Globally, Tuberculosis (TB) is one of the leading prevalent illnesses. Researchers have been working efficiently to prevent TB in recent decades. The innate and adaptive immune mechanism of host are compromised by diabetes mellitus (DM), which reduces their ability to eradicate Mycobacterium tuberculosis (MTB) bacilli. This may significantly enhance the likelihood for contracting TB. The WHO has suggested a number of important intervention techniques to lessen this dual burden, most notably the creation of cooperative control programs, TB diagnosis and treatment in people with DM, as well as DM detection and treatment in patients with TB. The co-occurrence of DM and TB is an increasing worldwide health problem, therefore serving as the rationale for this study. As DM reaches epidemic proportions globally and TB remains a major infectious cause of death, their bidirectional relationship poses a critical public health concern. TB patients are more prone to diabetes because of their weakened immune systems, in accordance to numerous research findings. Angiogenesis-inflammation nexus indicators, such as elevated levels of circulating inflammatory cytokines along with an increase in inflammation, are distinctive characteristics of DM co-morbidity with TB. In an effort to lessen the collective impact of both illnesses, it would be crucial to develop logical treatment decisions by comprehending more about the immunological foundation of TB with DM susceptibility.
Tuberculosis, Diabetes mellitus, Co-occurrence, Immune Mechanisms
Globally, Tuberculosis (TB) remains as the prevalent communicable illness. Researchers have been working efficiently to prevent TB in recent decades. The innate and adaptive immune mechanism of host are compromised by diabetes mellitus (DM), which reduces their ability to eradicate Mycobacterium tuberculosis (MTB) bacilli.1 As per the July 2023 report by the World Health Organisation (WHO), there are an estimated 10.6 million new active TB cases diagnosed globally each year.2 According to recent estimates, due to continuous epidemiological changes in an increasing number of nations worldwide, the global population of people with diabetes mellitus (DM) will likely exceed 552 million within 2023. The rapid increase in DM prevalence justifies its classification as an epidemic disease.3 The World Health Organisation reported 422 million diabetics globally in 2014, up from 108 million in 1980. Based on this, it was estimated that nearly 463 million people would be affected by 2019. Moreover, estimates indicate that the population is expected to grow from 578 million by 2030 and 700 million by 2045. The WHO indicate that DM is associated with approximately 15% of TB cases globally.4 By 2030, when 62-80 million people are likely to get diabetes, India has been predicted to become the “Diabetes Capital” of the entire world.5
In spite of this increasing incidence, DM is considered a potential challenge that should be controlled and treated in TB patients.6 However, several significant intervention strategies have been recommended by the WHO to reduce TB and DM comorbidity to alleviate the dual burden, emphasising on identifying and treating TB in DM patients, diagnosing and managing DM in TB patients, and establishing cooperative control strategies.7 As a result, understanding the scope and immunological mechanisms of comorbidity is crucial, particularly in low-income and middle-income nations.8 According to the level of incidence of TB with diabetes in different nations, India had the greatest prevalence (29%), followed by Korea (26.5%), Mexico-Texas (25%), and Ethiopia (15.8%).9
Even though the association among TB and DM has been recognised for a number of decades, the WHO and International Union Against Tuberculosis and Lung Disease stated that by 2011, all TB patients should have DM tests done due to a significant increase in DM globally.10 Moreover, in India and China, programs for screening TB in DM have already begun. However, worldwide responses to this crisis have been impeded by poor awareness of the technologies and the most suitable methods for screening in TB centres.11
Although the association with TB along with DM as well as their combined involvement in the development of human illness has been recognised for decades, it has only lately gained attention from both fundamental followed by clinical researchers.12 DM and pulmonary tuberculosis (PTB) are the two most prevalent co-morbid illnesses in different parts of the world, and their combination appears to represent a serious threat to healthcare facilities worldwide. A number of epidemiological and clinical investigations have shown diabetes mellitus as a contributing factor to active TB occurence.13 Moreover, the relationship between TB with diabetes in infected individuals has had an adverse impact on disease presentation and therapeutic responses. However, the available information is to draw definitive conclusions, as it has detrimental consequences on global public health, particularly for diabetic patients.14 As a result, the present review highlights the mechanism by which DM affects the immune system of TB patients and the consequences that follow.
