ISSN: 0973-7510
E-ISSN: 2581-690X
Fermented milk is considered as a good source of nutrition for many people. One of the most important substances that could be used in the fermentation of milk is kefir grains. Kefir fermented milk is very important in many health conditions such as bacterial infections, high blood pressure and some hepatic conditions. The aim of this study was to determine the antibacterial activity of fermented milk of kefir against certain pathogenic microorganisms such as Salmonella sp., Escherichia coli, Staphylococcus sp. and Candida albicans using agar diffusion method. The effectiveness of fermented milk of kefir has been studied as hepatoprotective against CCl4 inducing liver toxicity and as a protective agent against kidney and spleen damage using laboratory animal models. The results revealed that the fermented milk of kefir had a potent antibacterial activity against many pathogenic microorganisms and it also showed significant high protection in mice against the toxicity of CCl4. In conclusion, kefir milk can be used as an antibacterial supplement and as a protective agent against liver toxicity.
Kefir, Antibacterial, Carbon tetrachloride; Liver toxicity, Milk.
“Kefir is gotten from the Turkish word “keif” which signifies “nice feeling”1 and the drink started in the Caucasian heaps of Russia2,3,4. Kefir is obtained from the fermenting activity of Kefir grains5. Traditionally, it is fermented in goatskin bags for 24 hours6. Kefir contains many ingredients that demonstrate biological activity, such as some probiotic bacteria and bioactive peptides7 and onsets of activity varies according to the type of kefir and the time of fermentation8. It is self-carbonated fermented milk with a slightly acidic taste2. The kefir drink is produced from cow, goat, sheep3, camel, buffalo or soy milk4, 9 that could be whole fat, low-fat, skimmed or fat-free milk1. This difference in the milk type and methods of fermentation affects the amount of grain produced, food composition and flavor of kefir2. Kefir grains are considered to be the most important component in the production of fermented kefir and can be reused again10. It contains many types of bacteria in addition to proteins and polysaccharides11, 12, 13. Although the kefir drink can be found in many countries, in Egypt the grains are not commercially available and are culturally donated from person to person.
Partial sequencing of the gene encoding 16S rRNA was used for species identification14, 15. Fermented milk produced by kefir grains contains yeast and lactobacilli16, 17, 18. Kefir has many applications in a variety of medical conditions such as; high blood pressure, allergy problems and coronary heart disease. Also, it strengthens the immune system and improves the digestive health. Kefir antimicrobial activity is associated with the production of organic acids, peptides (bacteriocins), carbon dioxide, hydrogen peroxide, ethanol and diacetyl15, 19.
The main objective of this study was to investigate the antimicrobial activity of the fermented kefir in vitro against different pathogens and to evaluate its hepatoprotective effect in mice.
Preparation and characterization of kefir
Kefir grains were collected from Qena City, Egypt. They were varying in size (from 0.3 to 3.0 cm in diameter), irregular in shape20. 21 as shown in (Fig. 1), and white to yellowish-white in color17, 18, 19. Also, the grains were flexible, softer in texture, viscous12, 17, 18 and insoluble in water and common solvents. When milk was added, the grains swelled and produced a jellied called Kefiran22. Kefir has a pH of 4.2 – 4.6, an ethanol content of 0.5 – 2.0% (v/v), a lactic acid content of 0.8–1.0% (m/v), a carbon dioxide content of 0.08 – 0.2% (v/v) 23 The grains were recovered by sieving, mass of grain increased by 6% after the fermentation process and microorganisms found in the milk are different from those produced after the fermentation process15, 22.

Fig. 1. The physical appearance of Kefir grains
Kefiran was prepared by adding 100 g of kefir grains to 500 ml of purified milk at 25ºC in a dark place for 24 h – 48 h. Kefir grains were separated from the fermented milk by plastic sieve24, 25.
The antimicrobial activity of the fermented kefir
Screening for antibacterial activity of the Kefir fermented milk was done using agar diffusion method (26, 27) against Gram-positive bacteria (Staphylococcus aureus ATCC 44330 and Bacillus subtilis), Gram-negative bacteria (Escherichia coli ATCC 5087, Salmonella enteritidis and Pseudomonas aeruginosa), and yeast (Candida albicans).
The Kefir fermented milk was filter sterilized using 0.45 um membrane filter and the indicator microorganisms were incubated overnight in brain heart infusion broth (Oxide) at 37°C. The antimicrobial activity was done based on seeding inoculation of each indicator microorganism in 20 ml Muller Hinton agar (Oxide), and then cups were prepared using Wassermann tubes with an external diameter of 5 mm. A fixed amount of 50 µl, 100 µl and 150 µl of tested kefir solution was distributed to each well. The plates were incubated for 24 h at 37°C. A positive control of antibiotic ampicillin (10 mg/ml) was also tested. Estimation of antimicrobial activities was done by measuring diameters of zone of inhibitions.
Identification of microorganisms isolated from fermented kefir by partially sequencing of 16S rRNA gene
The Kefir fermented milk was platted on MRS agar (Oxide) and Brain heart agar (Oxide) for 2 days at 25°C to isolate major lactic acid bacteria and other microorganisms in kefir and then a DNA extraction was done followed by PCR and DNA sequencing.
Genomic DNA extraction and purification
Genomic DNA extraction was done according to27 with some modifications. Briefly, a 1.5 ml of culture was centrifuged for 10 min at 3,000 g, the supernatant was discarded and the pellets were resuspended in 200 µl spheroblast buffer (10% sucrose, 25 mM Tris pH 8.4, 25 mM EDTA pH 8.0, 2 mg/mL lysozyme and 0.4 mg/ml RNase A), vortexes and incubated at 37ºC for 10 minutes until cell lysis occurred. Then, 50 µl of 5% SDS (lysis buffer 1) and 5 M NaCl (lysis buffer 2) were added, mixed and incubated at 65ºC for 5 minutes. A 100 µl neutralizing buffer (60 ml 5M Potassium acetate, 11.5 ml glacial acetic acid, and 28.5 ml dH2O) was then added and put on ice for 5 min before centrifugation at 18,000 g at 4ºC for 15 minutes. The supernatant (approximately 400 µl) was transferred to a new tube, mixed with equal volume of isopropanol, left 5 minutes at room temperature and centrifuged at 18,000 g at room temperature for 15 minutes to precipitate the DNA. The resulting pellet was washed with 70% ethanol by centrifugation at 18,000 g at room temperature for 5 minutes. The final pellet was air-dried and resuspended in 50 µl 1 x TE buffer pH 8 and stored in the refrigerator at 4ºC.
