation activity. New Phytol. 2020;228(2):682-696.
Crossref - Jha Y. Potassium mobilizing bacteria: enhance potassium intake in paddy to regulate membrane permeability and accumulate carbohydrates under salinity stress. Braz J Biol Sci. 2017;4(8):333-344.
Crossref - Zorb C, Senbayram M, Peiter E. Potassium in agriculture: status and perspectives. J Plant Physiol. 2014;171(9):656-669.
Crossref - Singh G, Biswas DR, Marwaha TS. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): a hydroponics study under phytotron growth chamber. J Plant Nutr. 2010;33(8):1236-1251.
Crossref - Rajawat MVS, Singh S, Tyagi SP, Saxena AK. A modified plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere. 2016;26(5):768-773.
Crossref - Sattar A, Naveed M, Ali M, et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl Soil Ecol. 2019;133(1):146-159.
Crossref - Meena VS, Maurya BR, Verma JP. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res. 2014;169(5-6):337-347.
Crossref - Ajmal M, Ali HI, Saeed R, et al. Biofertilizer as an alternative for chemical fertilizers. J Agric Sci. 2018;7(1):1-7.
- Vassileva M, Azcon R, Barea JM, Vassilev N. Rock phosphate solubilization by free and encapsulated cells of Yarowia lipolytica. Process Biochem. 2000;35(7):693-697.
Crossref - Youssef SM, Shaaban A, Abdelkhalik A, et al. Compost and phosphorus/potassium-solubilizing fungus effectively boosted quinoa’s physio-biochemical traits, nutrient acquisition, soil microbial community, and yield and quality in normal and calcareous soils. Plants. 2023;12(17):3071.
Crossref - Lian B, Wang B, Pan M, Liu C, Teng HH. Microbial release of potassium from K-bearing minerals by thermophilic fungus Aspergillus fumigatus. Geochim Cosmochim Acta. 2008;72(1):87-98.
Crossref - Shanware AS, Kalkar SA, Trivedi MM. Potassium solubilisers: occurrence, mechanism and their role as competent biofertilizers. Int J Curr Microbiol Appl Sci. 2014;3(9):622-629.
- Gontia-Mishra I, Sapre S, Tiwari S. Zinc solubilizing bacteria from the rhizosphere of rice as prospective modulator of zinc biofortification in rice. Rhizosphere. 2017;3(Part 1):185-190.
Crossref - Khanghahi MY, Ricciuti P, Allegretta I, Terzano R, Crecchio C. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ Sci Pollut Res. 2018;25(26):25862-25868.
Crossref - Dinesh R, Srinivasan V, Hamza S, et al. Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma. 2018;321:173-186.
Crossref - Othman NMI, Othman R, Zuan ATK, et al. Isolation, characterization, and identification of zinc-solubilizing bacteria (ZSB) from wetland rice fields in Peninsular Malaysia. Agriculture. 2022;12(11):1823.
Crossref - Bist V, Niranjan A, Ranjan M, Lehri A, Seem K, Srivastava S. Silicon-solubilizing media and its implication for characterization of bacteria to mitigate biotic stress. Front Plant Sci. 2020;11:28.
Crossref - Raturi G, Sharma Y, Rana V, et al. Exploration of silicate solubilizing bacteria for sustainable agriculture and silicon biogeochemical cycle. Plant Physiol Biochem. 2021;166:827-838.
Crossref - Vasanthi N, Saleena LM, Raj SA. Concurrent release of secondary and micronutrient by a Bacillus sp. Am Eurasian J Agric Environ Sci. 2012;12(8):1061-1064.
Crossref - Kaur T, Devi R, Kumar S, Sheikh I, Kour D, Yadav AN. Microbial consortium with nitrogen-fixing and mineral solubilizing attributes for growth of barley (Hordeum vulgare L.). Heliyon. 2022;8(4):e09374.
Crossref - Sandini IE, Pacentchuk F, Franco DAS, Sandini AH. Bacterial consortium of Azospirillum brasilense and Pseudomonas fluorescens on the stimulation of growth of corn culture. Cienc Rural. 2024;54(9):e20230123.
Crossref - Herrmann L, Lesueur D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol. 2013;97(20):8859-8873.
Crossref - Rojas-Sanchez B, Guzman-Guzman P, Morales-Cedeno LR, et al. Bioencapsulation of microbial inoculants: mechanisms, formulation types and application techniques. Appl Biosci. 2022;1(2):198-220.
Crossref - Vassilev N, Vassileva M, Lopez A, et al. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl Microbiol Biotechnol. 2015;99(12):4983-4996.
Crossref - Vassilev N, Vassileva M, Martos V, et al. Formulation of microbial inoculants by encapsulation in natural polysaccharides: focus on beneficial properties of carrier additives and derivatives. Front Plant Sci. 2020;11:270.