Comorbidities and complications
According to a number of studies, TB patients are more likely to get diabetes because of a compromised defence mechanism. However, poor glucose tolerance is one of the main factors contributing to the occurrence of DM among TB affected individuals.15,16 The risk of developing TB remains in most of these cases even when the modified glucose tolerance level returned to normal after taking effective TB treatment. TB is a major contributor to pancreatitis, and as a result, hyperglycaemia may be the first sign of pancreatic tuberculosis.16 Pancreatic hypofunction is the primary factor in this course of action, even if some of the hyperglycemia associated with the elevated stress level due to the illness itself could possibly represent the cause of TB. However, TB-related hyperglycaemia affects the glycaemic control of diabetics and requires dosage adjustments for insulin.17
Role of hyperglycemia in TB disease progression
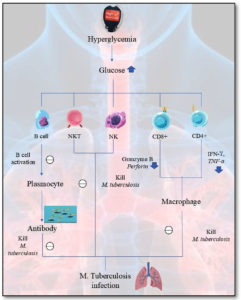
The adaptive and innate immunity of the host can be weakened by diabetes, which lowers the ability of the immune system to eradicate Mycobacterium tuberculosis (MTB) bacilli and elevates the likelihood of contracting TB.18 Initially, elevated glucose levels in the blood may cause M2 polarization of macrophages by diminishing their phagocytic function and leading to higher in vivo MTB burden. Furthermore, hyperglycaemia increases absolute neutrophils count thereby imparing their phagocytic ability.19 Third, an upsurge in natural killer cells, that release IL-17F, TNF-α and IL-2, is additionally associated to an aggravation of the disease or larger bacterial burdens. Fourth, DM reduces secretion of dendritic cells (DC), which lowers activation of CD4+ T cell as well as impairs adaptive immunological response. Hyperglycemia suppresses Th1 T cell production of cytokines including IFN-γ and TNF, which reduces the efficacy of the Th1 an immune reaction in clearing along with killing MTB.20 A decrease in IFN-γ might also hinder Th1-type cell ability to activate CD8+ T lymphocytes, which lowers the production of bactericidal chemicals including granzyme, lysozyme, and IFN-γ. This hinders cytotoxic T cells from being able to eradicate bacteria. As a result, adaptive and innate immune cell activity weakens in TB patients with DM, impairing their capacity to manage MTB and raising the risk of TB21 (Figure 1).
Figure 1. The impact of Hyperglycaemia on the immunity in tuberculosis patients
NKT – Natural killer T cells, NK – Natural Killer cells, CD8+ – Cluster of differentiation 8, CD4+ – Cluster of differentiation 4+, IFN-γ – Interferon gamma, TNF-α – tumor necrosis factor-alpha, and M. tuberculosis – Mycobacterium tuberculosis
Innate immune mechanism
The pathophysiology and susceptibility to tuberculosis are significantly impacted by the variations in innate immunity among diabetics together with non-diabetics.22 Research has shown that changes associated with DM severely affect the function of neutrophils, dendritic cells (DCs), NK cells, macrophages, as well as various other innate immune-related cells. As a result, dysfunction in defence mechanism may significantly influence the host’s ability to recover and its susceptibility to exogenic TB infections.23
Monocytes
The pathogenesis of TB is significantly influenced by blood monocytes. Following MTB infection, they quickly migrate to the lung where they differentiate into DCs and macrophages to produce cytokine and antigen presentation. Additionally, MTB has the ability to infiltrate monocytes and proliferate within them.24 Kumar et al. found that PTB patients with concomitant DM had significantly reduced frequencies of intermediate and classical monocytes. After the completion of anti-TB treatment, the characteristics of monocytes were analyzed, suggesting that TB with DM co-morbidity can reverse alterations in monocyte frequency. Therefore, further research into the role of monocytes in the co-morbidity of TB and DM is essential.25
Dendritic cells
Through their function in collecting, digesting, and presenting antigens, DCs are major players in bridging adaptive and innate immune responses; they are prominent sites of TB infections.26 Studies have shown that TB infections, which lead to naive T cell activation, rely on DCs migrating to lymph nodes during the disease progression.27,28 To better understand how DCs are impacted by TB, Kumar et al. investigated the ex vivo phenotypic characteristics of DCs in patients suffering from TB regardless of their DM status. Compared to patients with both DM and TB, the frequencies of myeloid and plasmacytoid DCs were considerably lower in TB patients having diabetes.29 As a result, anti-TB therapy reverses alterations in the proportions of DCs in TB patients with DM. Thus, hyperglycaemia and its associated factors may be the primary cause of altered DM incidence in TB patients.30