PCR amplification and sequencing of bacterial 16S rRNA gene
PCR was carried out in 50 µl reaction volume in sterile 200 µl PCR tube. The PCR reaction mixture consisted of 500 ng genomic DNA, 10 mM dNTPs mixture, 1 µl (20 uM of each primer); forward primer 5‘-TAACACATGCAAGTCGAACG-3‘ and reverse primer 5‘-AAACTYAAAKGAATTGACGG-3‘, 2.5 units of Taq DNA polymerase enzyme and 10 µl 5X reaction buffer. The PCR program included template denaturation at 94ºC (3 min), followed by 34 cycles of denaturing at 94ºC (30 sec.), annealing at 56ºC (30 sec.), and extension at 72ºC (60 sec.), and followed by completion of DNA synthesis at 72ºC (5 min). Primers were removed from the final PCR product prior to sequencing using QIAquick PCR purification kit (QIAGEN, Germany). The PCR product of interest was detected and purified by agarose gel electrophoresis using 1% (w/v) agarose gels with reference to 1 kbp DNA ladder. DNA was sequenced using the ABI Prism BigDye terminator sequencing ready reaction kit version 3.1 and analyzed with the ABI Prism 3100 generic analyzer.
Sequence manipulation and phylogenetic analysis
Searching for DNA sequence homology was done using BLAST tool at NCBI database (www.ncbi.nlm.nih.gov/blst) in order to assess the degree of DNA similarity. Multiple sequence alignment and molecular phylogeny were constructed using MEGA7 software28.
Evaluation of the protective ability of Kefir against carbon tetrachloride CCl4-induced liver toxicity in mice
Animal grouping and treatment
Three weeks old, clinically healthy, female Swiss albino mice (n=40) weighting 26–30 g were randomly divided into 4 groups (10 mice/group) after 7 days adaptation. They were housed in stainless-steel wire-mesh cages (four in a cage), at 24±2ºC temperature, 55% relative humidity and a 12 h light–dark cycle. The animals were provided a normal diet and tap water. The groups were separately treated for as following :
Group I, animals were sham-treated with 2 ml/kg distilled water through oral gavage, daily for 4 weeks; this group of animals served as the control.
Group II, animals were treated with 1.5 ml/kg body weight (b.w.) CCl4 dissolved in 1.5 ml corn oil through oral gavage, daily for 4 weeks.
Group III, animals were treated with 1.5 ml/kg b.w. CCl4 + 30 ml/kg b.w kefir through oral gavage, daily for 4 weeks.
Group IV, animals were treated with 30 ml/kg b.w. Kefir through oral gavage daily for 4 weeks.
Preparation of Fermented Kefir to feed animals
The compound was prepared by washing the kefir grains with distilled water and raw milk, after that heated to 90 °C for 10 min in a water bath, then cooled to inoculation temperature (25 °C) and 10% active kefir grains added. The mixture was placed in a plastic container with screen cloth as a cover and incubated at room temperature for 24–48 hrs. A plastic container is used because the acidity of fermented kefir may degrade metals such as aluminum and iron which could mix with the drink thereby causing harmful effects to the body29. After fermentation, kefir grains were sieved by filtration through a plastic sieve and washed for another process29. Kefir drink was maintained at 4 ± 1ºC for 24 h and then used for microbiological and chemical analyses before feeding the animals in group III, kefir samples which were stored for more than 3 days were not used. Animal treatment was continued for 4 weeks then the experiment was concluded and animals were killed under anesthesia, blood samples were collected and livers, kidneys and spleen were rapidly removed then weighted to calculate relative liver weight to body weight.
Biochemical analysis
Each blood sample was placed in dry clean centrifuge tube, and then centrifuged for 10 min at 3000 revolutions per minute (rpm) to separate the serum. Serum was carefully separated into clean dry Wasser man tubes by using a Pasteur pipette and used for determination of serum liver function tests; aspartate aminotransferase (AST) (biolab), alanine aminotransferase (ALT) (biolab) and alkaline phosphatase (ALP) (biolab)] using standard techniques by manufactures.
Histopathological examination
Tissue samples were collected from liver, kidney and spleen of all animals (Group I-IV). These samples were fixed in neutral formalin solution 10% for 72 hrs., after that fixed samples were processed and stained by Hematoxylin and Eosin according to30.
The antimicrobial activity of kefir against pathogenic microorganisms
Antibacterial activity of kefir, freshly fermented for 24 hrs and 48 hrs or stored for 72 hours at 4 – 8°C, was estimated by agar diffusion method. It was noted that Kefir has antibacterial activity against Staphylococcus aureus, E. coli and Salmonella enteritidis. For E.coli and S. enteritidis, the antimicrobial activity was superior to control antibiotics. The tested products exhibited no activity against P. aeruginosa and C. albican. The results demonstrated that kefir possesses high antibacterial potentials against Gram-negative and Gram-positive strains (Fig. 2).
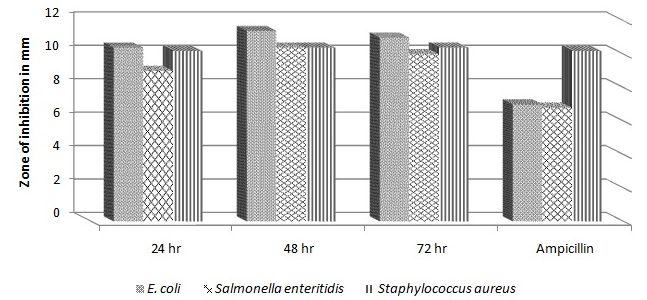
Fig. 2. The antimicrobial activity of fermented kefir after 24hr, 48hr and 72hr against E. coli, Salmonella enteritidis and Staphylococcus aureus in compared to Ampicillin.