Crossref - Sahu PK, Brahmaprakash GP. Formulations of Biofertilizers – Approaches and Advances. In: Singh, D., Singh, H., Prabha, R. (eds) Microbial Inoculants in Sustainable Agricultural Productivity. Springer, New Delhi. 2016;2:179-198.
Crossref - Kumareasan G, Sivasakthivelan P, Reetha D, Shanthi R. Efficacy of liquid, gel, and carrier formulation of Azospirillum inoculants on the growth of maize. Plant Arch. 2018;18:350-353.
- Mishra J, Arora NK. Bioformulations for Plant Growth Promotion and Combating Phytopathogens: A Sustainable Approach. In: Arora N, Mehnaz S, Balestrini R. (eds) Bioformulations: for Sustainable Agriculture. Springer, New Delhi. 2016:3-33.
Crossref - Stella M, Theeba M, Illani ZI. Organic fertilizer amended with immobilized bacterial cells for extended shelf-life. Biocatal Agric Biotechnol. 2019;20:101248.
Crossref - Mahanty T, Bhattacharjee S, Goswami M, et al. Biofertilizers: a potential approach for sustainable agriculture development. Environ Sci Pollut Res Int. 2017;24(4):3315-3335.
Crossref - Raimi A, Roopnarain A, Adeleke R. Biofertilizer production in Africa: current status, factors impeding adoption and strategies for success. Sci Afr. 2021;11:e00694.
Crossref - Macik M, Gryta A, Frac M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv Agron. 2020;162:31-87.
Crossref - Herridge DF, Peoples MB, Boddey RM. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil. 2008;311(1):1-18.
Crossref - Gutta S, Kumutha K, Anandham R, Kokila Devi E. Immobilization of aggregated and non-aggregated PGPR cells in chitosan beads to increase efficiency of plant growth promotion in rice [dissertation]. Coimbatore, India: Tamil Nadu Agricultural University. 2019.
- Kumari R, Singh DP. Nano-biofertilizer: an emerging eco-friendly approach for sustainable agriculture. Proc Natl Acad Sci India Sect B Biol Sci. 2020;90(4614):733-741.
Crossref - Ceglie FG, Bustamante MA, Ben Amara M, Tittarelli F. The challenge of peat substitution in organic seedling production: optimization of growing media formulation through mixture design and response surface analysis. PLoS One. 2015;10(6):e0128600.
Crossref - Brahmaprakash GP, Sahu PK. Biofertilizers for sustainability. J Indian Inst Sci. 2012; 92(1):37-62.
- Shravani K, Triveni S, Latha PC, Damodara CK. Evaluation of shelf life and quality of carrier and liquid based biofertilizers. Int J Microbiol Res. 2019;11(6):1598-1601.
- El-Ramady H, El-Ghamry A, Mosa A, Alshaal T. Nanofertilizers vs. Biofertilizers: New Insights. Environment, Biodiversity and Soil Security. 2018;2(1):40-50.
Crossref - Malusa E, Sas-Paszt L, Ciesielska J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J. 2012;2012(1):491206.
Crossref - Saha AK, Deshpande MV, Kapadnis BP. Studies on survival of Rhizobium in the carriers at different temperature using green fluorescent protein marker. Curr Sci. 2001;80(5):69-71.
- Sohaib M, Zahir ZA, Khan MY, et al. Comparative evaluation of different carrier-based multi-strain bacterial formulations to mitigate the salt stress in wheat. Saudi J Biol Sci. 2020;27(3):777-787.
Crossref - Kumar V, Sharma N. Fungal biofertilizers: present trends and future prospects. In: Sharma N, ed. Metabolomics, Proteomes and Gene Editing Approaches in Biofertilizer Industry. Springer Nature Singapore. 2023:189-204.
Crossref - Chaudhary T, Dixit M, Gera R, et al. Techniques for improving formulations of bioinoculants. 3 Biotech. 2020;10:1-9.
Crossref - Kaur R, Kaur S. Carrier-Based Biofertilizers. In: Kaur S, Dwibedi V, Sahu PK, Kocher GS. (eds) Metabolomics, Proteomes and Gene Editing Approaches in Biofertilizer Industry. Springer, Singapore. 2023:57-75.
https://doi.org/10.1007/978-981-99- 3561-1_4 - Stella D, Sivasakthivelan P. Effect of different organic amendments addition into Azospirillum bioinoculant with lignite as carrier material. Bot Res Int. 2009;2(4):229-232.
- Pathirana BKW, Yapa PN. Evaluation of different carrier substances for the development of an effective pelleted biofertilizer for rice (Oryza sativa L.) using co-inoculated bacteria and arbuscular mycorrhizal fungi. Asian J Biotechnol Bioresour Technol. 2020;6(1):1-10.