Neutrophils
The aetiology of TB infection as well as defence systems of the body are significantly influenced by neutrophils, another kind of innate cell. The natural immune response to TB fluctuates according to neutrophils, which are believed to mitigate infection by oxidatively destroying MTB.31 When the bacterial load is high, this promotes the growth of neutrophils, as these cells can also contribute to pathology. Active TB infection has been demonstrated to be correlated with a whole blood neutrophil-dominant interferon signature. Increased absolute neutrophil counts are a hallmark of TB with DM co-morbidity.32
Natural Killer Cells
The innate immunity effector cells, sometimes referred to as NK cells, undergo regulation by a set of either stimulating or inhibiting receptors. It is believed that the generation of NK cells plays a substantial role on the host’s defence against TB through the release of IFN-γ, IL-17, and IL-22.33 Recent research uncovered that TB with DM can be distinguished by the production of type 1 (TNF-α) and type 17 (IL-17A and IL-17F) cytokines, as well as an increase in NK cell counts triggered on by the TB antigen. However, among TB patients with DM, CD107a antigen-specific expression was significantly reduced in association with NK cells.34 Additionally, according to Zhang et al., patients with TB have significantly more peripheral blood mononuclear NK cells than healthy individuals.35
Antimicrobial peptides
Antimicrobial peptides (AMPs), which are predominantly found in phagocytic cells, play a substantial role in innate immunity to infections. They work by engulfing and killing pathogens and destroying their hosts.36 According to various studies, AMPs promising therapeutic agents due to their significant anti-mycobacterial efficacy and minimal immunogenicity.37-39 Among the AMP family, Human beta-defensin 2 (HBD2), human neutrophil peptide 1-3 (HNP1-3), cathelicidin (LL37) as well as granulysin are four of the most notable molecules.40 In comparisons between TB patients with DM and non-TB (NTB) or latent tuberculosis (LTB) infected patients, they found that HBD2, HNP1-3 and cathelicidin levels were elevated, while granulysin levels were decreased. However, the degree and severity of pulmonary illness, bacterial loads, cathelicidin, HBD2, and AMPs were also correlated with glycaemic indices.41 Gonzalez Curiel et al. also demonstrated that in contrast to LTB, the expression of the AMP gene was higher during active TB. In conclusion, decreased phagocytosis of TB and reduced gene expression in TB infection suggest that DM patients may have altered innate immunity.42
Adaptive Immune Mechanism
Numerous investigations comparing patients with TB and DM to those without DM have established the significance of adaptive immunity to MTB in the setting of DM. There has been a reduction in naturally existing T-regulatory cells such as CD127, CD4+, and CD25+, as well as an increase in cytokines like Th1 (IFN-γ, IL-2) along with Th17 (IL-17A, but not IL-22) in the plasma of patients with TB and DM.43 Since TB patients with diabetes also had greater levels of IL-10, it has been shown that their levels of pro-inflammatory and anti-inflammatory cytokines were higher than those of patients without diabetes.44 In addition, TB patients with diabetes mellitus exhibited a bronchoalveolar lavage with greater IL-10 levels and lower levels of IFN-γ, demonstrating an abnormal Th2 response within the lung compartment, as documented by Sun et al.45
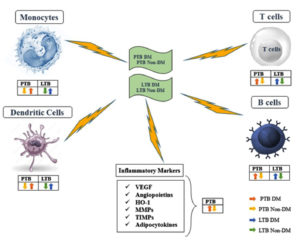
Generally, patients having TB with DM have hyperactive cell-mediated immunity to MTB antigens, based on the majority of adaptive immune mechanism-related study findings.46 Alternatively, research on patients having TB without diabetes marginally strengthen the idea that diabetics are more susceptible to TB due to compromised immune systems. However, additional investigation on this hyper-reaction would be intriguing to understand its connection with DM, as it may increase the susceptibility to TB and influence immunopathology. Furthermore, a few of these research investigations discovered that the IFN-γ to IL-10 ratios were significantly lower in those with both TB and DM47 (Figure 2).