Microorganisms identification by partially sequencing the 16S rRNA and phylogenetic analysis
Identification of isolates to the species level was based on sequencing of 16S rRNA gene. The PCR of 16S rRNA gene using specific primers was done and revealed positive reactions and correct amplicon sizes. DNA sequencing was done and the obtained sequences were analyzed using both Bioedit v. 7.0.9.0 and CLC sequence analyzer programs. The homology search of the obtained sequences using BLAST tool at NCBI database was done to categorize the microorganisms to the closest species as in (Table 1). The isolated microorganisms were identified as Micrococcus cohnii (isolate ID SK14), Lactobacillus kefiranofaciens ZW3 (isolate ID SK22) and Lactobacillus casei strain KF11 (isolate ID SK23).
Table (1):
Strains identifiers and their accession numbers
Strain ID |
Product size |
Closest isolate |
Accession number |
Max score |
Total score |
Query cover |
E value |
Max ident |
|---|---|---|---|---|---|---|---|---|
SK14 |
390bp |
Micrococcus cohnii strain WS4601 |
NR_117194.1 |
217 |
217 |
91 % |
4e-56 |
78 % |
SK22 |
560bp |
Lactobacillus kefiranofaciens ZW3, |
CP002764.1 |
2874 |
11492 |
100 % |
0.0 |
99 % |
SK23 |
518bp |
Lactobacillus casei strain KF11 |
KR816166.1 |
957 |
957 |
100% |
0.0 |
100% |
The resulted sequences were aligned to the closely related microorganisms by retrieving their sequences from the NCBI GenBank database and assembled in MEGA7 software for phylogenetic analysis using the Neighbor-Joining method and the evolutionary distances were computed using the Kimura 2-parameter method as seen in (Fig. 3).
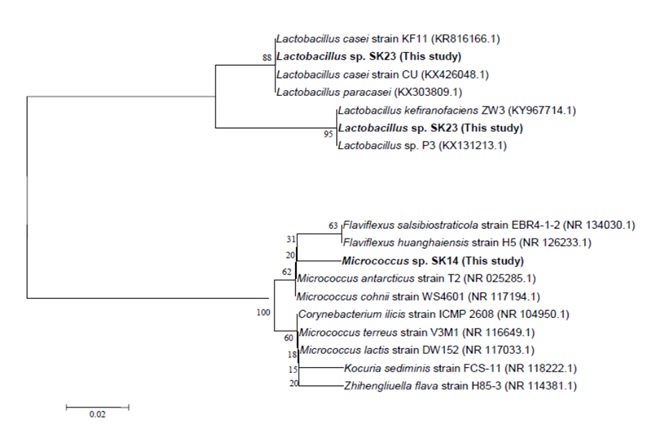
Fig. 3. Phylogenetic tree of the fermented kefir derived isolates based on partial 16S rRNA gene sequences. The phylogenetic tree was inferred using the Neighbor-Joining method (43). The distances were computed using the Kimura 2-parameter method (44) and are in the units of the number of base substitutions per site. Numbers at nodes indicate percentages of 1000 bootstrap re-samplings. Co-don positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA7 (45).
Protective effect of Kefir against risk of carbon tetrachloride-induced liver toxicity and other damages in mice
Effect of treatment on body weight and relative liver weight
Effect of treatment on body weight and relative liver weight to body weight were estimated as illustrated in (Table 2); the ratio of liver weight to 100 g body weight was significantly increased by sole administration of CCl4 (1.9900±.13565, p <0.05) compared to control animals showing (1.1070±.04842). Interestingly treatment with both kefir and CCl4 exhibited liver weight/100 g body weight ratio showing (1.3900±.08741) which is significantly lower than CCl4 group (p <0.05) and was close to normal value.
By comparing the total body weight at the end of experiments to its corresponding initial value, only CCl4 group exhibited a significant decrease compared to its corresponding initial weight (Table 2). Note northerly, the body weights exhibited by combination group had higher values compared to both initial body weight and body weights exhibited by CCl4-treated group, however, it is still less than the control values as illustrated in (Table 2).
Table (2):
Effects of kefir on liver weights and total body weights of mice treated with carbon tetrachloride (CCl4) at the end of study (4 weeks)
Treatment Groups |
Liver weight/100g body weight |
Body weight(%of initials) |
|---|---|---|
Control |
1.1070 (n= 10 ) ± 0.04842 |
27.2000 (n= 10 ) ± 0.48419 |
Kefir |
1.1190 (n=10 ) ± 0.04656 |
27.3100(n=10 ) ± 0.58356 |
CCl4 |
1.9900 (n= 5 ) ± 0.13565 a,b |
22.3000 (n= 5 ) ± 0.96954 a,b |
Combination |
1.3900 (n= 5 ) ± 0.08741 a,b,c |
24.4000 (n= 5 ) ± 0.87178 a,b,c |
Total |
1.3103 (n=30 ) ± 0.06545 |
25.9533 (n=30 ) ± 0.47766 |
Data are presented as mean ± standard error of 10 animals/group.
aSignificantly different from control value at p< 0.05.
bSignificantly different from Kefir value at p< 0.05.
cSignificantly different from CCl4 value at p< 0.05.
Data were calculated as relative weight of liver to 100 g animal body weight at the end of the experiment. Data are presented as mean ± standard error of 10 animals/group.
a, b or *, c indicates significant difference from control, kefir or corresponding initial body weight respectively at p<0.05 using Tukey’s test as post
ANOVA test and as showing in (Fig. 4).
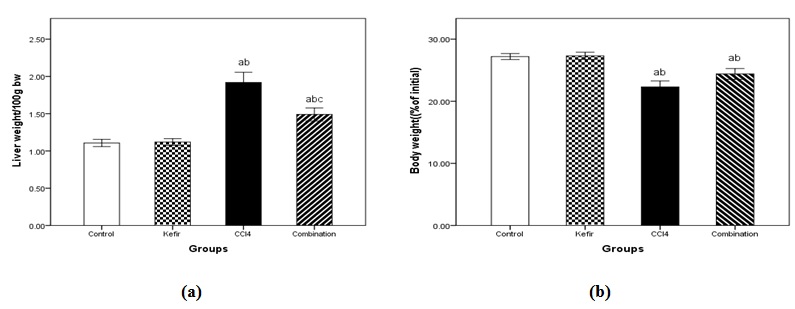
Fig. 4. Effects of kefir on (a) liver weights and (b) total body weights of mice treated with carbon tetrachloride (CCL4) after 4 weeks. Data are presented as mean ± standard error of 10 animals/group.a Significantly different from control value at p< 0.05.b Significantly different from Kefir value at p< 0.05.c Significantly different from CCl4 value at p< 0.05.