Crossref - Mugnier J, Jung G. Survival of bacteria and fungi in relation to water activity and the solvent properties of water in biopolymer gels. Appl Environ Microbiol. 1985;50(1):108-114.
Crossref - Singleton P, Keyser H, Sande E. Development and evaluation of liquid inoculants. Inoculants and Nitrogen Fixation of Legumes in Vietnam. 2002;109:52-66.
- Elsakhawy T, Ghazi A, Abdel-Rahman MA. Developing liquid rhizobium inoculants with enhanced long-term survival, storage stability, and plant growth promotion using ectoine additive. Curr Microbiol. 2021;78(10):282-291.
Crossref - Dayamani KJ. Formulization and Determination of Effectiveness of Liquid Inoculants of Plant Growth Promoting Rhizobacteria [doctoral dissertation]. University of Agricultural Sciences GKVK, Bangalore. 2010. https://krishikosh.egranth.ac.in/server/api/core/bitstreams/be6a202b-8f34-4104-a829-d1ebee87a06b/content
- Gopal KS, Baby A. Enhanced shelf life of Azospirillum and PSB through addition of chemical additives in liquid formulations. Int J Sci Environ Technol. 2016;5(4):2023-2029.
- Biradar BP, Santhosh GP. Cell protectants, adjuvants, surfactant and preservative and their role in increasing the shelf life of liquid inoculant formulations of Pseudomonas fluorescens. Int J Pure Appl Biosci. 2018;6(4):116-122.
Crossref - Sarbani NNMM, Yahaya N. Advanced development of bio-fertilizer formulations using microorganisms as inoculant for sustainable agriculture and environment – a review. Malaysian Journal of Science Health & Technology. 2022;8(1):92-101.
Crossref - Bashan Y, de-Bashan LE, Prabhu SR, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013). Plant Soil. 2014;378(1-2):1-33.
Crossref - Swapna G, Divya M, Brahmaprakash GP. Survival of microbial consortium in granular formulations, degradation and release of microorganisms in soil. Ann Plant Sci. 2016;5(5):1348-1352.
Crossref - Wright IJ, Dong N, Maire V, et al. Global climatic drivers of leaf size. Science. 2017;357(6354):917-921.
Crossref - Pyar H, Peh KK. Enteric coating of granules containing the probiotic Lactobacillus acidophilus. Acta Pharm. 2014;64(2):247-256.
Crossref - Cakmakcy R. A review of biological fertilizers current use, new approaches, and future perspectives. Int J Innov Stud Sci Eng Technol. 2019;5:83-92.
- Brindavathy RB, Gnanachitra M. Development of new formulations of phosphorus solubilizing bacteria using spray drying technology. Madras Agric J. 2017;104(7-9):304-307.
Crossref - Sahu PK. Development of Fluid Bed Dried (FBD) Inoculant Formulation of Consortium of Agriculturally Important Microorganisms (AIM) [doctoral dissertation]. University of Agricultural Science. 2012.
- Sahu PK, Lavanya G, Brahmaprakash GP. Fluid bed dried microbial inoculants formulation with improved survival and reduced contamination level. J Soil Biol Ecol. 2013;33:81-94.
- Beham MM, Kumutha K, Subhashini R, Kannan P. Comparison of different formulation of Rhizobia for cell viability. Pharma Innovation. 2021;10(11):260-630.
- Daud WRW. Fluidized bed dryers-recent advances. Adv Powder Technol. 2008;19(5):403-418.
Crossref - Patel DK, Murawala P, Archana G, Kumar GN. Repression of mineral phosphate solubilizing phenotype in the presence of weak organic acids in plant growth promoting fluorescent Pseudomonads. Bioresour Technol. 2011;102(3):3055-3061.
Crossref - Kalia A, Sharma SP, Kaur H. Nanoscale fertilizers: harnessing boons for enhanced nutrient use efficiency and crop productivity. Nanobiotechnol Appl Plant Prot. 2019;2:191-208.
Crossref - Abdelghany AM, El-Banna AA, Salama EA, et al. The individual and combined effect of nanoparticles and biofertilizers on growth, yield, and biochemical attributes of peanuts (Arachis hypogea L.). Agronomy. 2022;12(2):398.
Crossref - Panichikkal J, Prathap G, Nair RA, Krishnankutty RE. Evaluation of plant probiotic performance of Pseudomonas sp. encapsulated in alginate supplemented with salicylic acid and zinc oxide nanoparticles. Int J Biol Macromol. 2021;166:138-143.
Crossref - Sharma R, Malaviya P. Ecosystem services and climate action from a circular bioeconomy perspective. Renew Sustain Energy Rev. 2023;175:113164.
Crossref - An C, Sun C, Li N, et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: strategies towards sustainable agriculture. J Nanobiotechnol. 2022;20(1):11.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.