Figure 2. Immunology of Tuberculosis and Diabetes Mellitus.
PTB – Pulmonary tuberculosis, LTB – Latent tuberculosis, DM – Diabetes mellitus, VEGF – Vascular endothelial growth factor, HO-1 – Heme oxygenase-1, MMPs – Matrix metalloproteinases and TIMPs – Tissue inhibitor of metalloproteinases
Biomarkers related to tuberculosis with diabetes mellitus
There is a wide array of biomarkers for TB, specific to the pathogen or the host. The cytokines including IL-19, IL-20, IL-22, IL-24, and IL-26 constitute members of IL-20 subfamily-are essential for host’s defensive systems and glucose metabolism. The innate immunological responses of the host are significantly influenced by the IL-20 cytokine subfamily.48 For instance, it stimulates increased leucocyte recruitment and activation at the inflammation, which improves the function of the epithelial and mucosal surfaces as barriers and enhances the production of antibacterial peptides.49 Kumar et al. conducted research on the function of the inflammatory cytokine IL-20 subfamily in individuals with TB having DM in order to gain an improved comprehension of the interactions among TB and DM. The circulating plasma cytokines levels in each group were measured and compared between those with LTB-DM or PTB-DM and patients without DM. PTB in DM patients was linked to higher levels of circulating IL-10 and a decreased IL-19, IL-20, IL-22, and IL-24 levels. In LTB patients having DM, the circulation concentrations of IL-10, IL-19, IL-20, and IL-24 were lowered, whereas IL-22 levels were increased.50 Furthermore, among PTB and LTB patients, there was a strong inverse correlation among haemoglobin A1C (HbA1c) levels along with IL-10, IL-20, IL-19, IL-22 and IL-24 levels. IL-20 subfamily cytokines are involved in regulation of co-morbidity-related host immunological and metabolic processes because decreased IL-20 production of cytokines was a feature of co-occurring DM in LTB or PTB51 (Figure 2).
As markers of the actively active angiogenesis-inflammation nexus, the characteristics of TB with DM coexistence include an increase in inflammation and raised circulating inflammatory cytokines in the blood. The circulating levels of angiogenesis-promoting agents, including vascular endothelial growth factor A (VEGF-A), VEGF-C, VEGF-D, VEGF-R1, VEGF-R2, and VEGF-R3, as well as angiopoietins like Angiopoietin-2 and Tie2 receptor, were examined by Kumar et al. to investigate their relationships of TB with DM. TB-DM patients had much higher levels of all angiogenic factors compared to TB patients; however, angiopoietins such as Angiopoietin-1 and Angiopoietin-2 were markedly lower.52 Furthermore, VEGF-A, VEGF-C, VEGF-R2 and VEGF-R3 had considerably greater levels.