Effect of treatment on liver function
The serum levels of liver functions (AST, ALT, and ALP) are presented in (Table 3). In the CCl4 treated group, the serum levels of AST, ALT, and ALP p <0.05, increased to 1372.6367 ± 2.06498, 1410.2500 ± 2.60688 and 251.4583 ± 16.79796 respectively compared to negative control group values of 38.1200±.60255, 45.0820±.80311 and 67.8300 ±.50400 respectively. The pretreatment of CCl4-treated mice with kefir significantly p <0.05, decreased the CCl4 induced elevation of these markers levels to 561.5050±2.79362, 472.8833±1.85210, 112.5600 ±2.62721, respectively. Interestingly, kefir administration does not exhibit any significant change from control values of liver functions that is mean the Kefir protects liver against carbon tetrachloride as showing in (Fig. 5).
Table (3):
Effect of kefir and carbon tetrachloride on liver function test (AST, ALT and ALP) in mice after four weeks of treatment
Treatment Groups |
AST (IU/L) |
ALT (IU/L) |
ALP (IU/L) |
|---|---|---|---|
Control |
38.1200 (n= 10 ) ± 0.60255 |
45.0820 (n= 10 ) ± 0.80311 |
67.8300 (n= 10 ) ± 0.50400 |
Kefir |
39.3470 (n= 10 ) ± 0.35139 |
46.4830 (n= 10 ) ± 0.43301 |
68.5500 (n= 10 ) ± 0.56239 |
CCl4 |
1372.6367 (n= 6) ± 2.06498 a,b |
1410.2500 (n= 6) ± 2.60688 a,b |
251.4583 (n= 6) ± 16.79796 a,b |
Combination |
561.5050 (n= 6) ± 2.79362 a,b,c |
472.8833 (n= 6) ± 1.85210 a,b,c |
112.5600 (n= 6) ± 2.62721 a,b,c |
Total |
386.8600 (n= 32 ) ± 92.22711 |
381.7016 (n= 32 ) ± 93.40436 |
110.8722 (n= 32 ) ± 12.84858 |
aSignificantly different from control value at p< 0.05.
bSignificantly different from Kefir value at p< 0.05.
cSignificantly different from CCl4 value at p< 0.05.
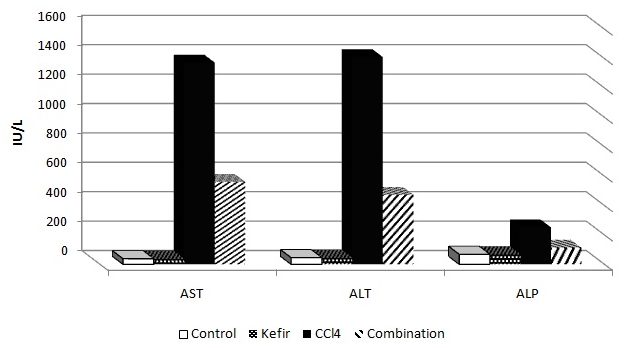
Fig 5: Effect of kefir and carbon tetrachloride on liver functions (AST, ALT and ALP) in mice after four weeks of treatment.
– Data were presented as mean ± standard error of 10 animals/group.
–a, b or c indicates significant difference from control, kefir or CCl4 respectively at p <0.05 using Tukey’s test as post ANOVA test.
– ALP: Alkaline phosphatase; AST: Aspartyl aminotransferase; ALT: Alanine aminotransferase.
Histopathological examination
Liver
Control group
In the control group, the liver was histologically normal without noticeable alterations (Fig. 6a) with well demarcated hexagonal lobules having a central vein with normal portal traits containing artery, vein and bile ducts. The hepatocytes within the hepatic lobules were arranged in cords radiating around the central veins and separated by hepatic sinusoids.
Carbon tetrachloride (CCl4) group
In this group, the liver of the animal showed variable degrees of degeneration, necrosis and inflammation. Moderate to severe vacuolation of hepatocytes (hydropic degeneration to fatty changes) were seen especially at the periphery of the lobules (Fig.6b), in contrast to those around central veins which appeared normal. Mild to moderate sinusoidal dilatation with active proliferation of van Kupffer cells were noticed in most animals (Fig. 6c). Necrotic changes accompanied with fatty changes were also found in some areas; in which the hepatocytes had pyknotic nuclei with strong eosinophilic cytoplasm or severe destruction (Fig. 6d). Mild to moderate portal tracts and hepatic parenchyma infiltration with leucocytes was evident (Fig. 6e) with congested blood vessels.
In CCl4-kafir treated group
Histopathological examinations proved mild to moderate improvements in the form of absence of hepatic necrosis, however, some cells showed degenerative changes (Fig. 6f &g). Sings of regeneration was noticed in some areas as some cells showed mitotic activities and binucleation in others. The hepatic sinusoids appeared also of normal appearance with mild congestion was seen in some animals. Compared to the CCl4 treated animals, much less damage could be seen especially in the peri-central hepatic cells with mild inflammatory changes.
