ISSN: 0973-7510
E-ISSN: 2581-690X
Central bearded dragons, Pogona vitticeps, are ectothermic heliotherms native to dry and arid regions and are often kept as exotic pets in colder less arid countries. They do however, often fall sick resulting in sudden death. This is now known to be caused by bacterial infections such as Listeria monocytogenes and Porphyromonas pogonae sp. This study therefore, aimed to identify microorganisms present in the buccal cavity of the central bearded dragon (P. vitticeps) in Namibia. In order to achieve this, oral swabs were taken from a bearded dragon found in a garden in Windhoek, Namibia and grown in nutrient agar. Colony morphology was described before Gram’s staining to differentiate the bacteria. The automated VITEK®2 system was used to identify the bacteria. On nutrient agar, opaque yellow circular colonies were observed after 24hrs incubation at 37°C. A gram-positive coccus shaped bacteria was observed. VITEK®2 analysis identified the bacteria as Micrococcus luteus. There has not been a study on the microbiota of the buccal cavity of the central bearded dragon in Namibia.
Pogona vitticeps; Micrococcus luteus, buccal microbiota; invasive diseases.
Central bearded dragons, Pogona vitticeps, are ectothermic heliotherms native to dry and arid regions. They are largely herbivorous but may consume insects. When kept as pets, bearded dragons require an optimum diet and living conditions. These include regular water supply and calcium fortified insects1,2. Despite optimum conditions, bearded dragons have been known to suffer from anorexia, lethargy and weight loss with symptoms including dehydration and limited reaction3. When untreated these cases usually lead to death however, sudden death has occurred in some cases soon after the emergence of signs and symptoms of ill health4. Tests done during illness or after death have often revealed gastrointestinal bacterial infections. Porphyromonas pogonae sp. (Porphyromonas spp sometimes known to infect humans as well) and Listeria monocytogenes are some of the common bacterial species found to infect bearded dragons (Fig. 1)2,5.
There is also a growing concern in the increase in fungal infections among reptiles. Bearded dragons are most likely to be infected by opportunistic fungi, however dermatomycoses have been noted to be on the rise6. On the other hand, adenoviral infections are relatively common among reptiles with the greatest number of cases being reported in bearded dragons7. Though the relationship between the bearded dragon and microorganisms is evident not much literature is present on bacterial relations particularly from the oral cavity. To the best of our knowledge, no study has been done to evaluate the oral microbiota of bearded dragons. Therefore, this study aimed to identify buccal cavity bacteria of the central bearded dragon.
Bacterial Isolation
A central bearded dragon (Fig. 1) found in a garden in Windhoek, Namibia was safely captured and transported in a brown bag to the Namibia University of Science and Technology Natural and Applied Sciences laboratory following the institutional rules for the use of animals in research. Oral swabs were taken from the roof, cheek and tongue oral cavity of the bearded dragon. Swabs were immediately inoculated onto nutrient agar plates and incubated at 37°C for 24 hours. The bearded dragon was subsequently released back into the garden. Six selected colonies were sub-cultured onto nutrient agar following a modified description by Kikillus, et al.8.
Identification
Descriptive colony morphology5 was done before differential staining using Gram’s staining9. In order to identify the isolated bacteria, automated biochemical tests were done using VITEK®2 (GP) cards were used depending on the stain result.
VITEK®2 Automated Analysis
Isolated bacteria samples were re-cultured on nutrient agar plates at 37°C for 24hrs before VITEK analysis. Thereafter, a few colonies from each sample were suspended in 0.45% saline solution. An optic density between 0.55 and 0.63 for analysis was preferred. The automated VITEK®2 system performed 43 biochemical tests (Table 1) over a period of 10hrs using gram-positive VITEK®2 cards.
