ISSN: 0973-7510
E-ISSN: 2581-690X
Seborrheic dermatitis (SD) is the most common superficial fungal skin infection affecting large number of the population worldwide. Prevalence of this disease has been increased. The present study aimed to formulate topical gel loaded with Ketoconazole (KTZ) as an antifungal agent and black seed oil with anti-inflammatory or antifungal activity to effectively treat seborrheic dermatitis (SD). The dispersion method was used to formulate gel preparations using gelling agents (Carbopol 934, Methylcellulose, and Xanthan gum). The prepared gels were evaluated for organoleptic properties, pH, spreadability, rheology, and drug content. The antifungal assay was performed using Malassezia furfur. In vitro permeation studies were performed on Franz diffusion cells. For drug-excipients compatibility studies, FTIR was performed. According to ICH guidelines, three months of stability studies were performed. The results indicated that gel formulations follow non-Newtonian shear-thinning pseudo-plastic type flow; F2 has high drug content (92.22 ± 0.32%), and the zone of inhibition (22 ± 1.25 mm), and highest drug release (91.88 ± 0.11%). No physicochemical interactions were found between the drug and polymer. Stability studies revealed that the formulations were stable at 25 °C. In conclusion, a gel of KTZ with oil exhibited a synergetic effect and offered a potential SD treatment strategy, suggesting it may be an alternative treatment for SD.
Seborrheic Dermatitis, Ketoconazole, Black Seed Oil, Malassezia furfur, Antifungal Activity, Stability Analysis
At present, fungal skin infections are the most common skin dermatosis. Seborrheic dermatitis (SD) is the most common superficial fungal skin infection affecting 5-10% of the population.1 SD is a chronic, reverting, and inflammatory skin disease in adults and infants on sebaceous regions such as the scalp, face, eyebrows, ears, nasolabial folds, upper chest, anterior back, axillae, and groin regions.2,3 The exact cause is unknown, but generally, yeast Malassezia furfur (previously called Pityrosporum ovale), present in normal flora, is diagnosed as a causative agent.4,5 Malassezia species activates lipase enzyme on the scalp, which causes oxidation of the triglycerides of sebum to produce free saturated and unsaturated fatty acids. As a result, fungi consume saturated fatty acids for self-growth. Unsaturated fatty acids such as oleic acid cause skin irritation, while arachidonic acid activates inflammatory response through prostaglandins and cytokines production and ultimately damage the stratum corneum.6
The other causes may include loss of skin barrier, altering the level of hormones, increased sebum production, neurological disorders, and environmental factors.7 The most common symptom in infants is cradle cap which means yellow and crusty scalp skin.8 Adults’ clinical manifestations include flakes, scaling, pruritus, erythema, yellowish and greasy plaques, and inflammation.9 While in dandruff, only flaking is involved, which makes the difference between SD and dandruff. This disease affects 1% to 3% of the general immunocompetent population while 34% to 83% of immunosuppressed persons and patients with neurological disorders. SD mostly appears in three age groups: during infancy, adolescence, and in persons between 30-60 years.10,11
Treatment interventions available for SD include oral antifungals, topical antifungals, and corticosteroids. In immunosuppressed patients, topical calcineurin inhibitors are used. Over-the-counter shampoos are also used, such as tea tree oil, selenium sulfide, coal tar, and zinc pyrithione.12,13 Nevertheless, these drugs have certain limitations due to side effects such as skin atrophy, hypopigmentation, and hypertrichosis. Furthermore, the use of corticosteroids increases the risk of recurrent infection.14 To overcome these problems, KTZ has been used to treat SD. KTZ is a synthetic broad-spectrum antifungal agent that belongs to azoles (imidazole), with a molar mass of 531 g/mol and a melting point of 146 °C. KTZ inhibits cytochrome P450 enzyme Lanosterol 14α-demethylase, thus inhibiting fungal ergosterol synthesis through inhibition of demethylation of lanosterol to ergosterol, due to this membrane function disrupts and permeability increases.15 KTZ is lipophilic and has a half-life of about 8 h. It belongs to BCS class II because it has low solubility (g/ml) and high permeability. Its absorption occurs in stomach acids and elimination through bile.16-19 Orally KTZ may cause hepatotoxicity; topical administration has been suggested to ascertain that KTZ targets the scalp explicitly and may play an imperative role in treating SD. KTZ has less aqueous solubility, resulting in less dissolution and efficacy, which is a limitation for the topical route. To overcome these problems, formulate gel using penetration enhancers; thus, drug release is faster, and drug and skin penetration contact increases.20