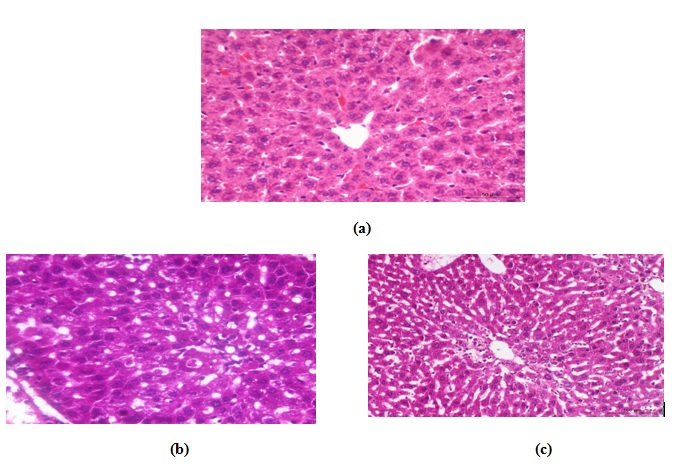
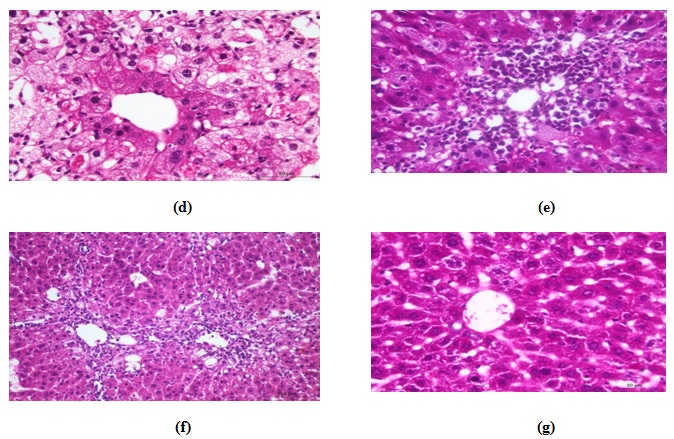
Fig. 6. Photomicrographs of hematoxylin and eosin stained histological section of (a) normal liver, (b) CCl4 –treated animals showing variable degrees of vacuolar degeneration, (c) CCl4 –treated animals showing widened sinusoids, active proliferated Von kupffer cells, (d) CCl4 –treated animals showing necrotic changes in the form of severe destruction of hepatocytes or karyolysis, (e) CCl4 –treated animals showing leuco-cytic infiltration in the portal area and hepatic parenchyma, (f) CCl4 –kefir treated animal showing much less damages in the hepatic parenchyma , more or less normal hepatocytes, and (g) CCl4 –kefir treated animal show-ing very mild vacuolar degeneration with no evidence of necrosis, mild congestion and no inflammatory changes.
Kidneys
Control group
The renal tissue of this group appeared of normal structure especially the renal cortex (Fig. 7a). Renal tubules and glomeruli were histologically normal. The tubules were linned by columnar epithelium and Bowman’s capsules were normal and had normal glomeruli. Minimal changes were noticed in some areas in renal medulla. The renal pelvis also appeared normal.
CCl4–treated group
Histopathological examination revealed mild pathological alterations. The most common changes were hydropic degeneration of renal epithelium of some tubules (Fig. 7b) with mild congestion of some blood vessels (Fig. 7c). Early necrotic changes were demonstrated in outer cortex. Glomerulonephritis was seen some areas (Fig. 7d). Marked vacuolation was evident in renal medulla. No signs of an inflammatory reaction could be seen except in one case, which appeared as focal mononuclear cell infiltration in the renal cortex (Fig. 7e).
CCl4-Kefir treated group
The histopathological changes in these animals were much less in comparing to the CCl4-treated group. Renal tubules had minimal pathological changes (hydropic degeneration) in most areas with mild congestion and without inflammatory changes (Fig. 7f&g). The glomeruli showed no histopathologic alterations.
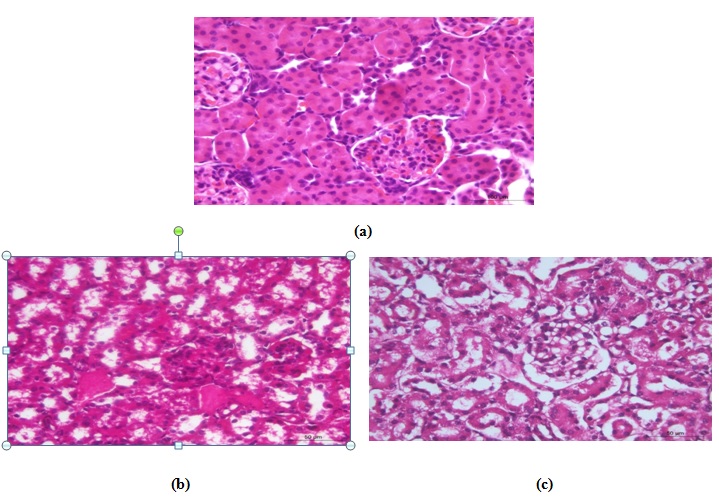
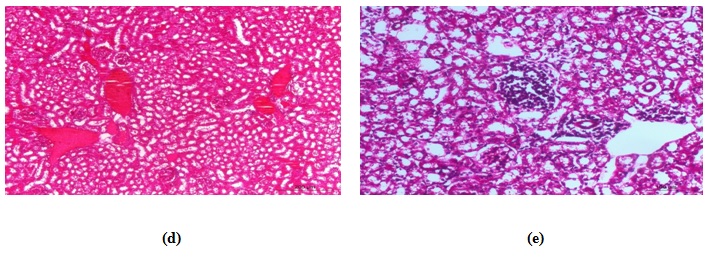
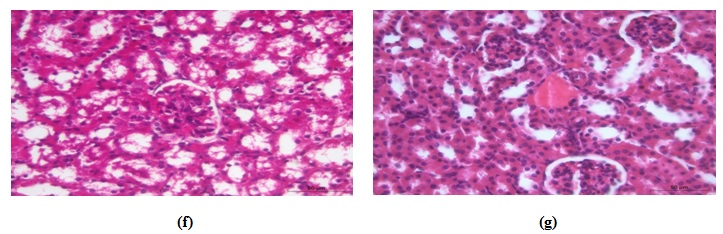 Fig. 7. Photomicrograph of kidney of (a) control mouse showing normal structure, (b) CCl4-treated mouse had vacuolar degenerative changes in the renal epithelium, (c) CCl4-treated mouse had early necrot-ic changes, (d) CCl4-treated mouse had congestion of renal blood vessels, (e) CCl4-treated mouse had focal leuco-cytic infiltrations, (f) CCl4-kefir treated mouse had moderate improvement of renal lesions and mild vacuolar degenerative changes in the renal epithelium with absence of necrotic changes, and (g) CCl4-kefir treated mouse had mild congestion of renal blood vessels with no focal inflammatory reaction.