among TB with DM patients presenting with haemoptysis or bilateral or cavitary disease, implying a relationship between the severity of the illness and poor clinical outcome. However, following six months of an effective anti-TB regimen, circulation levels of angiogenic factors were noticeably reduced, while angiopoietins were elevated. According to the authors, the aforementioned elements may serve as precise biomarkers for tracking therapy outcomes in co-morbidity in TB patients with DM.53
Diabetes and tuberculosis-disease progression and recovery
MTB infection progresses more quickly in people with diabetes compared with non-diabetic controls and is associated with a increased bacterial loads, a shorter lifespan, and greater risk of pulmonary and extrapulmonary conditions. Additionally, miliary TB, increased bacterial loads, and disease progression result in significantly higher mortality rates among those with TB and DM.54 Inducing hyperglycaemia enhances the incidence of MTB shedding from the airways, irrespective when there are number of cavities, as demonstrated by studies conducted in laboratories on animals. An upward trend in the pulmonary bacterial load along with alterations in the diabetic airway milieu may be the cause of this phenomenon. Research indicates that TNF-α and IL-1 expressions were higher in chronically hyperglycaemic guinea pigs than in NDM controls, with the extrapulmonary bacterial load also increasing, which worsened the severity and progression of TB.55-57 As a result, the immunological response of cells to MTB infection and the consequent altered the formation particular cytokines could potentially be an influential variable in the disease’s severity. When diabetes and TB coexist, increased granulocytic infiltration likely contributes to higher IL-8 and IL-17 levels. In addition to oxidative stress, these effects of chronic hyperglycemia trigger a pro-inflammatory reaction that worsens inflammation and raises the risk of TB in people with DM.58
However, before developing an adaptive immune response to MTB, those suffering from diabetes with TB have faster progression of the disease. It happens as a result elevated IL-4 concentrations as well as cytokines that reduce inflammation by blocking IFN-γ expression, therefore rendering it unable to regulate bacterial growth.59 Cohort studies demonstrate that after receiving TB treatment, the mortality rate is greater for those with diabetes than for those without the illness. It is believed that a higher bacterial load upon diagnosis is correlated with delayed TB clearance among DM patients as well as the possibility of TB failure during treatment. This might be because DM patients have delayed immune response kinetics, and TB patients with DM have changed anti-TB medication pharmacokinetics involving drug absorption, metabolism, distribution, and excretion.60
Current challenges and future opportunities
Despite being a long-standing infectious disease, TB is increasingly being recognized as an immune-related condition. The direction in which the disease develops is determined by the conflict between MTB’s invasiveness and the host’s resistance. MTB will become active if its invasiveness is greater than the host’s immunity. LTB infections will be detected if the two forces are balanced and if MTB’s invasiveness is weaker than the host’s immunity, it will be eliminated.61
The growing involvement of DM in TB offers a new viewpoint and a chance for investigators to learn more about the disease’s aetiology. With the cooperation of fundamental scientific and epidemiological research teams, an algorithm to categorise million individuals with diabetes according to their risk of MTB or TB is expected to be developed in the future. Based on this, individuals with the highest benefit-to-risk ratio would be recommended for LTB infection therapy, while those with the poorest prognoses would receive tailored TB treatment.62,63
In this review, we have outlined the immunological changes associated with TB and DM. Public health is impacted, especially in countries where both diseases are widespread, as the increasing risk of DM exacerbates the severity of TB. Several investigations have demonstrated that hyperglycaemia impairs the defence mechanism of host, which facilitates the development followed by TB spread and leads to markedly a poor therapeutic prognosis. On the other hand, an array of research investigations suggests that TB patients with DM have altered immune systems. Further thorough investigation into the pathways that are pathogenic underlying TB associated DM is required for completely comprehending the manner in which hyperglycaemia affects immune system of host and to provide appropriate therapy. Thus, it would be advantageous to improve our understanding of the immunological underpinnings concerning TB with DM susceptibility to develop effective treatment methods that would lessen the combined burden of both conditions.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Paleckyte A, Dissanayake O, Mpagama S, Lipman MC, McHugh TD. Reducing the risk of tuberculosis transmission for HCWs in high incidence settings. Antimicrob Resist Infect Control. 2021;10(1):106.
Crossref - Tuberculosis. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Accessed July 27, 2023.
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203-234.
Crossref - TB and Diabetes. World Health Organization. https://www.who.int/publications/digital/global-tuberculosis-report-2021/featured-topics/tb-diabetes. Accessed July 27, 2023.
- Gupta S, Chakraborty A. Vulnerability toward the development of diabetes mellitus type 2 among adults from a rural community of West Bengal, India. J Health Res. 2021;36(4):738-745.
Crossref - Lee MR, Huang YP, Kuo YT, et al. Diabetes Mellitus and Latent Tuberculosis Infection: A Systematic Review and Metaanalysis [published correction appears in Clin Infect Dis. 2017;65(2):356]. Clin Infect Dis. 2017;64(6):719-727.