In this study, we formulated a gel of KTZ with the herbal oil of Nigella sativa. The use of oil has been suggested to increase the therapeutic efficacy due to its synergistic antimicrobial effect with KTZ. Nigella sativa, commonly called black seed or black cumin, belongs to the family Ranunculaceae. It was found that black seed oil has antimicrobial activity against Malassezia furfur.21 It is a spice used to remedy certain diseases such as asthma, blood pressure, inflammation, headache, eczema, and dermatitis. The main active constituents found include thymoquinone, dithymoquinone, thymohydroquinone, thymol, and p-cymene. In addition, anti-inflammatory antioxidant, and antimicrobial activities are due to active components such as 3% thymoquinone, low free fatty acids and thymol, etc.22,23 Nigella sativa can also be used as permeation enhancer for lipophilic drugs.24
Different natural or synthetic polymers are used to give the essential structural network required for gels. Carbopol is a synthetic carboxy vinyl anionic polymer of acrylic acid used between 0.5%-2%. It is hydrophilic and used as a gelling agent in gel formulations. It is acidic but viscous formulations after neutralization.25 Methylcellulose (MC) is a cellulose derivative, semi-synthetic non-ionic polymer. MC has thickening, emulsifying, and film-forming properties used in the pharmaceutical industry.26 Xanthan gum (XG) is a natural polysaccharide produced from gram-negative bacterium Xanthomonas campestris NRRL B-1459. It is anionic, non-toxic, and does not cause allergic skin reactions. It is used in gel formulations and this gelling ability is due to different interactions with the water molecules.27,28 As described before, treatment methods include oral or topical therapy.29 Black seed oil was used due to its antimicrobial and penetration enhancing properties.30 Based on the above literature, the present study aims to develop a gel containing KTZ and black seed oil.
Materials
KTZ and Carbopol (934) were received from Briell Pharmaceuticals, Pakistan. Black seed oil was purchased from Sigma Aldrich, Germany. Methylcellulose, xanthan gum, was obtained from Sigma-Aldrich, Germany. Glycerin, propylene glycol, Methylparaben, and Propylparaben were received from Merck, Germany. All other solvents and chemicals, such as Methanol and triethanolamine (for adjustment of pH) used in this research were of analytical grade.
Methods
Preparation of Polymeric Gels
Polymeric gels were prepared by previously published method.31 Briefly, the Carbopol gel was prepared using Carbopol 934 concentrations of 1.25%, 1.5%, and 1.75% (Table 1). Carbopol 934 was soaked in distilled water for 24 h. After 24 h, this gel base was stirred using a homogenizer (IKA Eurostar 40 Digital) at 1200 rpm for 30-60 min.
Table (1):
Formulation design of topical gels, used various combination of formulation components
Ingredients |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
Control |
|---|---|---|---|---|---|---|---|---|---|---|
Ketoconazole (w/w) |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
Black seed oil (w/w) |
0.25 |
0.35 |
0.45 |
0.25 |
0.35 |
0.45 |
0.25 |
0.35 |
0.45 |
– |
Carbopol 934 (%) |
1.25 |
1.5 |
1.75 |
– |
– |
– |
– |
– |
– |
1.5 |
Methyl cellulose (%) |
– |
– |
– |
– |
– |
– |
1.0 |
1.5 |
2.0 |
– |
Xanthan gum (%) |
– |
– |
– |
1.0 |
1.5 |
2.0 |
– |
– |
– |
– |
Glycerin |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
Propylene glycol |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
Methyl paraben (%) |
0.03 |
0.03 |
0.03 |
0.03 |
0.03 |
0.03 |
0.03 |
0.03 |
0.03 |
0.03 |
Propyl paraben (%) |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
Distilled water |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
The same procedure was repeated for MC and XG gel base, but their concentrations, soak time, and mixing rate differed. The MC gel was prepared by using concentrations 1.0%, 1.5% and 2.0%. MC was soaked in distilled water for 30-120 min. After 2 h, this gel base was stirred using a homogenizer (IKA Eurostar 40 Digital) at 800-1200 rpm for 30-60 min. The XG gel was prepared using 1.0%, 1.5%, and 2.0% concentrations. XG was soaked in distilled water for 30-120 min. After 2 h, this gel base was stirred using a homogenizer (IKA Eurostar 40 Digital) at 600-800 rpm for 30-60 min. KTZ and black seed oil were dissolved in glycerin and propylene glycol. Then this drug solution was added slowly to the gel base with continuous stirring by a homogenizer for 10 min. After that, preservatives, methyl, and propylparaben were added. The final weight of the gel was made by using a sufficient quantity of distilled water. The acidic nature of Carbopol was neutralized by adding triethanolamine dropwise till a clear gel was obtained. The gel was left for 24 h for equilibration.32,33 The gel was stored in a glass container and placed in a dark place till further analysis. The schematic diagram for the preparation of polymeric gels is shown in supplementary Figure 1.