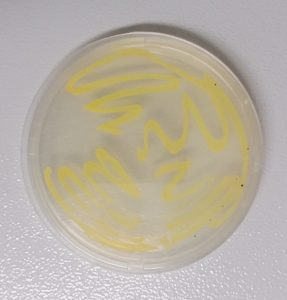
The study aimed to identify microorganisms present in the buccal cavity of P. vitticeps. The selected colony was observed to be yellow with opaque circular colonies. The colonies had entire edges and raised elevations. Gram-staining showed the cells to be gram positive and coccus shaped, with a few tetrads clustered cells. VITEK®2 GP analysis identified the bacteria as Micrococcus luteus. Figure 2 shows the colonies on nutrient agar while Table 1 shows a summary of the results.
Micrococcus luteus is a coccus shaped gram-positive bacteria. It is an aerobic exopolysaccharide producing bacteria that may be found on the skin, mouth and sometimes in the throat of mammals13,14. Oral microbial flora of reptiles includes bacteria from Proteus, Porphyromonas, Micrococcus, Salmonella and Staphylococcus genera5,8,15. Variations, however, may be present among bearded dragons depending on the diet based on whether they are kept as pets (captive) or in the wild16. As such, the oral cavity of snakes and other reptiles alike are more often than not infected by aerobic bacteria17.
Table (1):
Results summary for M. luteus.
| ID | Morphology | Gram stain | Shape | VITEK result | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G4.2 | Yellow, opaque circular colonies with raised elevations and entire edges. | Positive | Coccus | Micrococcus luteus | |||||||
| Biochemical tests | |||||||||||
| Reactions | Result | Acid Production | Result | Growth in 6.5% NaCl | Result | Antibiotic resistance | Result | ||||
| Phosphati-dylinositol phospholipase C | Amygdalin | Single test | – | Polymyxin B | + | ||||||
| Arginine dihydrolase (two tests) | + | Xylose | + | Optochin | + | ||||||
| β-galactosidase | + | α-cyclodextrin | + | Novobiocin | + | ||||||
| α-glucosidase | + | Sorbitol | – | O129 | – | ||||||
| Alanine-phenylalanine-proline arylamidase | – | Galactose | Bacitracin | – | |||||||
| L-aspartic acid | Ribose | + | |||||||||
| Aryl-amidase | Lactate | ||||||||||
| β-galactosidase | + | Lactose | + | ||||||||
| α-mannosidase | N-acetyl-glucosamine | + | |||||||||
| Alkaline phosphatase | Maltose | + | |||||||||
| L-leucine aryl-amidase | Mannitol | + | |||||||||
| Proline arylamidase | Mannose | + | |||||||||
| β-glucuronidase (two tests) | Methyl-β-d-glucopy-ranoside | – | |||||||||
| α-galactosidase | – | Pullulan | – | ||||||||
| L-pyroglutamic acid arylamidase | Raffinose | + | |||||||||
| Alanine arylamidase | Salicin | + | |||||||||
| Tyrosine arylamidase | Sucrose | + | |||||||||
| Andurease | Trehalose | + | |||||||||
Table 1 also indicates the biochemical test results [10–12].
Described species of Micrococcus are often responsible for invasive diseases such as pneumonia, peritonitis and endocarditis among others18. M. luteus has been isolated from the surface of human skin, water and soil19. It has been noted to be an opportunistic bacterium and immunocompromised patients pose the greatest risk of infection20,21. Treatment of M. luteus infection typically involves the use of antibiotics such as penicillin, vancomycin and gentamicin22. Cefoxitin has also been successfully used to treat peritonitis as a result of M. luteus infection23. The pathogenicity of M. luteus is not only of concern to humans. Swabs were collected from crocodiles which were dying suddenly on a crocodile farm. Strains including those of M. luteus isolated from the crocodiles dying suddenly were identified as being pathogenic. Though another bacterium (Edwardsiella tarda) caused death, the presence of M. luteus and other species contributed to ill health24.