Crossref - Collaborative Framework for Care and Control of Tuberculosis and Diabetes. Geneva: World Health Organization. https://theunion.org/sites/default/files/2020-08/collaborative-framework_tb-diabetes.pdf. Accessed January 25, 2025
- Joshi R, Behera D, Di Tanna GL, Ameer MA, Yakubu K, Praveen D. Integrated Management of Diabetes and Tuberculosis in Rural India – Results From a Pilot Study. Front Public Health. 2022;10:766847.
Crossref - Ayelign B, Negash M, Genetu M, Wondmagegn T, Shibabaw T. Immunological Impacts of Diabetes on the Susceptibility of Mycobacterium tuberculosis. J Immunol Res. 2019;2019(1):6196532.
Crossref - Lu P, Zhang Y, Liu Q, et al. Association of BMI, diabetes, and risk of tuberculosis: a population-based prospective cohort. Int J Infect Dis. 2021;109:168-173.
Crossref - Hewage S, Somasundaram N, Ratnasamy V, et al. Active screening of patients with diabetes mellitus for pulmonary tuberculosis in a tertiary care hospital in Sri Lanka. PLoS One. 2021;16(4):e0249787.
Crossref - Singh SP, Singh SP, Kishan J, Kaur S, Ramana S. Association of tuberculosis and diabetes Mellitus: an analysis of 1000 consecutively admitted cases in a tertiary care hospital of North India. Pan Afr Med J. 2016;24:4.
Crossref - Venkatesan B, Vajravelu LK, Ravi S, Thulukanam J, Muthamilan OL. Analysis of robust immune response among diabetic and non-diabetic individuals against SARS-COV-2 vaccination. J Pure Appl Microbiol. 2023;17(1):395-402.
Crossref - Gautam S, Shrestha N, Mahato S, Nguyen TPA, Mishra SR, Berg-Beckhoff G. Diabetes among tuberculosis patients and its impact on tuberculosis treatment in South Asia: a systematic review and meta-analysis. Sci Rep. 2021;11(1):2113.
Crossref - Nyirenda JLZ, Wagner D, Ngwira B, Lange B. Bidirectional screening and treatment outcomes of diabetes mellitus (DM) and Tuberculosis (TB) patients in hospitals with measures to integrate care of DM and TB and those without integration measures in Malawi. BMC Infect Dis. 2022;22(1):28.
Crossref - Menon S, Rossi R, Dusabimana A, Zdraveska N, Bhattacharyya S, Francis J. The epidemiology of tuberculosis-associated hyperglycemia in individuals newly screened for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):937.
Crossref - van Crevel R, Critchley JA. The Interaction of Diabetes and Tuberculosis: Translating Research to Policy and Practice. Trop Med Infect Dis. 2021;6(1):8.
Crossref - Chandra P, Grigsby SJ, Philips JA. Immune evasion and provocation by Mycobacterium tuberculosis. Nat Rev Microbiol. 2022;20(12):750-766.
Crossref - Jagatia H, Tsolaki AG. The Role of Complement System and the Immune Response to Tuberculosis Infection. Medicina. 2021;57(2):84.
Crossref - Abbas U, Masood KI, Khan A, et al. Tuberculosis and diabetes mellitus: Relating immune impact of co-morbidity with challenges in disease management in high burden countries. J Clin Tuberc Other Mycobact Dis. 2022;29:100343.
Crossref - Ssekamatte P, Sande OJ, van Crevel R, Biraro IA. Immunologic, metabolic and genetic impact of diabetes on tuberculosis susceptibility. Front Immunol. 2023;14:1122255.
Crossref - Ferlita S, Yegiazaryan A, Noori N, et al. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium tuberculosis. J Clin Med. 2019;8(12):2219.
Crossref - de Martino M, Lodi L, Galli L, Chiappini E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front Pediatr. 2019;7:350.
Crossref - Lavalett L, Ortega H, Barrera LF. Infection of Monocytes From Tuberculosis Patients With Two Virulent Clinical Isolates of Mycobacterium tuberculosisInduces Alterations in Myeloid Effector Functions. Front Cell Infect Microbiol. 2020;10:163.