Characterization of polymeric gels
Organoleptic properties
Each gel formulation was placed in a glass container and visually observed for its colour, odour, homogeneity (means no lumps present), grittiness (presence of any sandy particle), and consistency. Consistency was checked by applying gel on the backside of the hand.
pH determination
The pH of each formulation was determined by using a digital pH meter (Horiba Scientific) at 25 °C. The gel was placed in a container, and dipped the pH meter’s electrode in the jar for one minute to calibrate it. The average of three readings was taken and calculated as a result.34
Spreadability test
This test was performed to check the degree of spread of gel on the skin surface. A suitable quantity of each formulation was placed between two glass slides. One glass slide was marked with a circle, and a 0.5-1 g formulation was placed. The standard weight of 125 g was placed on the upper slide and waited for 5 min. After 5 min, the time required to separate glass slides was measured. Three readings were taken, and spreadability was computed by using the formula:
S = M × L / T
Where,
S = Spreadability
M = Standard weight on the upper slide (grams)
L = Length of a glass slide (cm)
T = Time taken by slides for separation (sec)
Rheological studies
In this, the viscosity of each gel formulation was determined by using a rotational viscometer (Brookfield RVDV-II+P). The gel was placed in a beaker. This test was carried out by using spindle 6 at 5-100 rpm at 25 °C. All the data were presented as mean ± standard deviation (±SD). A rheogram was plotted between rpm and viscosities.35
Drug content
The drug content of the best formulations was measured by taking a known quantity of gel in 10 ml of phosphate buffer solution of pH 6.8 and mixing thoroughly for 15 min to dissolve the drug completely. From this solution, 1 ml was taken and filtered by using membrane filters. Then it was further diluted by using 100 ml of phosphate buffer solution of pH 6.8. The absorbance was measured at a wavelength of 246 nm using a UV-Vis spectrophotometer (UV-1602 of BMS) and then calculated % age drug content.
Antifungal assay
The antifungal study of best formulations was performed using Malassezia furfur’s well-diffusion method. Sabouraud dextrose agar media was used as a medium inoculated with test fungus strain by streak plate method in a triplicate manner. Wells were made in inoculated Petri dishes, and each formulation was placed in these wells. The Petri dishes were placed in an incubator at 37 ± 2 °C for 24-48 h. After this, the zone of inhibition was measured in millimetres and compared with the positive control formulation, which has no black seed oil.36,37
In vitro permeation studies
The in vitro permeation studies were performed on best formulations in Franz diffusion cell (PermeGear) through cellulose acetate membrane. This membrane was mounted between the donor and receptor compartment, and 1 ml of gel was placed above the membrane in the donor compartment. The receptor compartment contains a phosphate buffer solution of pH 6.8 with continuous stirring at 600 rpm at 37 ± 1 °C. In the next step, 1-2 ml of aliquot was taken at regular intervals of 0.5, 1, 1.5, 2, 3, 4, and 6 h. After taking each sample, fresh phosphate buffer solution was added to the dissolution medium to maintain the constant volume. Samples were diluted, and absorbance was measured using a UV-Vis spectrophotometer (UV-1602 of BMS) at 246 nm. The percentage of drug release was plotted against time.