This is of particular concern as domestic reptiles kept as pets harbor pathogenic bacteria which may be transferred to humans. Bacteria from the Salmonella species, though intestinal, are the most common pathogens associated with captive reptiles. This runs the risk of infecting other pets within the same household15. Bacterial pathogens in the oral cavity of bearded dragons becomes of great concern in the event that a bite occurs. Lizard bites rarely occur as there are limited human-lizard interactions. However, bacterial infections from lizard bites can occur with one such case being fatal as a result of septicemia25. The same concern is raised in snakes as venom is not the only challenge presented by venomous snakes. As such bacterial infections are often reported following snake bite incidents in cases were the patient survives the bite16,17.
Central bearded dragons harbor aerobic bacteria in their oral cavity, some pathogenic. In this study M. luteus was identified in the buccal cavity of P. vitticeps. Currently, there are no reported cases on bearded dragon bites on humans. However, they are often kept as pets with close interactions with humans therefore, caution should be exercised when handling them, cleaning their cages and feeding them to prevent the possibility of contracting M. luteus. Future work with regards to the P. vitticeps could include identifying more microorganisms from the buccal cavity and gut with special attention to possible pathogenicity.
ACKNOWLEDGMENTS
The authors acknowledge Dr Renatus Peter Shilangale for allowing access to his Laboratory.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
Support from Namibia University of Science and Technology is acknowledged. The grant number is S102.
ETHICS STATEMENT
This article does contain studies with animals performed by the authors in a humane way following approved guide lines by our institutional Ethics committee.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Oldfield CL. Bearded Dragons: Common Husbandry and Nutrition-Related Problems. Vet Nurs J. 2014;29(11):354–7.
Crossref - Matt CL, Ramachandran A, Allison RW, Wall CR, Dieterly AM, Brandao J. Listeria monocytogenes in an Inland Bearded Dragon (Pogona vitticeps). J. Exot. Pet. Med., 2019; 30: 76–81.
Crossref - Collins SN. Diagnostic Challenge: Gastric Neuroendocrine Carcinoma in a Bearded Dragon (Pogona Vitticeps). J. Exot. Pet. Med. [Internet], 2019; 30: 7–11.
Crossref - Schilliger L, Veronique M, Marschang RE, Nicolier A, Richter B. Triple infection with agamid adenovirus 1, Encephaliton cuniculi-like microsporidium and enteric coccidia in a bearded dragon (Pogona vitticeps). Tierarztl Prax Ausgabe K Kleintiere – Heimtiere., 2016; 44(5): 1–4.
Crossref - Kawamura Y, Kuwabara S, Kania SA, Kato H, Hamagishi M, Fujiwara N, et al. Porphyromonas pogonae sp. nov., an anaerobic but low concentration oxygen adapted coccobacillus isolated from lizards (Pogona vitticeps) or human clinical specimens, and emended description of the genus Porphyromonas Shah and Collins 1988. Syst. Appl. Microbiol. [Internet], 2015; 38(2): 104–9.
Crossref - Schmidt V. Fungal Infections in Reptiles-An Emerging Problem. J. Exot. Pet. Med. [Internet], 2015; 24(3): 267–75. https://doi.org/10.1053/j.jepm.2015.06.014
- Hyndman TH, Howard JG, Doneley RJ. Adenoviruses in free-ranging Australian bearded dragons (Pogona spp.). Vet. Microbiol. [Internet], 2019; 234: 72–6.
Crossref - Kikillus KH, Gartrell BD, Motion E. Prevalence of Salmonella spp., and serovars isolated from captive exotic reptiles in New Zealand. N.Z. Vet. J. 2011; 59(4): 174–8.
Crossref - Khalid M, Hassani D, Bilal M, Butt ZA, Hamayun M, Ahmad A, et al. Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch. Oral. Biol. [Internet], 2017; 81: 175–85.
Crossref - Bascomb S, Manafi M. Use of enzyme tests in characterization and identification of aerobic and facultatively anaerobic gram-positive cocci. Clin. Microbiol. Rev., 1998; 11(2): 318–40.