Crossref - Nathella PK, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152(1):13-24.
Crossref - Sernoskie SC, Jee A, Uetrecht JP. The Emerging Role of the Innate Immune Response in Idiosyncratic Drug Reactions. Pharmacol Rev. 2021;73(3):861-896.
Crossref - Roberts LL, Robinson CM. Mycobacterium tuberculosis infection of human dendritic cells decreases integrin expression, adhesion and migration to chemokines. Immunology. 2014;141(1):39-51.
Crossref - Sohail MU, Mashood F, Oberbach A, Chennakkandathil S, Schmidt F. The role of pathogens in diabetes pathogenesis and the potential of immunoproteomics as a diagnostic and prognostic tool. Front Microbiol. 2022;13:1042362.
Crossref - Kumar NP, Moideen K, Sivakumar S, et al. Modulation of dendritic cell and monocyte subsets in tuberculosis-diabetes co-morbidity upon standard tuberculosis treatment. Tuberculosis. 2016;101:191-200.
Crossref - Eckold C, Kumar V, Weiner J, et al. Impact of Intermediate Hyperglycemia and Diabetes on Immune Dysfunction in Tuberculosis. Clin Infect Dis. 2021;72(1):69-78.
Crossref - Borkute RR, Woelke S, Pei G, Dorhoi A. Neutrophils in Tuberculosis: Cell Biology, Cellular Networking and Multitasking in Host Defense. Int J Mol Sci. 2021;22(9):4801.
Crossref - Mily A, Sarker P, Taznin I, et al. Slow radiological improvement and persistent low-grade inflammation after chemotherapy in tuberculosis patients with type 2 diabetes. BMC Infect Dis. 2020;20(1):933.
Crossref - Parra JAC, Zuniga NM, Zamudio LAJ, Alvarez LAJ, Lara CS, Zuniga J. Memory of Natural Killer Cells: A New Chance against Mycobacterium tuberculosis?. Front Immunol. 2017;8:967.
Crossref - Harris LD, Khayumbi J, Ongalo J, et al. Distinct Human NK Cell Phenotypes and Functional Responses to Mycobacterium tuberculosisin Adults From TB Endemic and Non-endemic Regions. Front Cell Infect Microbiol. 2020;10:120.
Crossref - Zhang C, Song X, Zhao Y, et al. Mycobacterium tuberculosis Secreted Proteins As Potential Biomarkers for the Diagnosis of Active Tuberculosis and Latent Tuberculosis Infection. J Clin Lab Anal. 2015;29(5):375-382.
Crossref - Zhang QY, Yan ZB, Meng YM, et al. Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res. 2021;8(1):48.
Crossref - Lei J, Sun L, Huang S, et al. The antimicrobial peptides and their potential clinical applications. Am J Transl Res. 2019;11(7):3919-3931
- Ryu M, Park J, Yeom JH, Joo M, Lee K. Rediscovery of antimicrobial peptides as therapeutic agents. J Microbiol. 2021;59(2):113-123.
Crossref - Pfalzgraff A, Brandenburg K, Weindl G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front Pharmacol. 2018;9:281.
Crossref - Duarte-Mata DI, Salinas-Carmona MC. Antimicrobial peptides´ immune modulation role in intracellular bacterial infection. Front Immunol. 2023;14:1119574.
Crossref - Kumar NP, Moideen K, Viswanathan V, et al. Heightened circulating levels of antimicrobial peptides in tuberculosis-Diabetes co-morbidity and reversal upon treatment. PLoS One. 2017;12(9):e0184753.
Crossref - Gonzalez-Curiel I, Castaneda-Delgado J, Lopez-Lopez N, et al. Differential expression of antimicrobial peptides in active and latent tuberculosis and its relationship with diabetes mellitus. Hum Immunol. 2011;72(8):656-662.
Crossref - Ngo MD, Bartlett S, Ronacher K. Diabetes-Associated Susceptibility to Tuberculosis: Contribution of Hyperglycemia vs. Dyslipidemia. Microorganisms. 2021;9(11):2282.