Drug release kinetics
The in vitro drug release data obtained from experiments were analyzed using equations of zero order, first order, Higuchi, Hixson-Crowell, and Korsmeyer-Peppas models to determine the drug release kinetics linear regression analysis. In the Korsmeyer-Peppas model, the value of n represents release kinetics; if the value of n < 0.5 or equal to 0.5, it indicates Fickian diffusion, but if the value of n is 0.5-1, then it indicates Non-Fickian diffusion.38
Fourier Transform Infrared spectrophotometer (FTIR)
The Fourier Transform Infrared spectrophotometer (Bruker) was used to check the compatibility between drugs and polymers. IR spectra of pure KTZ, black seed oil, polymer, and best formulations were recorded. The samples were prepared in KBr discs of IR grade (2 mg sample in 200 mg KBr). These discs were then scanned at wavenumber 4000-400 cm-1 with resolution 4 cm-1.39
Stability studies
According to ICH guidelines, the best gel formulation was chosen for accelerated stability studies. Therefore, this formulation was stored for three months at 8 °C, 25 °C, and 40 °C. The formulation was then observed for organoleptic properties, pH, spreadability, rheological studies, and drug content.40
Synthesis and characterization of gels
Topical gels were prepared by dispersion by adding drug solution into soaked gelling agent solutions (Carbopol, MC, and XG) at different concentrations with continuous stirring. The final formulation also contained glycerin and propylene glycol as a moistening agent, methyl or propylparaben as a preservative, and triethanolamine to maintain the pH of Carbopol gel.
Organoleptic properties
The gel formulations were evaluated for organoleptic properties shown in supplementary Table 1. All the gels were odorless, had no grittiness, and were homogeneous except F6, clumpy. The color was inspected visually against the white background. The F1, F2, and F3 were white, while all remaining was light yellow to yellow translucent. All the gels have semisolid consistency except F4 and F5, which were thin, while F9 was thick inconsistency. The texture of the gel is based on the amount of gelling agent.
pH determination
The pH of gels was checked to determine that either the gel is acidic or basic. The pH values of the prepared gels were found to be 4.9-6.0. The pH of gel formulations is given in in supplementary Table 1. The pH range is near the skin; hence, the formulations were non-irritant except F4, out of range 4.9. Carbopol is acidic; therefore, its pH was adjusted using a pH neutralizer, triethanolamine. These pH values are acceptable to avoid irritation upon application to the skin.41
Spreadability test
This test was performed to check the degree of spread of gel on the skin’s surface. All gel preparations’ spreadability was 13.3-16.7 g.cm/s as shown in supplementary Table 1, except F9, which was 10.40 g.cm/s because of its high viscosity. Spreadability also plays a role in patient compliance and helps in the uniform application of gel to the skin. It depends on the type and quantity of the polymers used.42 When measured in g.cm/s, if the viscosity is less than the slides require lesser time to separate, and spreadability is high; there is an inverse relation between them. This value showed that greater force is required to spread the gel on the skin surface. The Carbopol gel has optimized spreadability because of its suitable viscosity.43
Rheological studies
A rheogram was constructed between rpm and viscosities. The viscosities of all prepared gel formulations were found between 1044-13445 cps. The values of viscosities of all formulations indicated that they followed non-Newtonian shear thinning pseudo-plastic type flow means viscosity decreased with the increase in rpm or shear rate (Figure 1). Viscosity also depends upon the concentration and type of polymer used. As the polymer concentration increased, it was supposed that the viscosity also increased.
Figure 1. Rheological Behaviors of (A) Carbopol Gel, (B) Xanthan Gum and (C) Methyl Cellulose, respectively at various rpm
Different gelling agents were used to select the best gelling agent. Gels containing 1.0% and 1.5% of XG form a fragile gel that liquefies within 6 h. The better gel was prepared with a 2.0% gelling agent, but it also softens after 24 h and forms clumps. Gels containing 1.0% and 1.5% MC showed phase separation and were rejected. Compared to a 2.0% gelling agent, the gel was thick and more sticky and could not be adequately spread out as indicated by spreadability and viscosities values. Carbopol 934 formed more uniform gels that do not liquefy upon keeping and showed better visual appearance, pH, spreadability, and viscosity. Thus, Carbopol 934 gels were considered optimized and used for further studies. The rheological studies were performed, valid for the therapeutic effect of storage conditions and application on the skin surface.44 When rpm had increased, the particles of gelling agent aligned due to this internal resistance decreased, and hence the viscosity decreased. F1, F2, and F3 were considered optimized and selected for further studies based on the above characterization parameters.