Fig. 7. Photomicrograph of kidney of (a) control mouse showing normal structure, (b) CCl4-treated mouse had vacuolar degenerative changes in the renal epithelium, (c) CCl4-treated mouse had early necrot-ic changes, (d) CCl4-treated mouse had congestion of renal blood vessels, (e) CCl4-treated mouse had focal leuco-cytic infiltrations, (f) CCl4-kefir treated mouse had moderate improvement of renal lesions and mild vacuolar degenerative changes in the renal epithelium with absence of necrotic changes, and (g) CCl4-kefir treated mouse had mild congestion of renal blood vessels with no focal inflammatory reaction.Spleen
Control
In the control group, spleen appeared more or less normal at the level of white and red pulps (Fig. 8a).
CCl4-treated group
With regards to the spleen histopathological examination, splenic changes involved hyperplasia of lymphoid follicles of the white pulp (Fig. 8b). In some follicles mild lymphocyte destruction (rarefication) was found. Moreover, the red pulp of splenic tissues showed significant congestion of blood vessels and sinusoids with mild edematous changes (Fig. 8c).
CCl-kefir treated group
The remedy effect of Kefir was observed in splenic tissues that revealed no prominent lymphoid hyperplasia in the white pulp or congestion in the red pulp; so it appeared to be more or less normal (Fig. 8d).
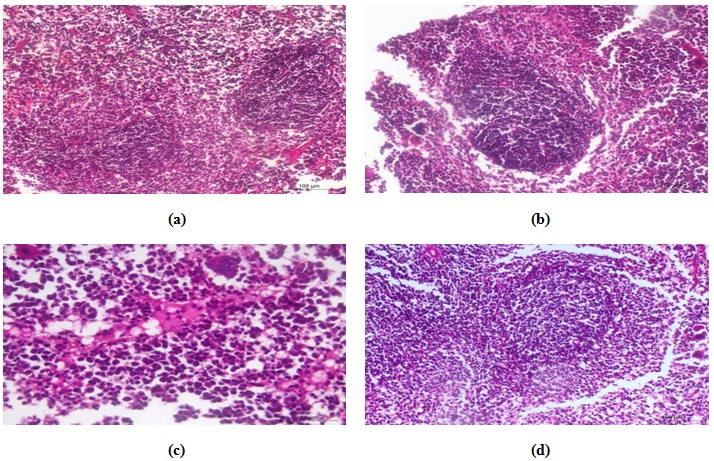
Fig. 8. Photomicrograph of spleen of (a) control animal showing normal histological structures, (b) CCl4 treated animal had hyperplasia of lymphoid follicle with rarefication of lymphoid elements, (c) CCl4 treated animal showing eosinophilic edematous changes, and (d) CCl4-kefir treated mouse had more or less nor-mal histological structures.
Statistical analysis
All data were expressed as means ± standard error of the mean (S.E.M). Statistical analysis was done using statistical packages for social sciences (SPSS) computer software (version 22), IBM software, USA. One-way analysis of variance (ANOVA) test was used to elucidate significance among group means, followed by Tukey’s post-hoc test to compare mean values pair-wise. Differences were considered significant at p <0.05.
Recently, there is a strong focus on beneficial foods with probiotic microorganisms and functional organic substances especially the commercial use of kefir. It may act as a matrix in the effective delivery of probiotic microorganisms in different types of products as it has a biological activity due to the presence of kefir’s exopolysaccharides, known as kefiran22. Kefir is mainly considered a probiotic resource because of its composition31. According to definition “Probiotics are microbial cell preparations or components of microbial cells with a beneficial effect on the health of the host”. Some studies suggest that probiotic bacteria in kefir consumers’ gut are increased and play an important role in health improvement9, 32.
The probiotic species, particularly lactobacilli are equipped for creating an extensive variety of antimicrobial mixes, counting natural acids (lactic and acidic acids), carbon dioxide, hydrogen peroxide, ethanol, diacetyl and peptides (bacteriocins) that can be helpful not just in diminishing sustenance pathogens and bacterial harm amid capacity and sustenance consumption, additionally in the treatment and counteractive action of gastrointestinal and vaginal infection25 kefiran has more advantages, comparing to other polysaccharides, such as bactericidal, fungicidal, antitumor properties33, 34 anti-inflammatory and promote healing35, 36, immunomodulation or epithelium protection37 and antioxidant activity38.
Our results demonstrated that after 24 h as well as 48h, fermented kefir possesses high antibacterial activity against Gram-negative and Gram-positive including Staphylococcus aureus, E. coli and Salmonella Enteritidis. The antimicrobial activity was superior to control antibiotic, although exhibited no activity against P. aeruginosa and C. albican. These results agree with previous study which showed that kefir as a probiotic can restrain the action of coliform microscopic organisms, and some entero pathogenic microscopic organisms like Shigella sp., Salmonella sp., and of Gram-positive microorganisms, for example, S. aureus, Bacillus cereus, Clostridium sp. and Listeria monocytogenes39.
In this study, isolated microorganism from kefir were closely related to Micrococcus cohnii (isolate ID SK14), Lactobacillus kefiranofaciens ZW3 (isolate ID SK22) and Lactobacillus casei strain KF11 (isolate ID SK23).
Micrococci, like many other representatives of the Actinobacteria, can be metabolically versatile, with the ability to utilize a wide range of unusual substrates, such as pyridine, herbicides, chlorinated biphenyls, and oil. They are likely involved in detoxification or biodegradation of many other environmental pollutants Other Micrococcus isolates produce various useful products, such as long-chain (C21-C34) aliphatic hydrocarbons for lubricating oils40.
An exopolysaccharide (EPS) producing strain, ZW3, was isolated from Tibet kefir grain and was identified as Lactobacillus kefiranofaciens. FT-IR spectroscopy revealed the presence of carboxyl, hydroxyl, and amide groups, which correspond to a typical hetero polymeric polysaccharide. The GC analysis of ZW3 EPS revealed that it was glucogalactan in nature34.