Crossref - Tegegne BS, Mengesha MM, Teferra AA, Awoke MA, Habtewold TD. Association between diabetes mellitus and multi-drug-resistant tuberculosis: evidence from a systematic review and meta-analysis. Syst Rev. 2018;7(1):161.
Crossref - Sun Q, Zhang Q, Xiao H, Cui H, Su B. Significance of the frequency of CD4+CD25+CD127- T-cells in patients with pulmonary tuberculosis and diabetes mellitus. Respirology. 2012;17(5):876-882.
Crossref - Chai Q, Wang L, Liu CH, Ge B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol Immunol. 2020;17(9):901-913.
Crossref - Aravindhan V, Bobhate A, Sathishkumar K, Patil A, Kumpatla S, Viswanathan V. Unique Reciprocal Association Seen Between Latent Tuberculosis Infection and Diabetes Is Due to Immunoendocrine Modulation (DM-LTB-1). Front Microbiol. 2022;13:884374.
Crossref - Moniruzzaman M, Wang R, Jeet V, McGuckin MA, Hasnain SZ. Interleukin (IL)-22 from IL-20 Subfamily of Cytokines Induces Colonic Epithelial Cell Proliferation Predominantly through ERK1/2 Pathway. Int J Mol Sci. 2019;20(14):3468.
Crossref - Johnstone KF, Herzberg MC. Antimicrobial peptides: Defending the mucosal epithelial barrier. Front Oral Health. 2022;3:958480.
Crossref - Kumar NP, Banurekha VV, Nair D, Kumaran P, Dolla CK, Babu S. Type 2 diabetes – Tuberculosis co-morbidity is associated with diminished circulating levels of IL-20 subfamily of cytokines. Tuberculosis. 2015;95(6):707-712.
Crossref - Kaushik SR, Sahu S, Guha H, et al. Low circulatory Fe and Se levels with a higher IL-6/IL-10 ratio provide nutritional immunity in tuberculosis. Front Immunol. 2023;13:985538.
Crossref - Prada-Medina CA, Fukutani KF, Pavan Kumar N, et al. Systems Immunology of Diabetes-Tuberculosis Comorbidity Reveals Signatures of Disease Complications. Sci Rep. 2017;7(1):1999.
Crossref - Blankley S, Berry MPR, Graham CM, Bloom CI, Lipman M, O’Garra A. The application of transcriptional blood signatures to enhance our understanding of the host response to infection: the example of tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130427.
Crossref - Sinha R, Ngo MD, Bartlett S, et al. Pre-Diabetes Increases Tuberculosis Disease Severity, While High Body Fat Without Impaired Glucose Tolerance Is Protective. Front Cell Infect Microbiol. 2021;11:691823.
Crossref - Podell BK, Ackart DF, Obregon-Henao A, et al. Increased severity of tuberculosis in Guinea pigs with type 2 diabetes: a model of diabetes-tuberculosis comorbidity. Am J Pathol. 2014;184(4):1104-1118.
Crossref - Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis.J Immunol. 2010;184(11):6275-6282.
Crossref - Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37(5):518-524.
Crossref - Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45-63.
- Carabali-Isajar ML, Rodriguez-Bejarano OH, Amado T, et al. Clinical manifestations and immune response to tuberculosis. World J Microbiol Biotechnol. 2023;39(8):206.
Crossref - Riza AL, Pearson F, Ugarte-Gil C, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. 2014;2(9):740-753.
Crossref - Sia JK, Rengarajan J. Immunology of Mycobacterium tuberculosisInfections. Microbiol Spectr. 2019;7(4):10.1128/microbiolspec.GPP3-0022-2018.
Crossref - Ahmad SR, Yaacob NA, Jaeb MZ, Hussin Z, Wan Mohammad WMZ. Effect of Diabetes Mellitus on Tuberculosis Treatment Outcomes among Tuberculosis Patients in Kelantan, Malaysia. Iran J Public Health. 2020;49(8):1485-1493.
Crossref - Villar-Hernandez R, Ghodousi A, Konstantynovska O, Duarte R, Lange C, Raviglione M. Tuberculosis: current challenges and beyond [published correction appears in Breathe (Sheff). 2023 Jun;19(2):225166]. Breathe (Sheff). 2023;19(1):220166.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.