Drug content
The %age of drug content was observed between 85.5-92.2% (Table 2), It is necessary to ascertain the topical formulation’s drug content and make sure the right amount is present in product. The formulation F2 has the highest drug content of 92.22 ± 0.32%., indicated a uniformity of content in the gel formulations.45,46
Table (2):
Drug Content (%) and Zone of Inhibition of Gels
Formulations |
Drug Content (%) |
Zone of Inhibition (mm) |
|---|---|---|
F1 |
85.54 ± 1.01 |
20 ± 1.25 |
F2 |
92.22 ± 0.32 |
22 ± 1.25 |
F3 |
90 ± 0.35 |
21 ± 1.25 |
F4 |
– |
19 ± 1.25 |
Antifungal assay
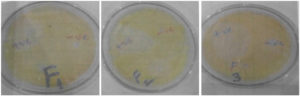
The antifungal assay of selected formulation based on above results was performed and compared with positive control (no black seed oil) Figure 2. The results were in an acceptable range, F2 with a high inhibition zone of 22 ± 1.25 mm, while the positive control has less zone of inhibition of 19 ± 1.25 mm (Table 2). This difference was because of black seed oil presence in F2. Hence, adding black seed oil with KTZ gel potentiates the antifungal results to treat dermatological infections.47
Figure 2. Antifungal activity of F1, F2 and F3 gels against Malassezia furfur’s well-diffusion method
In vitro permeation studies
The F1 and F3 release 70.88% ± 0.54 and 58.77% ± 0.69 drugs in 6h; on the other hand, the F2 releases 91.88% ± 0.11 drugs in 6 h Supplementary Figure 2. After 6 h, F2 has the highest percentage of drug release of 91.88 ± 0.11%, while F1 has 70.88 ± 0.11% and F3 has 58.77 ± 0.11%. Although F3 has a high quantity of oil compared to F2, the polymer concentration was high in this formulation, which halted the release of a drug because of increased viscosity or difficulty in water penetration. In F1, the polymer concentration was less; hence, drug release was approximately high compared to F3, but there was also difficult due to less oil quantity.
Drug release kinetics
Different dissolution kinetics models were applied to optimized formulations, as shown in Table 3, and the results indicated that F2 follows the first-order kinetic model because it had a high R2 value.48 The kinetic results indicated that F1 and F3 followed Higuchi kinetic model because its R2 value was higher than the other kinetics models. In contrast, F2 followed the first-order kinetic model due to its high R2 value. Additionally, the data were also fitted in Korsmeyer-Peppas kinetic model. The value of n described the release process of the drug. For F1 and F2, the exponent value was noted as n < 0.5, and it showed the Fickian diffusion, while for F3, n > 0.5, it followed the Non-Fickian distribution.38
Table (3):
Kinetic Evaluation of F1, F2 and F3 formulation Gels
| Formulations | Zero Order | First Order | Higuchi Order | Hixson-Crowell | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| k0 | R2 | k1 | R2 | kH | R2 | kHC | R2 | kKP | N | |
| F1 | 15.06 | 0.59 | 0.27 | 0.91 | 30.74 | 0.98 | 0.07 | 0.85 | 32.43 | 0.45 |
| F2 | 20.40 | 0.32 | 0.58 | 0.96 | 42.39 | 0.93 | 0.16 | 0.90 | 49.80 | 0.36 |
| F3 | 11.76 | 0.83 | 0.17 | 0.96 | 23.42 | 0.97 | 0.05 | 0.93 | 20.87 | 0.59 |
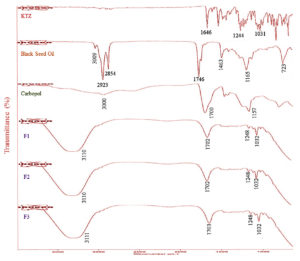
Fourier Transform Infrared Spectrophotometer (FTIR)
The FTIR spectra of pure KTZ, black seed oil, Carbopol, F1, F2, and F3 are shown in Figure 3. The IR spectrum of pure KTZ powder showed characteristic absorption peaks at 1646 cm-1, 1244 cm-1, and 1031 cm-1 for C=O, cyclic and aliphatic ether stretching, as shown in Figure 3.49,50 The FTIR spectra of optimized gels were slightly different from the pure drug, but no such interaction was observed.