Lactobacillus casei is a species of the genus Lactobacillus found in the human intestine and mouth. This particular species of Lactobacillus is documented to have a wide pH and temperature range, and complements the growth of L. acidophilus, a producer of the enzyme amylase (a carbohydrate-digesting enzyme)25.
Kefir was found to have a protective effect against CCI4-induced damage in liver, spleen and kidney. Histopathologically, compared to CCl4 treated mice, mild to moderate improvements in the form of absence of neither hepatic degeneration nor necrosis with sings of regeneration (increased mitotic activities and bi-nucleation). The hepatic sinusoids appeared also of normal appearance with mild. Renal tissues showed minimal degeneration. Spleen also showed marked improvement comparing to CC14 treated animals.
Kefir has a histopathological preventive attribute in animal model as it lower the necrobiotic changes in acute renal injury. The adverse findings of CCl4 (hepatocellular damage and apoptosis) were reduced with kefir administration; this indicating that kefir has a protective role at liver damage41. Also it has been found that no toxic effect of L. kefiranofaciens M1 was seen at the gross and microscopic histopathology of the organs (heart, liver, kidney, adrenal glands, spleen, ovary, and testis)42.
Kefir was chosen in our study as a potential protective agent because of its antioxidant and hepatoprotective activity. According results of this study, CCl4 induced liver toxicity in mice and it is harmful to other organs such as kidney and spleen which was in the form of increased liver weight to body weight, elevated liver enzymes and alkaline phosphatase, an indication of structural and functional defects in liver cells. Marked improvement was evident with treatment with kefir as indicated by estimation of body weight and relative liver weight to body weight; the ratio of liver weight to 100 g body weight was significantly increased by sole administration of CCl4 (1.9900±.13565, p <0.05) compared to control animals (1.1070±.04842). Interestingly treatment with both kefir and CCl4 exhibited liver weight/100 g body weight ratio (1.3900±.08741) which is significantly lower than CCl4 group (p <0.05) and was close to normal value.
At the end of experiment, comparing the animal total body weight to its corresponding initial value, only CCI4 group exhibited a significant decrease compared to its corresponding initial weight. Note northerly, the body weights exhibited by combination group had higher values compared to both its initial body weight and body weights exhibited by CCl4-treated group, however, it is still less than the control value. Data were calculated as relative weight of liver to 100 g animal body weight at the end of the experiment. Data are presented as mean ± standard error of 10 animals/group.
Kefir effectively has protection against CCl4-induced hepatotoxicity in mice. These protections are approved via the serum levels of liver functions (AST, ALT, and ALP). In the CCl4 treated group, the serum levels of AST, ALT, and ALP p <0.05, were increased to 1372.6367 ± 2.06498, 1410.2500 ± 2.60688 and 251.4583 ± 16.79796 respectively compared to negative control group values of 38.1200±.60255, 45.0820±.80311 and 67.8300 ±.50400 respectively. The pretreatment of CCl4-treated mice with kefir significantly p <0.05, decreased the CCl4 induced elevation of these markers levels to 561.5050±2.79362, 472.8833±1.85210, 112.5600 ±2.62721, respectively. Interestingly, kefir administration does not exhibit any significant change from control values of liver functions that is mean the Kefir protects liver against carbon tetrachloride.
In conclusion, our findings revealed that kefir has antimicrobial activity against pathogenic microorganisms and protective properties against CCl4-induced hepatotoxicity. These protective effects included anti-inflammatory effect and inhibition of CCl4 activity with improving of liver functions. So, kefir may have the potential for clinical applications to the prevention and/or treatment of liver toxicity.
- Adriana, P., Socaciu, C. Probiotic activity of mixed cultures of kefir’s lactobacilli and non-lactose fermenting yeasts. Bull. UASVM. Agric., 2008; 65: 329–334.
- Saloff-Coste, C. Kefir. Danone World Newsletter, 1996; 11(4): 27.
- Garrote, G.L., Abraham, A.G., De Antoni, G.L. Characteristics of kefir prepared with different grain [ratio] milk ratios. J. Dairy Res., 1998; 65(01): 149–154.
- Loretan, T., Mostert, J., Viljoen, B. Microbial flora associated with South African household kefir. S. Afr. J. Sci., 2003; 99(1–2): 92–94.
- Garrote, G.L., Abraham, A.G., De Antoni, G.L. Inhibitory power of kefir: the role of organic acids. J. Food Protec., 2000; 63(3) 364 – 369.
- Duitschaever, C., Kemp, N., Emmons, D. Pure culture formulation and procedure for the production of kefir. Milchwissenschaf, 1987; 42(2): 80-82.
- Farnworth, E.R. Keûr–a complex probiotic. Food Sci. Technol. Bull., 2006; 2(1): 1–17.
- Dong, H., Dana, J., Hyunsook, K., Byeong, K. Antimicrobial Activity of Kefir against Various Food Pathogens and Spoilage Bacteria. Korean J. Food Sci. Anim. Resour., 2016; 36(6): 787–790.
- Abraham, A.G., Antoni, G.L. Characterization of kefir grains grown in cows’ milk and in soya milk. J. Dairy Res., 1999; 66(02): 327–333.
- Rattray, D.D., O’Connell, C.M., Baskett, T.F. Acute disseminated intravascular coagulation in obstetrics: a tertiary centre population review (1980 to 2009). J. Obstet. Gynaecol. Can., 2012; 34(4): 341–347.
- Farnworth, E.R., Mainville, I. Kefir: a fermented milk product. Handbook of fermented functional foods, 2003; 2: 89–127.
- Farnworth, E.R. The evidence to support health claims for probiotics. J. Nutr., 2008; 138(6): 1250-1254.
- Garrote, G.L., Abraham, A.G., De Antoni, G.L. Microbial Interactions in Kefir: A Natural Probiotic Drink. Biotechnology of Lactic Acid Bacteria: Novel Applications, 2010; 327.
- Mainville, I., Robert, N., Lee, B., Farnworth, E.R. Polyphasic characterization of the lactic acid bacteria in kefir. Syst. Appl. Microbiol., 2006; 29(1): 59–68.
- Rattray, F., O’Connell, M. Fermented milks kefir. Encyclopedia of dairy sciences, 2011; 2: 518–524.