Crossref - Funke G, Funke-Kissling P. Performance of the new VITEK 2 GP card for identification of medically relevant gram-positive cocci in a routine clinical laboratory. J. Clin. Microbiol., 2005; 43(1): 84–8.
Crossref - Wallet F, Loiez C, Renaux E, Lemaitre N, Courcol RJ. Performances of VITEK 2 colorimetric cards for identification of gram-positive and gram-negative bacteria. J. Clin. Microbiol., 2005; 43(9): 4402–6.
Crossref - Pekala A, Pazdzior E, Antychowicz J, Bernad A, Glowacka H, Wiecek B, et al. Kocuria rhizophila and Micrococcus luteus as emerging opportunist pathogens in brown trout (Salmo trutta Linnaeus, 1758) and rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Aquaculture, 2018; 486: 285–9.
Crossref - Garcia-Sanchez AM, Machado-Moreira B, Freire M, Santos R, Monteiro S, Dias D, et al. Characterization of microbial communities associated with ceramic raw materials as potential contributors for the improvement of ceramic rheological properties. Minerals, 2019; 9(316).
Crossref - Ebani VV. Domestic reptiles as source of zoonotic bacteria: A mini review. Asian Pac. J. Trop. Med. [Internet]. 2017; 10(8): 723–8.
Crossref - Artavia-Leon A, Romero-Guerrero A, Sancho-Blanco C, Rojas N, Umana-Castro R. Diversity of Aerobic Bacteria Isolated from Oral and Cloacal Cavities from Free-Living Snakes Species in Costa Rica Rainforest. Int. Sch. Res. Not., 2017; 2017: 1–9.
Crossref - Dehghani R, Sharif MR, Moniri R, Sharif A, Kashani HH. The identification of bacterial flora in oral cavity of snakes. Comp Clin Path., 2016; 25(2): 279–83.
Crossref - Hetem DJ, Rooijakkers SHM, Ekkelenkemp MB. Staphylococci and Micrococci. In: Cohen J, Powderly WG, Opal SM, editors. Infectious Diseases. Elsevier, 2017. p. 1509-1522.e2.
Crossref - Yang S, Sugawara S, Monodane T, Nishijima M, Adachi Y, Akashi S, et al. Micrococcus luteus Teichuronic Acids Activate Human and Murine Monocytic Cells in a CD14- and Toll-like Receptor 4-dependent Manner. Infect Immun. 2001;69(4):2025–30.
Crossref - Babic MN, Zalar P, Enko B, Schroers HJ, Dzeroski S, Gunde-Cimerman N. Candida and Fusarium Species known as Opportunistic Human Pathogens from Customer-Accessible Parts of Residential Washing Machines. Fungal Biol. 2015;119(2–3):95–113.
Crossref - Marashdeh MQ, Gitalis R, Levesque C, Finer Y. Endodontic Pathogens Possess Collagenolytic Properties that Degrade Human Dentine Collagen Matrix. Int Endod J. 2019; 52(4):416–23.
- Martin Guerra JM, Martin Asenjo M, Rodriguez Martin C. Bacteraemia by Micrococcus luteus in an Immunocompromised Patient. Med Clin (Barc) [Internet]. 2019;152(11):469–70.
Crossref - Han SA, Shin BC, Kim H lee, Chung JH. A Case of Micrococcus luteus Relapsing Peritonitis in a Continous Ambulatory Peritoneal Dialysis Patient. Med. J. Chosun Univeristy, 2016; 41(3).
- Guo G, Jiang J, Yang N, Wang P, Zhang L, Wang Y, et al. An investigation of sudden death in farmed infant siamese crocodiles during winter and spring in Hainan, China. Indian J. Anim. Res., 2018; 52(7): 1058–62.
- Kushwaha AS, Pathak A, Kushwaha N. Fatal Monitor Lizard Bite: A Case Report. Int J Nurs Heal Sci. 2015;2(6):70–2.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.