The IR spectrum of black seed oil showed a band at 3009 cm-1, which weak stretching the alkene group. Due to aliphatic stretching, it showed two firm peaks at 2923 cm-1 and 2854 cm-1. Two peaks were found due to the ketone C=O group stretching at 1746 cm-1 and the C-H scissoring effect at 1463 cm-1. Two different peaks, at 1165 cm-1 due to C-O and at 723 cm-1, were also found, shown in Figure 3.51 In the IR spectrum of Carbopol 934, a band due to the stretching of -OH group of Carboxylic acid was shown in 3000-1940 cm-1. A characteristics absorption band due to C=O stretching appeared at 1700 cm-1. A prominent peak was found at 1157 cm-1 due to C-C bonding stretching.52 Best formulations (F1, F2, and F3) were also scanned for the same region, and the IR spectrum was shown in Figure 3. No such shift was indicated in all KTZ and Carbopol absorption band formulations, while a shift appeared in black seed oil absorption peaks. However, this shift was slightly more prominent in F3, which may be due to the increased concentration of polymer. The band’s appearance at 1248 cm-1, 1032 cm-1, and 1702 cm-1 also revealed the non-reactivity of KTZ and Carbopol. The intensity of peaks reduced but no such interactions were found between drug, oil and polymer.
Stability studies
The accelerated stability studies of the F2 formulation were performed at various temperatures Supplementary Table 2. There were no changes observed in organoleptic properties, pH, spreadability, rheology and drug content at 8 °C and 25 °C, but at 40 °C, slightly notable changes occurred in color, consistency and rheology (Supplementary Table 2). Hence, 25 °C is considered the appropriate temperature for topical gel storage.53
In conclusion, the current study revealed that the gel formulation of KTZ shows a synergistic affect along with black seed oil. The formulated gels were evaluated for organoleptic properties, pH, spreadability, rheology, drug content, antifungal activity, in vitro permeation studies, FTIR and stability studies. Among all the formulated gels, F2 showed the best results with high drug content, antifungal activity, and percent drug release. The antifungal activity of the F2 showed an excellent therapeutic efficacy towards the treated organism. The stability studies results indicated that 25 °C is the suitable temperature for storage. The results are encouraging, and further studies are required to explore its true potential. Hence, it may be concluded that F2 can be used to cure patients suffering from SD.
Additional file: Additional Table S1-S2 and Figure S1-S2.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA, for funding this research work through the project number NBU-FFR-2025-3336-02.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MIS conceptualized the study and performed supervision. AA, HWA, SAA, AWA, ZR, and MI wrote the manuscript. HWA, SAA, AWA, ZR, KR and MI reviewed, edited and approved the final manuscript for publication.
FUNDING
This study was funded by Deanship of Scientific research at Northern Border University, Arar, KSA, through the project number NBU-FFR-2025-3336-02.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Han SH, Hur MS, Kim MJ, et al. In vitro Anti-Malassezia Activity of Castanea crenata Shell and Oil-Soluble Glycyrrhiza Extracts. Ann Dermatol. 2017;29(3):321-326.
Crossref - Harada K, Saito M, Sugita T, Tsuboi R. Malassezia species and their associated skin diseases. J Dermatol. 2015;42(3):250-257.
Crossref - Dessinioti C, Katsambas A. Seborrheic dermatitis: Etiology, risk factors, and treatments: Facts and controversies. Clin Dermatol. 2013;31(4):343-351.
Crossref - Sowell J, Pena SM, Elewski BE. Seborrheic dermatitis in older adults: Pathogenesis and treatment options. Drugs Aging. 2022;39(5):315-321.
Crossref - Saunte DML, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;10:112.
Crossref - Gupta AK, Richardson M, Paquet M. Systematic review of oral treatments for seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2014;28(1):16-26.
Crossref - Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet], 2020.
- Dall’Oglio F, Nasca MR, Gerbino C, Micali G. An overview of the diagnosis and management of seborrheic dermatitis. Clin Cosmet Investig Dermatol. 2022:1537-1548.
Crossref - Wang J, Zhang Y, Li B, et al. Eczema, facial erythema, and seborrheic dermatitis symptoms among young adults in China in relation to ambient air pollution, climate, and home environment. Indoor Air. 2022;32(1):e12918.
Crossref - Wikramanayake TC, Borda LJ, Miteva M, Paus R. Seborrheic dermatitis-Looking beyond Malassezia. Experimen Dermatol. 2019;28(9):991-1001.
Crossref - Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2):1-22.
Crossref - Mangion SE, Mackenzie L, Roberts MS, Holmes AM. Seborrheic dermatitis: Topical therapeutics and formulation design. Eur J Pharm Biopharm. 2023;185:148-164.
Crossref - Thomas LM, Khasraghi AH. Topical treatment of seborrhoeic dermatitis and dandruff: An overview. Ann Trop Med Pub Health. 2020;23(11):231-823.