- Shikongo-Nambabi, M.N.N.N., Shoolongela, A., Schneider, M. ontrol of bacterial contamination during marine fish processing. Journal of Biology and Life Science, 2011; 3(1): 2157–6076.
- Magalhães, K.T., Pereira, G.V.d.M., Campos, C.R., Dragone, G., Schwan, R.F. Brazilian kefir: structure, microbial communities and chemical composition. Braz. J. Microbiol., 2011; 42(2): 693–702.
- Rea, M., Lennartsson, T., Dillon, P., Drinan, F., Reville, W., Heapes, M. Cogan, T. Irish kefir like grains: Their structure, microbial composition and fermentation kinetics. J. Appl. Bacteriol., 1996; 81(1): 83–94.
- Leite, A.M.d.O., Miguel, M.A.L., Peixoto, R.S., Rosado, A.S., Silva J.T., Paschoalin, V.M.F. Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz. J. Microbiol., 2013; 44(2): 341–349.
- Garrote, G., Abraham, A. De Antoni, G. Preservation of kefir grains, a comparative study. LWT- Food Sci. Technol., 1997; 30(1): 77–84.
- Kaufmann, K.D. Kefir rediscovered. Alive Book, Burnahy, 1997; 86.
- Wszolek, M., Kupiec-Teahan, B., Guldager, H.S., Tamine, A. Production of kefir, koumiss and other related products. Fermented milks, 2006; 174–216.
- Odet, G. Fermented milks. Bulletin-Int. Dairy Federation, 1995; (300): 98–100.
- Lazar, V., Balotescu, M.C., Vassu, T., Barbu, V., Smarandache, D., Sasarman, E., Israil, A., Bulai, D., Alexandru, I. Cernat, R. Experimental Study on Rats of the Probiotic Effect of Some Lactic Acid Bacteria Previously Selected tor Their In Vitro Capacity to Interfere with Salmonella enteritidis Infection. Roum. Biotech. Lett., 2005; 10: 2123–2133.
- Chifiriuc, M.C., Stecoza, C., Dracea, O., Larion, C., Israil, A. Antimicrobial activity of some new O-acyloximino-dibenzo [b, e] thiepins and O-acyloximino-dibenzo [b, e] thiepin-5, 5-dioxides against planktonic cells. Roman. Biotechnol. Lett., 2010; 15(2): 5134-5139.
- Ahmed S.H., Amin, M.A., Saafan, A.E., El-Gendy, A.O., ul Islam, M. Measuring susceptibility of Candida albicans biofilms towards antifungal agents. J. Microbiol. and Biotechnol. Res., 2017; 3(1): 149-156.
- Ahmad, M.S., El-Gendy, A.O., Ahmed, R.R., Hassan, H.M., El-Kabbany, H.M., Merdash, A.G. Exploring the Antimicrobial and Antitumor Potentials of Streptomyces sp. AGM12-1 Isolated from Egyptian Soil. Front. Microbiol., 2017; 8.
- Tamura, K. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molec. Biol. Evolu., 2007; 24(8): 1596-1599
- Aspiras, B.E.E., Flores, R.F.A.C., Pareja, M.C. Hepatoprotective effect of Fermented Water Kefir on Sprague-Dawley rats (Rattus norvegicus) induced with sublethal dose of Acetaminophen. Int. J. Curr. Sci., 2015; 17: 18–28.
- Bancroft, J.D., Gamble, M. Theory and practice of histological techniques. Elsevier Health Sciences, 2008.þ
- Nalbantoglu, U., Cakar, A., Dogan, H., Abaci, N., Ustek, D., Sayood, K., et al. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol., 2014; 41: 42–51.
- Zheng, Y., Lu, Y., Wang, J., Yang, L., Pan, C., Huang, Y. Probiotic properties of Lactobacillus strains isolated from Tibetan Kefir grains. PLoS ONE, 2013; 8:e69868.
- Cevikbas, A., Yemni, E., Ezzedenn, F.W., Yardimici, T., Cevikbas, U., Stohs S.J. Antitumoural, antibacterial and antifungal activities of kefir and kefir grain. Phytother. Res., 1994; 8: 78–82.
- Wang, Y., Ahmed, Z., Feng, W., Li, C., Song, S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol., 2008; 43: 283–288.
- Rodrigues, K.L., Caputo, L.R.G., Carvalho, J.C.T., Evangelista, J., Schneedorf. J. M. X Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents, 2005a; 25: 404–408.
- Rodrigues, K.L., Carvalho, J.C.T., Schneedorf, J.M. Anti-inflammatory properties of kefir and its polysaccharide extract. Inflammopharmacology, 2005b; 13: 485–492.
- Serafini F., Turroni, P., Ruas-Madiedo, G.A., Lugli, C., Milani, S., Duranti, N., et al. Kefir fermented milk and kefiran promote growth of Bifidobacterium bifidum PRL2010 and modulate its gene expression. Int. J. Food Microbiol, 2014; 17850–59.
- Chen Z., Shi J., Yang X., Nan B., Liu Y., Wang Z. Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J., 2015; 43: 15–21.
- Van Wyk, J. The inhibitory activity and sensory properties of kefir, targeting the low-income African consumer market, Stellenbosch: Stellenbosch University, 2000.
- Yanping, W., Jingrui, W., Zaheer, A., Xiaojia, B. Complete Genome Sequence of Lactobacillus kefiranofaciensZW3. J. Bacteriol., 2011; 193(16): 4280–4281.
- Ozsoy, ^.Y. The Protective Effect of Kefir on Carbon Tetrachloride-induced Histopathological Changes in the Livers of Rats. Kafkas Universitesi Veteriner Fakultesi Dergisi, 2016; 22(3): 403-408.
- Owaga, E.E., Chen, M.J., Chen, W.Y., Chen, C.W., Hsieh, R.H. Oral toxicity evaluation of kefir-isolated Lactobacillus kefiranofaciens M1 in Sprague-Dawley rats. Food Chem. Toxicol., 2014; 70:157-62.
- Saitou, N., Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molec. Biol. Evolu., 1987; 4(4): 406-425.
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol., 1980; 16(2): 111-120.
- Kumar, S., Stecher, G. Tamura, K. MEGA7 2016: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular biology and evolution, 2016; 54.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