Crossref - Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91(3):185-190.
- Endo EH, Makimori RY, Companhoni MVP, Ueda-Nakamura T, Nakamura CV, Filho BPD. Ketoconazole-loaded poly-(lactic acid) nanoparticles: Characterization and improvement of antifungal efficacy in vitro against Candida and dermatophytes. J Mycol. 2020;30(3):101003.
Crossref - Lopes MS, Catelani TA, Nascimento ALCS, Garcia JS, Trevisan MG. Ketoconazole: compatibility with pharmaceutical excipients using DSC and TG techniques. J Thermal Anal Calorim. 2019;141(4):1-8.
- Choi FD, Juhasz MLW, Mesinkovska NA. Topical ketoconazole: a systematic review of current dermatological applications and future developments. J Dermatol Treat. 2019;30(8):760-771.
Crossref - Jacobs GA, Gerber M, Malan MM, du Preez JL, Fox LT, du Plessis J. Topical delivery of acyclovir and ketoconazole. Drug Deliv. 2016;23(2):631-641.
Crossref - Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45(6):737-779.
Crossref - Namburi BBN, Yadav HKS, Hemant S, Ahmed A, Sureddy VL, Shivakumar HG. Formulation and evaluation of polymeric nanoparticulate gel for topical delivery. Int J Polym Mater Po.. 2014;63(9):476-485.
Crossref - Ogen-Shtern N, Margarita Y, von Oppen-Bezalel L. Antimicrobial activity by a unique composition of cold pressed Nigella sativa seed (black cumin) oil. Food Sci Nutr Res. 2021;4(2):1-9.
Crossref - Singh S, Das SS, Singh G, Schuff C, de Lampasona MP, Catalan CAN. Composition, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.). Biomed Res Int. 2014;2014(1): 1-10.
Crossref - Von Oppen-Bezalel L, Jurenka JS. Irritated, Itchy, Scaly, Seborrheic Scalp: Causes and Relief with a Proprietary, Cold-Pressed Nigella sativa (Black Seed) Oil Standardized to 3% Thymoquinone. SOFW Journal. 2022;148.
- Amra K, Momin M, Desai N, Khan F. Therapeutic benefits of natural oils along with permeation enhancing activity. Int J Dermatol. 2022;61(4):484-507.
Crossref - Das B, Nayak AK, Nanda U. Topical gels of lidocaine HCl using cashew gum and Carbopol 940: preparation and in vitro skin permeation. Int J Biol Macromol. 2013;62:514-517.
Crossref - Jain S, Sandhu PS, Malvi R, Gupta B. Cellulose derivatives as thermoresponsive polymer: an overview. J Appl Pharm Sci. 2013;3(12):139-144.
- Prajapati VD, Jani GK, Moradiya NG, Randeria NP. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohyd Polym. 2013;92(2):1685-1699.
Crossref - Petri DFS. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J Appl Polym Sci. 2015;132(23).
Crossref - Thomas LM, Khasraghi AH. Nanotechnology-Based Topical Drug Delivery Systems for Management of Dandruff and Seborrheic Dermatitis: An overview. Iraqi J Pharm Sci. 2020;29(1):12-32.
Crossref - Mirzaii M, Yaeghoobi M, Afzali M, Amirkhalili N, Mahmoodi M, Sajirani EB. Antifungal activities of quince seed mucilage hydrogel decorated with essential oils of Nigella sativa, Citrus sinensis and Cinnamon verum. Iranian J Microbiol. 2021;13(3):352-359.
Crossref - Ubaid M, Ilyas S, Mir S, et al. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An Acad Bras Cienc. 2016;88(4):2303-2317.
Crossref - Helal DA, El-Rhman DA, Abdel-Halim SA, El-Nabarawi M. Formulation and evaluation of fluconazole topical gel. Int J Pharm Pharm Sci. 2012;4(5):176-183.
- Kaur M, Singh K, Jain SK. Luliconazole vesicular based gel formulations for its enhanced topical delivery. J Liposome Res. 2019;30(4):388-406.
Crossref - Sadozai SK, Khan SA, Baseer A, Ullah R, Zeb A, Schneider M. In vitro, ex vivo, and in vivo evaluation of nanoparticle-based topical formulation against Candida albicans infection. Front Pharmacol. 2022;13:909851.
Crossref - Shetty S, Jose J, Kumar L, Charyulu RN. Novel ethosomal gel of clove oil for the treatment of cutaneous candidiasis. J Cosmet Dermatol. 2019;18(3):862-869.
Crossref - Borse VA, Gangude AB, Deore AB. Formulation and evaluation of antibacterial topical gel of doxycycline hyclate, neem oil and tea tree oil. Indian J Pharm Educ. 2020;54(1):206-212.
Crossref - Tripathi H, Choukse R, Patel R, Sonwane A. Formulation and Evolution of Ketoconazole Hydrogel Gel Topical Delivery System against Fungal Infection. Int J Pharm Life Sci. 2021;12(9):74.
- Aslani A, Malekpour N. Design, formulation, and physicochemical evaluation of periodontal propolis mucoadhesive gel. Dent Res J. 2016;13(6):484.
Crossref - Kacso I, Rus LM, Martin F, Miclaus M, Filip X, Dan M. Solid-state compatibility studies of Ketoconazole-Fumaric acid co-crystal with tablet excipients. J Thermal Anal Calorim. 2020;143(1):1-8.
Crossref - Mahtab A, Anwar M, Mallick N, Naz Z, Jain GK, Ahmad FJ. Transungual delivery of ketoconazole nanoemulgel for the effective management of onychomycosis. AAPS PharmSciTech. 2016;17(6):1477-1490.
Crossref - El-Housiny S, Eldeen MAS, El-Attar YA, et al. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study. Drug Deliv. 2018;25(1):78-90.
Crossref - Maslii Y, Ruban O, Kasparaviciene G, et al. The influence of pH values on the rheological, textural and release properties of Carbomer Polacril® 40P-based dental gel formulation with plant-derived and synthetic active components. Molecules. 2020;25(21):5018.
Crossref - Imam SS, Gilani SJ, Zafar A, Jumah MN, Alshehri S. Formulation of miconazole-loaded chitosan-carbopol vesicular gel: optimization to in vitro characterization, irritation, and antifungal assessment. Pharmaceutics. 2023;15(2):581.
Crossref - Jain S, Patel N, Madan P, Lin S. Formulation and rheological evaluation of ethosome-loaded carbopol hydrogel for transdermal application. Drug Dev Ind Pharm. 2016;42(8):1315-1324.
Crossref - Nikumbh KV, Sevankar SG, Patil MP. Formulation development, in vitro and in vivo evaluation of microemulsion-based gel loaded with ketoprofen. Drug Deliv. 2015. 22(4):509-515.
Crossref - Bayan MF, Chandrasekaran B, Alyami MH. Development and Characterization of Econazole Topical Gel. Gels. 2023;9(12):929.
Crossref - Coneac G, Vlaia V, Olariu I, et al. Development and evaluation of new microemulsion-based hydrogel formulations for topical delivery of fluconazole. Aaps PharmSciTech. 2015;16(4):889-904.
Crossref - Singh S, Verma D, Mirza MA, et al., Development and optimization of ketoconazole loaded nano-transfersomal gel for vaginal delivery using Box-Behnken design: In vitro, ex vivo characterization and antimicrobial evaluation. J Drug Deliv Sci Technol. 2017;39:95-103.
Crossref - Amra K, Momin M. Formulation evaluation of ketoconazole microemulsion-loaded hydrogel with nigella oil as a penetration enhancer. J Cosmet Dermatol. 2019;18(6):1742-1750.
Crossref - Jadhao UT, Sayali RP, Gunesh DN, Shital SD, Sneha LS. Formulation and evaluation of nanosponge gel containing ketoconazole. Innov Pharm Pharmacother. 2021;9(1):15-24.
- Mohammed SJ, Amin HHH, Aziz SB, et al. Structural characterization, antimicrobial activity, and in vitro cytotoxicity effect of black seed oil. Evid-Based Compl Alt. 2019;2019(1):6515671.
Crossref - Ali A, Khalid I, Minhas MU, et al. Preparation and in vitro evaluation of Chondroitin sulfate and carbopol based mucoadhesive controlled release polymeric composites of Loxoprofen using factorial design. Eur Polym J. 2019;121:109312.
Crossref - Viljoen JM, Botes D, Steenekamp JH. Formulation and evaluation of selected transmucosal dosage forms containing a double fixed-dose of acyclovir and ketoconazole. Eur J Pharm Sci. 2018;111:503-513.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.